Abstract
Some fruits are used as promising anticancer agents due to their antioxidant and antimutagenic properties. This work explores the potential of the fruit of Pourouma cecropiifolia Mart. as a source of compounds with anticancer activity. The research covers: (i) phytochemical profiling of hydroethanolic extracts from the peel, pulp, and seeds of P. cecropiifolia Mart. using liquid ultra-performance chromatography quadrupole time-of-flight mass spectrometry (UPLC-QTOF-MS); (ii) evaluation of antioxidant and anticancer potential of the extracts against carcinoma cell lines (HeLa, RKO, MCF-7, and T47D); and (iii) in silico docking analyses with human cytochrome P450 1A1 (CYP 1A1) and CYP 1B1. A total of 18 compounds were tentatively identified by UPLC-QTOF-MS, including flavonoids, phenolic glycosides, lignans, phenolic acids, terpenes, iridoid glycosides, proanthocyanidins, curcuminoids, and naphthodianthrone. Molecular docking simulations identified nortracheloside and epicatechin as potential inhibitors of CYP 1A1 and CYP 1B1, suggesting cytotoxic activity. The antiproliferation assay showed that pulp extracts had moderate activity against human breast ductal cancer cells.
Similar content being viewed by others
Cancer is among the leading causes of death worldwide, with 19.3 million new cases and 10.0 million deaths reported in 20201. Breast cancer in particular is rising in prevalence, with an estimated 2.3 million new global cases, according to the Global Cancer Statistics 2020 report2,3. Despite advancements in cancer diagnosis and treatment, barriers to clinical care and predictive diagnosis persist. Multimodal treatment approaches, including radiation, surgery, and chemotherapy, remain the standard for managing advanced cancer4. However, these treatments are often inadequate, as they cannot eliminate all cancer cells, necessitating the development of alternative therapies.
Research has shown natural products to be promising alternative anticancer treatments2,5,6. Many plant-derived bioactive molecules have the potential to contribute to the medical, agricultural, and nutritional fields7. Phytochemicals, which are specialized metabolites, are known to modulate multiple biological pathways in living tissue, offering a range of benefits, such as antioxidant, antimicrobial, anti-inflammatory, anti-diabetic, and anti-cancer properties8. These phytochemicals are categorized into major classes—phenolic compounds, carotenoids, alkaloids, terpenes, vitamins, and anthocyanins—based on their physiochemical properties, distribution in nature, and biosynthetic pathways9.
The Amazon rainforest, one of the world’s most biodiverse areas, is home to approximately 40,000 plant species10. In particular, Ecuador is recognized as one of 17 megadiverse countries owing to its high plant and animal biodiversity11. Many of its botanical species remain underexplored for their phytochemical composition. Among these, the Cecropiaceae family has shown considerable potential given its phenolic compound-rich chemical profile12,13,14.
In this context, Pourouma cecropiifolia Martius (commonly known as uvilla, uva de monte, and orsacha uvillas)15 was selected for investigation. This tropical plant, native to the Ecuadorian Amazon (distributed throughout the Pastaza, Sucumbíos, Napo, Zamora Chinchipe, and Morona Santiago provinces) at altitudes of 200–900 masl, produces sweet, juicy fruit typically consumed fresh or made into jams and wine16. The immature fruits are green, which turn purple after ripening. Fruits are spherical drupes 2–4 cm in diameter with a sweet, mucilaginous flesh containing one seed14. However, its susceptibility to mechanical damage and high perishability pose challenges for broader commercial use17.
Previous studies have partially characterized the phenolic profile of P. cecropiifolia Mart. (Cecropiaceae), identifying compounds such as rutin, quercetin derivatives, catechin, epicatechin, procyanidin B, and four hydroxycinnamic acid derivatives16. Notably, peel extracts of P. cecropiifolia Mart. have shown cytotoxic effects on cancer cell lines16. However, comprehensive data on the chemical composition and biological activities of hydroethanolic extracts from the peel and seed remain limited. This study aims to address these gaps by investigating the cytotoxic and antioxidant activity of hydroethanolic extracts made from P. cecropiifolia Mart. pulp, peel, and seeds and profiling their bioactive compounds using ultra-performance liquid chromatography quadrupole time-of-flight mass spectrometry (UPLC-QTOF-MS). The findings contribute to a deeper understanding of the potential applications of P. cecropiifolia Mart. in nutraceutical development.
Results and discussion
Bioactive compounds
Flavonoids and phenolic compounds derived from botanical sources are well-known for their extensive pharmacological activities18,19. As shown in Table 1, TPC ranged from 0.30 ± 0.01 to 23.18 ± 0.04 mg gallic acid equivalent (GAE) g−1, flavonoids 1.02 to 2.3 mg quercetin equivalent (QE) g−1, yellow flavonoids from 0.69 to 1.46 mg QE g−1, and anthocyanin from 0.16 ± 0.00 to 8.43 ± 0.04 mg g−1 d.w. Among the fruit parts, seeds exhibited the highest total TPC (23.18 ± 0.04 mg GAE g−1) and flavonoid content (0.31 ± 0.00 mg QE g−1 d.w.). The highest anthocyanin content (8.43 ± 0.040 mg g−1) was observed in the peel extract, while pulp exhibited the lowest TPC (0.30 ± 0.01 mg GAE g−1) and total flavonoid content (0.03 ± 0.00 mg QE g−1). The overall phenolic content of the fruit was higher than the flavonoid content. In contrast to our findings, Luts et al.16 found anthocyanins primarily in the peel of P. cecropiifolia Mart., and Ordonez et al.20 reported high TPC in its peel and seeds. We observed slightly higher TPC and total anthocyanin content in the seed extract, which is possibly attributable to variations in cultivars, climatic conditions, fruit ripeness stage, and the type of extraction solvent used.
The chromatographic analysis of extracts from the peel, pulp, and seed of P. cecropiifolia Mart. revealed the presence of ascorbic acid and trans-resveratrol. Ascorbic acid is an essential nutrient that serves as a cofactor for collagen prolyl hydroxylases, playing a crucial biological role21. It exhibits notable antioxidant22 and anticancer23,24,25 properties, including enhancing TET2 dioxygenase activity in myeloid neoplasia26. According to the results, ascorbic acid was detected exclusively in the P. cecropiifolia Mart. peel extract (4.67 ± 0.28 mg 100 g−1 d.w.) (Table 1). The ascorbic acid content was comparable to levels found in fruits such as Pyrus communis L. (2.8 mg 100 g−1), Eriobotrya japonica Lindl. (3.2 mg 100 g−1), and Eugenia malaccensis L. (3.8 mg 100 g−1)27.
Trans-Resveratrol (3,4′,5-trihydroxystilbene), a stilbenoid polyphenol, is widely distributed among plant taxa, including grape, pine, blueberry, cranberry, bilberry, and peanuts28. It exhibits diverse pleiotropic health benefits against various chronic diseases29,30,31, and in-vitro screening has identified it as a first-generation sirtuin-activating compound32 that activates nicotinamide adenine dinucleotide-dependent silent information regulator 2 (SIR2) deacetylase33, contributing to its therapeutic effects.
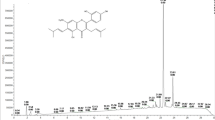
For the first time, this study identified the presence of trans-resveratrol in P. cecropiifolia Mart. using UPLC-ESI-QTOF-MS analysis. Deprotonated [M–H] ions were identified at m/z 227.97 (Fig. 1). The highest trans-resveratrol concentration was found in the peel extract (21.00 ± 0.30 µg g−1), with lower levels detected in the pulp (2.60 ± 0.00 µg g−1) and none in the seeds. Comparatively, the trans-resveratrol content in the peel of P. cecropiifolia Mart. was higher than that found in the peels of Vitis labrusca (3.54 µg g−1) and Syzygium cumini L. (11.19 µg g−1)34. However, higher trans-resveratrol levels have been reported in M. rubra fruit (50.61 µg g−1 d.w.)34. Together, these findings suggest that the trans-resveratrol and ascorbic acid identified in P. cecropiifolia Mart. are promising bioactive compounds that contribute to its antioxidant capacity.
Untargeted metabolic profiling
Peel, pulp and seeds extracts from P. cecropiifolia Mart. were analyzed using UPLC-QTOF-MS. The total ion chromatograms obtained in negative ionization modes are presented in Fig. 2, while Table 2 summarizes the tentative identification of metabolites, including molecular ions [M–H]−, retention times (min), accurate masses, and ion molecular formulas. The analysis revealed the tentative identification of 18 metabolites as predominant compounds in the peel, pulp, and seed extracts, including flavonoids (7), phenolic glycosides (1), lignans (2), phenolic acids (1), terpenes (1), iridoid glycosides (1), proanthocyanidins (2), curcuminoids (1), and naphthodianthrone (1).
Flavonoids
Three flavonoids (1, 2, and 4) were tentatively identified in the peel extract. Compound 1, with a retention time (tR) of 3.03 min, exhibited a deprotonated molecular ion [M−H]− at m/z 909.2027 and was identified as carthamin. Compound 2 displayed [M−H]− at m/z 547.1420, with typical fragment ions at m/z 437.08740, 143.03290, and 329.08650; this compound was tentatively identified as mirificin (C26H28O13). Compound 4, with [M−H]− at m/z 289.0714 (Rt = 7.18 min), was characterized as (-)-epicatechin.
Four additional flavonoids were tentatively identified in the seed extracts of P. cecropiifolia Mart. Compound 12 exhibited [M−H]− at 433.0768, with fragmentations at m/z 303.08185, 343.07626, and 373.10287, and was identified as quercetin-3-arabinopyranoside. Fraisse et al.35 isolated quercetin-3-arabinopyranoside from Alchemilla xanthochlora, a well-known medicinal plant recognized for its anti-inflammatory and astringent properties36. Furthermore, this compound has been reported as an α-glucosidase inhibitor37. Compound 13, with an tR of 7.04 min, displayed a deprotonated molecule at m/z 503.1227 and was tentatively identified as neocomplanoside based on electrospray ionization (ESI)-MS² analysis. The negative ESI-MS spectrum of Compound 16 generated a molecular ion peak [M–H]− at 579.1704 (Rt = 11.24 min) and was identified as narirutin, a compound previously reported in citrus species38. Finally, in the MS spectrum, Compound 17 was noted with [M–H]− at m/z 610.1932 (C28H34O15) and was identified as neohesperidin.
Lignans
Two lignans were tentatively identified in this study. The negative ESI-MS spectrum of the reference standard neoolivil-4-O-glucopyranoside (Compound 3), a deprotonated molecular ion, was detected at m/z 353.0875 (tR = 5.88 min) (C26H33O11). Similarly, the UPLC-ESI-QTOF-MS analysis identified nortracheloside (Compound 5) with a deprotonated ion at m/z 535.1826 (tR = 10.66 min) (C26H32O12). A peak in the negative ESI mode at tR = 10.66 min with [M−H]− at m/z 355.1627 was identified as denudatin B (Compound 5), previously reported as a nitric oxide synthase inhibitor39. According to the literature, nortracheloside exhibits anticancer properties40.
Proanthocyanidins
Two proanthocyanidins were tentatively identified in the P. cecropiifolia Mart. extracts. The negative ESI-MS spectrum of procyanidin B2 (Compound 9) at tR = 6.73 min showed a deprotonated molecular ion at m/z 577.1350. Procyanidin B2 has been reported to exhibit antioxidative, anti-inflammatory, and antitumor activity41. Mahuannin J (Compound 10), detected with [M−H]− at m/z 555.0951, showed parent ions at m/z 334.01500 and 391.04033.
Phenolic acid
According to the MS spectra, [M–H]− at m/z 353.0876 and two parent ions at m/z 191.05595 and 179.03457 correspond to chlorogenic acid (CGA). This compound, also found in tea and green coffee extracts, is synthesized by plants via the shikimic acid pathway. CGA was first isolated from coffee beans by K. Freudenberg in 192042, with its structure identified as 3-O-caffeoylquinic acid by Fischer and Dangschat in 193243. In clinical settings, chlorogenic acid exhibits multiple pharmacological properties, including antimicrobial, hepatoprotective, anticancer, immunomodulatory, DNA protective, neuroprotective, antioxidant, and antidiabetic activities44,45,46. Many in vitro and in vivo studies have also demonstrated that CGA has a pharmacological effect against myocardial ischemia–reperfusion injury47.
Antioxidant capacity
Antioxidants are compounds that scavenge highly reactive oxygen species, preventing chain reactions that can damage cell components such as proteins, DNA, and membrane phospholipids48. The literature has extensively documented the substantial contribution of various phytochemical components to the antioxidant activity (AA) of fruits, with phenolic compounds recognized as key contributors49,50,51. In this study, the antioxidant capacity of P. cecropiifolia Mart. was evaluated using different assays—the 2,2-diphenyl-1-picrylhidrazyl (DPPH), 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), and ferric reducing antioxidant power (FRAP) assays—as well as electrochemical methods measuring the antioxidant index 50 value (AI50) and electrochemical index (EI) to provide a comprehensive assessment of its antioxidant potential.
Table 3 shows the free radical scavenging activity of the hydroethanolic extracts from the peel, pulp, and seed of P. cecropiifolia Mart. Among the extracts, the seed and peel displayed the highest AA. Potential AA was quantified using the Trolox equivalent antioxidant capacity (TEAC) assay value. These values determined by the DPPH assay ranged from 1.34 ± 0.01 to 212.61 ± 2.92, while those obtained with the ABTS assay ranged from 2.70 ± 0.01 to 350.60 ± 2.20. The extracts’ antioxidant capacities obeyed the following order: seed > peel > pulp. The AA of P. cecropiifolia Mart. was further evaluated using the FRAP assay, which measures the ability of the extracts to reduce Fe(III) to Fe(II). The FRAP values ranged from 0.011 to 0.402 ± 0.010 mmol Fe2+ g−1 d.w. As shown in Table 3, the pulp extract demonstrated the lowest AA, whereas the seed extract showed the highest. Notably, the seed and peel extracts from P. cecropiifolia Mart. exhibited significant AA, in contrast to the results of previous studies20.
Phytochemicals are electroactive compounds with small redox potentials, reflecting their AA. Electrochemical methods are practical, rapid, and complementary tools for evaluating AA given their high sensitivity and low detection limits52. Figure 3 presents the cyclic voltammograms of O2•− in the absence (blank) and presence of hydroethanolic extracts of P. cecropiifolia Mart.
In Fig. 3a, b, c the anodic peak current intensity of the voltammograms decreases significantly upon the addition of P. cecropiifolia Mart. ethanolic extracts (peel, pulp, and seed). This decrease is because the O2•− formed by the reduction of O2 reacts with molecules in the extracts possessing antioxidant properties, resulting in a reduced oxidation signal of O2•− to O253. From the voltammograms in Fig. 3, the AI50 values were calculated by fitting the plot of (\(({{Ip}}_{a}^{0}-{{Ip}}_{a}^{s})/{{Ip}}_{a}^{0}\)) versus the extract concentration (Fig. 4). According to Table 3, the seed extract exhibited the lowest AI50 value (0.75 ± 0.02 mg L−1), followed by the peel (1.53 ± 0.03 mg L−1) and pulp (1.60 ± 0.03 mg L−1). Overall, the cyclic voltammetry (CV) analysis demonstrates the seed extract of P. cecropiifolia Mart. is a promising source of antioxidants with strong superoxide radical scavenging activity. It is noteworthy that lower AI50 values corresponded to higher antioxidant properties of the sample.
To further investigate the antioxidant properties of hydroethanolic extracts of P. cecropiifolia Mart., the EI was determined using differential pulse voltammetry (DPV). Figure 5. reveals an anodic peak with a slight shoulder for the seed extract between 0.235 and 0.546 V (vs. Ag/AgCl), while the peel extract signal extends from 0.323 to 0.638 V (vs. Ag/AgCl), and that for the pulp from 0.409 to 0.766 V (vs. Ag/AgCl). Lower potential (Epa < 0.45 V) values correspond to species with high capacity for electron donation, indicating higher AA54. Thus, the electroactive molecules in the P. cecropiifolia Mart. seed extract exhibits greater AA, as they react at lower potentials. The EI results are summarized in Table 3; EI values ranged from 3.81 ± 0.00 to 14.19 ± 0.10 μA V−1. The seed extract demonstrated the highest antioxidant potential, which is consistent with the results of the FRAP, TEACDPPH, and TEACABTS analyses.
Correlation analysis
Phenolic compounds have been shown to exhibit a positive relationship with AA55,56. Pearson’s correlation coefficients between total polyphenol content (TPC) and AA assays are presented in Table 4. The analysis revealed a strong positive linear relationship between TPC and AA assays of P. cecropiifolia Mart. (TPC-TEACDPPH: R2 = 1.000; TPC-TEACABTS: R2 = 1.000; TPC-FRAP: R2 = 1.000). Moreover, the correlation between spectroscopic and electrochemical AA assays also demonstrated a strong positive linear correlation, consistent with findings in previous studies57,58. AI50, being inversely proportional to AA, showed a moderate negative linear correlation with TPC, TEACDPPH, TEACABTS, FRAP, and EI values.
Effect of P. cecropiifolia Mart. extracts on cancer tumor cell proliferation
The inhibition of cell proliferation of hydroethanolic extracts from P. cecropiifolia Mart. (peel, pulp, and seed) on cell lines from cervix, colon, and breast tumors were also evaluated (Table 5). The results indicate that P. cecropiifolia extracts have selective cytotoxic activity on the cancer cell lines evaluated. The greatest activity was observed in the T47D cell line (ductal breast cancer), especially with the pulp extract. Low activity in the HeLa cell line suggests differences in the mechanisms of action or the sensitivity of the cells. While all 3 extracts were effective against T47D, the most active extract was pulp breast ductal cancer cells. This effect of pulp extract can be attributed to the presence of specialized metabolites, including procyanidin B2 and CGA. Previous clinical reports have shown that both procyanidin B259 and CGA60 exhibit anti-proliferative effects against breast cancer cells. In addition, acceptable IC50 values for cytotoxicity (<100 µg mL−1, according to NCI guidelines) have been reported for anthocyanin-rich extracts from the pericarp of P. cecropiifolia Mart. against HEp-2 (larynx), MKN-45 (gastric), and MCF-7 (breast) cancer cell lines14.
Molecular docking and dynamics
In the search for anticancer agents, human cytochrome P450 1A1 (CYP 1A1) and human cytochrome P450 1B1 (CYP 1B1) drug target proteins have come into a focal point in research. CYP 1A1 plays an important role in the etiology of breast carcinoma as key regulator of 2-hydroxylation of estrogen61,62. Although its implication in cancer is not fully understood yet, reduction of CYP1A1 levels by inhibition or other means impairs proliferation in breast cancer cells63. CYP1B1, on the other hand, is considered as an “universal tumor antigen” involved the estrogen metabolism and the activation of procarcinogens64,65,66,67,68. Previous studies have reported that several synthetic small molecules inhibit CYP 1A1 and 1B1 therapeutic targets in cancer cells69,70.
Molecular docking studies were conducted with eight majors naturally occurring compounds from P. cecropiifolia Mart extracts based on the total ion chromatogram as ligands and docked against CYP 1A1 and 1B1 (Table 6). The docking results showed that four compounds produced positive docking scores for either one or both human cytochrome P450 enzymes (CYP 1A1 and 1B1), suggesting they may not interact with the active sites of these enzymes. Upon examining the docking poses, procyanidin B2, neoolivil, mirificin, and carthamin were found to be too large to fit into the active sites of CYP 1A1 and 1B1, leading to several clashes with the enzymes. Consequently, these molecules were excluded as CYP targets.
Caffeic acid and epicatechin were the most active compounds with the highest binding energy towards CYP 1A1 and 1B1 underscoring their possible involvement in apoptotic signaling modulation. Previous docking studies have unveiled that epicatechin hold the potential to interact with specific targets, such as estrogen receptor beta, a potential target for triple-negative breast cancer71. These findings may support the promising synergistic activity of P. cecropiifolia Mart extracts against CYP 1A1 and 1B1 enzymes. In contrast, nortracheloside and istanbulin showed a higher affinity for CYP 1A1 than for CYP 1B1, suggesting selectivity toward this enzyme.
To understand the pharmacological activity of that most active compounds of P. cecropiifolia Mart extracts against CYP 1A1 and 1B1 enzymes, we focused our analysis on the dynamic stability of nortracheloside and epicatequin as ligands. According to the results from the simulations, the root-mean-square deviation (RMSD) values for the CYP 1A1 and 1B1 complexes with nortracheloside and epicatechin increased during the first few nanoseconds, gradually stabilizing toward the end of the simulation (Fig. 6a, b). The RMSD values fluctuated between 0.3 and 0.4 nm for all the studied systems, with slightly higher values for CYP 1B1 compared to CYP 1A1. This behavior was expected, as the protein undergoes conformational changes when a different ligand binds to the active site.
Analysis of the RMSD values for the different ligands revealed an RMSD value of 0.2 nm for the complex between nortracheloside and both CYPs (Fig. 7). In contrast, the RMSD value for epicatechin was below 0.1 nm (Fig. 7), indicating the structure maintained its conformation with minimal conformational changes throughout the simulation. The studied complexes exhibited stable dynamics, as RMSD values ≤ 0.2 nm confirm the accuracy of the docking method72.
A hydrogen bond (HB) analysis showed that for CYP 1A1, nortracheloside (Fig. 8a) formed approximately two HBs throughout the simulation, while epicatechin (Fig. 8b) formed three HBs during most of the simulation. Both ligands were able to form up to six HBs at certain points during the simulation. At the end of the simulation, nortracheloside formed HBs with S122 and the main chain of N222, N223, F224, G316, and L496. Epicatechin formed HBs with N255 and the main chain of N223, F251, and L312.
In CYP 1B1, both ligands formed, on average, two HBs during the 200 ns simulation. At the end of the simulation, both compounds remained in the active site of the CYPs. Examining the HBs at the end of the simulation, nortracheloside (Fig. 9a) formed HBs with D333 and the main chain of S127. Interestingly, epicatechin (Fig. 9b) formed the same two HBs (S127, D333) and an additional HB with the main chain of D326 (Fig. 9b). The identified HBs networks can guide the exploration of novel potential drug targeting strategies.
Finally, the binding energy results (Table 7) indicate that all values were negative, suggesting possible interactions. When comparing the two compounds, nortracheloside appeared to bind more tightly than epicatechin to both CYPs. Furthermore, the binding energy was consistent with the docking scores: for nortracheloside, the binding energy was 9.48 kcal mol−1 higher for CYP 1A1 than for CYP 1B1, implying a stronger inhibition of CYP 1A1. In contrast, epicatechin showed better docking scores and binding energy for CYP 1B1. The difference in energy (4.04 kcal mol−1) was smaller than that for nortracheloside, which aligns with the observed docking score differences. In summary, these findings suggest that nortracheloside, epicatechin, along with other bioactive compounds in the extracts, hold promise as CYP 1A1 and 1B1 inhibitors.
In this study, the anticancer and antioxidant potential of hydroethanolic peel, pulp, and seed extracts of P. cecropiifolia Mart. were investigated. The findings of this study, which include UPLC-ESI-QTOF-MS and in silico analysis, are notable as they reveal that extracts of P. cecropiifolia Mart contain bioactive compounds of medicinal importance. Our findings revealed that pulp extracts of P. cecropiifolia Mart. shows moderate anticancer activity against breast ductal cancer cells. Furthermore, the results of an in silico study of the naturally occurring compounds of P. cecropiifolia Mart revealed that they can inhibit CYP 1A1 and 1B1 synergistically to prevent cancer. Nonetheless, the potential of these plant extracts and selected compounds implicated in treating cancer cell lines necessitates further pharmacological studies to validate the in vitro and in silico explorations. Finally, the current findings may provide the scientific base to conduct more studies on these plant extracts.
Methods
Chemicals, reagents, and cell lines
The following compounds were used in this study: DPPH, ferric chloride, ethanol, methanol, aluminum chloride, sodium nitrite, gallic acid, quercetin, tetrabutylammonium hexafluorophosphate, dimethyl sulfoxide, and tetrabutylammonium hexafluorophosphate (Bu4NPF6), all of which were extra-pure grade (>99%) and obtained from Sigma Aldrich (St. Darmstadt, Germany). Authentic phenolic compound standards used in the chromatographic analysis, including ascorbic acid (>99%) and trans-resveratrol (>99%), were also purchased from Sigma Aldrich. The chromatographic solvents used were of RP-HPLC-DAD grade. Ultrapure water (18 MΩ cm−1 conductivity) was prepared by purifying deionized water using a Thermo ScientificTM BarnsteadTM GenPureTM water purification system.
Cervix adenocarcinoma (Hela, CRM-CCL-2), colon (RKO, CRL-2577), breast cancer (MCF-7, HTB-22), and ductal breast cancer T-47D (HTB-133) were purchased from the American Type Culture Collection (Manassas, VA, USA). 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS) was purchased from Promega, Madison, Wisconsin, USA.
Fruit collection and extraction
Samples of P. cecropiifolia Mart. were purchased from a local supplier in Parroquia Puerto Napo, Tena, Napo Province (1°02'19.2“S, 77°45'41.8“W) during the fruiting season (January–March 2022). The fruits were placed in sterile plastic bags and transported to the laboratory inside an icebox. For analysis, the peel, pulp, and seeds of the fruits were used. The samples were lyophilized using an SP Scientific-Genevac (BTP-9E LOVE) for 72 h until a constant weight was achieved. The lyophilized material was kept in an ultra-low freezer at −20 °C. Subsequently, the dried samples were ground into a fine powder using a laboratory mill. For extraction, powdered samples were soaked in a hydroethanolic solution (ethanol:H2O, 40:60, v/v) at room temperature at a ratio of 1:10 (w/v). The resulting mixtures were then vortexed for 120 min under light-protected conditions (Supplementary Table 1). The supernatants were collected in 5 mL amber vials and stored in a refrigerator at −4 °C for further analysis73.
Determination of TPC
The TPC of the extracts was evaluated following the method described by Kupina et al.74. Briefly, 100 µL of the extract was added to a test tube containing 40 µL of Folin–Ciocalteu reagent and 860 µL of water. The mixture was vigorously shaken and allowed to stand for 5 min. Subsequently, 100 µL of 7% (w/v) sodium carbonate and 900 µL of water were added. The resulting mixture was kept in the dark for 30 min. The absorbance was measured at 750 nm using a UV–vis spectrophotometer (Shimadzu UV-1280). TPC was expressed as milligrams of GAE per gram of dried extract (mg GAE g−1 d.w.).
Anthocyanin content
Anthocyanins were extracted using a 1:50 (m:v) ratio with 1.5 M HCl prepared in 85% ethanol. The extract was stored for 24 h at 4 °C75. After filtration, the absorbance was measured using a UV–vis spectrophotometer (UV-DR6000, Hach, Loveland, CO, USA). Anthocyanin content was calculated using the absorption coefficient of 982 (g 100 mL−1)−1 cm−1 75.
Total flavonoid content
The total flavonoid content of the hydroethanolic extracts was quantified using the aluminum chloride colorimetric assay as described by Chang et al.76 The calibration curve was prepared by dissolving quercetin in methanol. In brief, 0.1 mL of the hydroethanolic extract or quercetin standard was mixed with 0.1 mL of 10% (w/v) aluminum chloride solution and 0.1 mL of 0.1 mM potassium acetate solution. The mixture was incubated for 30 min at room temperature. Absorbance was measured at 415 nm using a UV–vis spectrophotometer (UV-DR6000, Hach, Loveland, CO, USA). The results were expressed as milligrams of QE per gram of dried sample (mg QE g−1 d.w.).
Analysis of ascorbic acid and trans-resveratrol using HPLC
Ascorbic acid and trans-resveratrol were quantified using high-performance liquid chromatography (HPLC). The analysis was performed using a Hitachi LaChrom Elite® liquid chromatograph equipped with an autosampler and UV detector. A reverse-phase TC C-18 analytical column (150 mm × 4.6 mm, 5 μm particle size, Agilent, USA) was used for the separation.
Ascorbic acid was extracted using the method described by Chebrolu et al. with minor modifications77. An aqueous solution of 3% metaphosphoric acid (MPA) was used as the extraction solvent, and extractions were conducted at room temperature. Lyophilized fruit samples were mixed with the extraction solution at a 1:10 (w/v) ratio, vortex-stirred for 2 min, and sonicated for 2 min. The mixture was then centrifuged at 3000 rpm, and the resulting extracts were stored in sealed dark-glass vials at −4 °C prior to analysis. Separation of ascorbic acid was achieved via isocratic elution using a mobile phase of methanol and water (15:85 v/v) with a pH of 2.5 (adjusted with MPA). The flow rate was set at 1.2 mL min−1, and UV detection was performed at 245 nm (Supplementary Fig. 1).
Trans-resveratrol extraction followed the method described by Sun et al.78 with modifications. Briefly, 50 mg of lyophilized fruit was mixed with 2 mL of ethanol/water (70:30 v/v) as the extraction solvent. The mixture was then filtered through 0.45 μm PVDF filters. Separation was performed using isocratic elution of water/acetonitrile/acetic acid (70:29.9:0.1 v/v/v) at a flow rate of 1 mL min−1. Detection was conducted at 306 nm, and the column oven temperature was set to 30 °C (Supplementary Fig. 2).
Analysis of trans-resveratrol via UPLC-QTOF-MS
Resveratrol was verified using UPLC-QTOF-MS (Waters Acquity UPLC Xevo G2-XS Q/TOF) with a scanning range of m/z 100 to 1200 Da in negative ionization mode. Separation was performed using an Acquity UPLC BEH C18 column (2.1 × 50 mm, 1.7 µm particle size, Waters, Milford, MA, USA) with a gradient mobile phase consisting of 0.01% formic acid in water (Solvent A) and 100% acetonitrile (Solvent B). Gradient elution conditions were as follows: 1% B (1 min), 1–20% B (10 min), 20–25% B (10 min), and 35–50% B (5 min), followed by column rebalancing. The flow rate was maintained at 0.25 mL min−1. The optimized ESI parameters were: a capillary voltage of 0.5 kV, cone gas flow of 30 L h−1, desolvation gas flow of 900 L h−1, source temperature of 140 °C, desolvation temperature of 450 °C, sample cone voltage of 40 V, and source offset of 80 V. MS/MS analysis was conducted with a collision energy of 20 eV with the Acquity UPLC BEH C18 column. Data collection and analysis were carried out using UNIFITM v1.8 software on the MassLynx NT 4.1 platform (Waters, Milford, MA, USA).
UPLC-QTOF-MS analysis
Metabolites in the hydroethanolic extract of P. cecropiifolia Mart. were identified using a UPLC-ESI-QTOF-MS system. The analysis was conducted in negative ionization mode with a scanning range of m/z 100–1200 Da. Chromatographic separation was achieved with an Acquity UPLC BEH C18 column (2.1 × 50 mm, 1.7 µm particle size, Waters, Milford, MA, USA). The LC mobile phase A consisted of 0.1% formic acid in water, while mobile phase B constituted 0.1% formic acid in acetonitrile. The gradient elution was as follows: 1% A (0–1 min), 1–20% A (10 min), 20–25% A (10 min), 25–35% A (5 min), 35–50% A (5 min), and 1% A (4 min). MS/MS parameters were as follows: a capillary voltage of 0.5 kV, source temperature of 140 °C, desolvation temperature of 450 °C, and sampling cone and source offset voltages of 40 and 80 V, respectively. The collision energy ramp was set to a low energy of 6 eV and a high energy range of 20–30 eV. Mass spectral data collection and analysis followed the previously described methodology for UPLC-ESI-QTOF-MS/MS analysis of trans-resveratrol.
DPPH free radical scavenging activity
The DPPH radical scavenging capacity was carried out in triplicate following the method of Gulati et al.79 with minor modifications. Six different concentrations of the extracts were mixed with 0.6 µM DPPH prepared in ethanol (96% w/w). Each reaction consisted of 50 µL of DPPH working solution, 100 µL of sample dilutions (ethanol served as the blank), and 100 µL of ethanol. The fruit extracts were prepared in test tubes containing ethanol to give 1001.5 to 4280.0, 6011.3 to 29040.0, and 20.2 to 199.9 µg mL−1 solutions for peel, pulp, and seed, respectively. The mixtures were incubated in a dark room on an orbital shaker (Orbit™ LS Low-Speed Laboratory Shaker, Labnet) at 60 rpm. The absorbance of each concentration was measured at 517 nm using a spectrophotometer (Promega, Glomax Discover System). Trolox was used as the positive control. The percent radical scavenging activity of the extracts was calculated using the following Eq. (1):
where Abs control is the absorbance of DPPH + ethanol and Abs sample is the absorbance of DPPH radical + sample (sample or standard). The IC50 values can be calculated with GraphPad Prism® software using a nonlinear regression as follows, Eq. (2):
The results were expressed as TEAC, was estimated using the equation below80, Eq. (3):
ABTS free radical scavenging activity
The ABTS radical cation decolorization assay was performed following the method of Re et al.81 Briefly, a solution of 7.07 mM ABTS was prepared by mixing it with 2.45 mM potassium persulfate to produce the ABTS radical cation. The mixture was incubated in the dark at room temperature for 16 h. The resulting ABTS radical solution was diluted with ethanol (96% w/w) to achieve an absorbance of 0.7 at 734 nm. A reaction mixture was prepared by combining 2850 µL of the ABTS reagent with 150 µL of either standard (Trolox) or extract solutions at concentrations of 800.0 to 448.8, 1106.2 to 29040.0, and 21.0 to 116.5 µg mL−1 for peel, pulp, and seed extracts, respectively. Trolox was used as a positive control, with a concentration range of 10 to 320 μM. All measurements were carried out at least three times. TEAC was calculated using the same equation as that for the DPPH free radical scavenging activity.
FRAP assay
The FRAP of the hydroethanolic extracts was assessed according to the method outlined by Benzie and Strain82. Briefly, the reaction mixture was prepared by mixing 300 µL of FRAP reagent, 30 µL of distilled water, and 10 µL of sample in a 96-well microtiter plate reader. The mixture was incubated at 37 °C for 4 min in the dark, and the absorbance was measured at 600 nm using a Promega Glomax Discover System spectrophotometer. Antioxidant capacity was expressed as µmol Fe2+ g−1 d.w.
Antioxidant capacity assessment via CV
Antioxidant capacity was evaluated by analyzing changes in the current of the superoxide anion radical in the presence of increasing concentrations of antioxidants (0.51 to 2.57 mg mL−1). The AI50 was determined from the plot of the dimensionless parameter (\(({{Ip}}_{a}^{0}\)-\({{Ip}}_{a}^{s})/{{Ip}}_{a}^{0}\)) vs. antioxidant concentration, where, \({{Ip}}_{a}^{0}\) is the peak current in the oxidative scan without the antioxidant in the medium, and \({{Ip}}_{a}^{s}\) is the peak current with the antioxidant. The AI50 value represents the concentration of an antioxidant necessary to decrease the anodic peak current by 50%. In this assay, the lower the AI50 value, the higher the antioxidant potency, signifying greater capacity to scavenge superoxide anion radicals83,84.
CV measurements were performed according to Barrietos et al.85 using a computer-controlled electrochemical system (CH Instruments) equipped with a standard three-electrode cell assembly. A glassy carbon electrode (3 mm in diameter) served as the working electrode, Ag/AgCl as the reference electrode, and a graphite rod as the counter electrode. The working solution consisted of 0.05 M tetrabutylammonium hexafluorophosphate in dimethylformamide. Before each measurement, the working electrode was polished with alumina of decreasing granulometry (0.3, 0.1, and 0.05 μm), rinsed with deionized water, sonicated for 1 min, rinsed with acetone, and dried with air. The cell was saturated with O2 by bubbling air through the solution for 1 min, generating the superoxide anion radical (O2•−) through the one-electron reduction of molecular oxygen. Voltammetric signals were recorded at room temperature using a scan rate of 0.1 V s−1 and potential window from −0.2 to −1.0 V (vs. Ag/AgCl).
Antioxidant capacity assessed using DPV
The antioxidant capacity of the extracts was further evaluated using the electrochemical index (EI)calculated through DPV as per Eq. (4)86:
where ipa represents the current value and Epa the potential value for each major anodic peak in the DPV voltammogram. DPV measurements were carried out using the same electrochemical setup as described previously. The electrolytic solution consisted of 0.1 M acetate buffer (pH = 5). Prior to each measurement, nitrogen purging was performed for 30 s. DPV was conducted in a potential window of −1.2 to 1.5 V (vs. Ag/AgCl) and a scan rate of 17 mV s−1.
Antiproliferation assay
Cells Hela, RKO, MCF7 y T47D were cultured in DMEM and T-47D in RPMI. Mediums were supplemented with 100 units mL−1 penicillin G, 100 μg mL−1 streptomycin, 0.25 μg mL−1 amphotericin B, 2 mM L-glutamine, and 10% fetal bovine serum. The cells were grown in a humidified incubator (37 °C, 5% CO2). Cultures were maintained in a humidified incubator at 37 °C with 5% CO2. Cell viability was assessed using the MTS assay, which measures mitochondrial respiratory activity as an indicator of inhibited growth of human cancer cell lines. A total of 3 × 103 cells were seeded per well in a 96-well plate. After 24 h, the cells were treated with P. cecropiifolia Mart. extracts (peel, pulp, and seed) for an additional 24 h. for 5 different concentrations, ranging from 2 to 200. DMSO was used as the vehicle at a final concentration of 0.1% v/v, serving as the negative control. Doxrubicin was used as a positive control in ranges of 0.1 to 10. Each concentration was performed three times in triplicate. Cells were incubated with the treatments for 48 h. At 44 h, MTS solution (5 mg mL−1) was added, and cells were further incubated for 4 h at 37 °C. Absorbance was measured at 570 nm with a reference wavelength of 650 nm. The percentage of inhibition was calculated using the following formula: viability (%) = (absorbance of treated cells/absorbance of control cells) × 100%.
Molecular docking and dynamics
To further investigate the anticancer activity of P. cecropiifolia Mart., molecular docking and molecular dynamics simulations were performed. The eight most predominant compounds identified from the chemical profile—three from the pulp and five from the peel—were selected for analysis, as cytotoxicity effects are likely attributed to compounds present in greater quantities. For the receptor, the X-ray crystal structures of human cytochrome P450 1A1 (CYP 1A1)87 and human cytochrome P450 1B1 (CYP 1B1)88 were selected as possible targets for cytotoxicity based on previous studies89. Docking studies were performed with an exhaustiveness parameter of 32, 1 Å spacing, full ligand flexibility, and a calculation box focused on the active sites of the selected CYPs. Structures were prepared using AutoDockTools90, and calculations run using AutoDock Vina91. The results of docking calculations were used as input for the molecular dynamic simulation. The topology of the enzymes was built using the AMBER99SB-ILDN force field92, while ligand topology was generated using the ACPYPE server93 with the Generalized Amber Force Field94. The ligand–enzyme complexes were solvated using the three-point water model (TIP3P) and neutralized with Cl− or Na+ ions as needed. The system was energy-minimized and equilibrated at 300 K for 100 ps following NVT and NPT protocols95. Simulations were run at 300 K and 1 bar for 200 ns using GROMACS 201996. Free binding energy was calculated from the trajectory data using the g_mmpbsa tool, applying the Molecular Mechanics Poisson–Boltzmann Surface Area method97.
Statistical analysis
All experiments were performed in triplicate, and the results were expressed as mean ± standard deviation. We conducted a one-way analysis of the variance (ANOVA) followed by Tukey’s test (p > 0.05) to identify statistical differences using R v.3.6.2 (R development). The relationships among DPPH, ABTS, FRAP, EI, AI50, and TPC were evaluated using correlation analysis. IC50 values were calculated via nonlinear regression analysis using GraphPad Prism v. 10.4.1 (GraphPad Software Inc., USA).
Data availability
Data is provided within the manuscript or supplementary information files.
Code availability
No applicable.
Abbreviations
- UPLC-ESI-QTOF-MS:
-
ultra-performance liquid chromatography with electrospray ionization quadrupole time-of-flight mass spectrometry
- AA:
-
antioxidant activity
- DPPH:
-
2,2-diphenyl-1-picrylhidrazyl radical
- ABTS:
-
2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)
- FRAP:
-
ferric reducing antioxidant power
- TEAC:
-
Trolox equivalent antioxidant capacity
- AI50 :
-
antioxidant index 50
- EI:
-
electrochemical index
- CV:
-
cyclic voltammetry
- DPV:
-
differential pulse voltammetry
- TPC:
-
total phenolic content
- GAE:
-
gallic acid equivalent
- TFC:
-
Total flavonoid content
- TAC:
-
Total anthocyanin content
- QE:
-
quercetin equivalent
- CGA:
-
chlorogenic acid
- MPA:
-
metaphosphoric acid
- MTS:
-
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2h-tetrazolium, inner salt
- RMSD:
-
root-mean-square deviation
- CYP 1A1:
-
Cytochrome P450 1A1
- CYP 1B1:
-
Cytochrome P450 1B1
References
Karges, J. Encapsulation of Ru(II) polypyridine complexes for tumor-targeted anticancer therapy. BME Front. 4, 0024 (2023).
Mazurakova, A. Anti-breast cancer effects of phytochemicals: primary, secondary, and tertiary care. EPMA J. 13, 315–354 (2022).
Liang, X. et al. Metal-organic framework-based photodynamic combined immunotherapy against the distant development of triple-negative breast cancer. Biomater. Res. 27, 120 (2023).
Park, H. S. et al. Bifunctional tumor-targeted bioprobe for Phothotheranosis. Biomater. Res. 28, 0002 (2024).
Wu, H. et al. Ginsenoside Rg3 nanoparticles with permeation enhancing based chitosan derivatives were encapsulated with doxorubicin by thermosensitive hydrogel and anti-cancer evaluation of peritumoral hydrogel injection combined with PD-L1 antibody. Biomater. Res. 26, 77 (2022).
Gahtori, R. et al. Anticancer plant-derivatives: deciphering their oncopreventive and therapeutic potential in molecular terms. Future J. Pharm. Sci. 9, 14 (2023).
Howes, M. R. et al. Molecules from nature: Reconciling biodiversity conservation and global healthcare imperatives for sustainable use of medicinal plants and fungi. Plants People Planet 2, 463–481 (2020).
Garagounis, C., Delkis, N. & Papadopoulou, K. K. Unraveling the roles of plant specialized metabolites: using synthetic biology to design molecular biosensors. N. Phytol. 231, 1338–1352 (2021).
Messinese, E. et al. By-products as a sustainable source of bioactive compounds for potential application in the field of food and new materials for packaging development. Food Bioprocess Technol. https://doi.org/10.1007/s11947-023-03158-2 (2023).
Silva, D. H. S., Castro-Gamboa, I. & Bolzani, V. D. S. Plant diversity from Brazilian Cerrado and Atlantic forest as a tool for prospecting potential therapeutic drugs. In Comprehensive Natural Products II 95–133 (Elsevier, 2010). https://doi.org/10.1016/B978-008045382-8.00061-7.
Maldonado, Y. E. A new essential oil from the native Ecuadorian species Steiractinia sodiroi (Hieron.) S.F. Blake (Asteraceae): chemical and enantioselective analyses. Sci. Rep. 13, 17180 (2023).
Ortiz, O. O., Rivera-Mondragón, A., Pieters, L., Foubert, K. & Caballero-George, C. Cecropia telenitida Cuatrec. (Urticaceae: Cecropieae): Phytochemical diversity, chemophenetic implications and new records from Central America. Biochem. Syst. Ecol. 86, 103935 (2019).
Kasangana, P., Haddad, P. & Stevanovic, T. Study of polyphenol content and antioxidant capacity of Myrianthus Arboreus (Cecropiaceae) root bark extracts. Antioxidants 4, 410–426 (2015).
Barrios, J. et al. Chemical analysis and screening as anticancer agent of anthocyanin-rich extract from Uva Caimarona (Pourouma cecropiifolia Mart.) Fruit. J. Agric. Food Chem. 58, 2100–2110 (2010).
Yánez, P. Distribución geográfica y aspectos etnobotánicos de tres especies delgénero pourouma (“uva de monte”), Cecropiaceae, en la región amazónica de Ecuador. Rev. Forest. Venez. 43, 103–109 (1999).
Lopes-Lutz, D., Dettmann, J., Nimalaratne, C. & Schieber, A. Characterization and quantification of polyphenols in Amazon Grape (Pourouma cecropiifolia Martius). Molecules 15, 8543–8552 (2010).
Lim, T. K. Pourouma cecropiifolia. In Edible Medicinal And Non-Medicinal Plants 446–449 (Springer Netherlands, Dordrecht, 2013). https://doi.org/10.1007/978-94-007-5628-1_50.
Fagbemi, K. O. et al. Bioactive compounds, antibacterial and antioxidant activities of methanol extract of Tamarindus indica Linn. Sci. Rep. 12, 9432 (2022).
Osman, A. et al. Dietary polyphenols drive dose-dependent behavioral and molecular alterations to repeated morphine. Sci. Rep. 13, 12223 (2023).
Ordoñez, E., Leon-Arevalo, A., Rivera-Rojas, H. & Vargas, E. Quantification of total polyphenols and antioxidant capacity in skins and seeds from cacao (Theobroma cacao L.), tuna (Opuntia ficus indica Mill), grape (Vitis Vinífera) and uvilla (Pourouma cecropiifolia). Sci. Agropecu. 10, 175–183 (2019).
Ebata, K. T. Vitamin C induces specific demethylation of H3K9me2 in mouse embryonic stem cells via Kdm3a/b. Epigenetics & chromatin. 10, 1–12 (2017).
Carr, A. C. & Frei, B. Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. Am. J. Clin. Nutr. 69, 1086–1107 (1999).
Rao, A. et al. TET family dioxygenases and the TET activator vitamin C in immune responses and cancer. Blood 136, 1394–1401 (2020).
Gustafson, C. B. et al. Epigenetic reprogramming of melanoma cells by vitamin C treatment. Clin. Epigenet.7, 51 (2015).
Agathocleous, M. et al. Ascorbate regulates haematopoietic stem cell function and leukaemogenesis. Nature 549, 476–481 (2017).
Guan, Y. et al. Context dependent effects of ascorbic acid treatment in TET2 mutant myeloid neoplasia. Commun. Biol. 3, 493 (2020).
Abreu-Naranjo, R. et al. Bioactive compounds, phenolic profile, antioxidant capacity and effectiveness against lipid peroxidation of cell membranes of Mauritia flexuosa L. fruit extracts from three biomes in the Ecuadorian Amazon. Heliyon 6, e05211 (2020).
Bhattacharya, S. Therapeutic role of resveratrol against hepatocellular carcinoma: A review on its molecular mechanisms of action. Pharmacol. Res. 6, 100233 (2023).
Okudaira, N., Ishizaka, Y. & Tamamori-Adachi, M. Resveratrol blocks retrotransposition of LINE-1 through PPAR α and sirtuin-6. Sci. Rep. 12, 7772 (2022).
Wei, J., et al. Exploring the biomolecular mechanism of resveratrol in the treatment of nephrotic syndrome based on network pharmacology. Pharmacol. Res. Chin. Med. 3, 100114 (2022).
Zhang, L.-X. et al. Resveratrol (RV): A pharmacological review and call for further research. Biomed. Pharmacother. 143, 112164 (2021).
Hou, X., Rooklin, D., Fang, H. & Zhang, Y. Resveratrol serves as a protein-substrate interaction stabilizer in human SIRT1 activation. Sci. Rep. 6, 38186 (2016).
Craveiro, M. et al. Resveratrol stimulates the metabolic reprogramming of human CD4 + T cells to enhance effector function. Sci. Signal. 10, eaal3024 (2017).
Shrikanta, A., Kumar, A. & Govindaswamy, V. Resveratrol content and antioxidant properties of underutilized fruits. J. Food Sci. Technol. 52, 383–390 (2015).
Fraisse, D., Heitz, A., Carnat, A., Carnat, A.-P. & Lamaison, J.-L. Quercetin 3-arabinopyranoside, a major flavonoid compound from Alchemilla xanthochlora. Fitoterapia 71, 463–464 (2000).
Falchero, L. et al. Essential oil composition of lady’s mantle (Alchemilla xanthochlora Rothm.) growing wild in Alpine pastures. Nat. Prod. Res. 23, 1367–1372 (2009).
Fan, P., Terrier, L., Hay, A.-E., Marston, A. & Hostettmann, K. Antioxidant and enzyme inhibition activities and chemical profiles of Polygonum sachalinensis F.Schmidt ex Maxim (Polygonaceae). Fitoterapia 81, 124–131 (2010).
Huber, R. et al. In vitro antiallergic effects of aqueous fermented preparations from Citrus and Cydonia fruits. Planta Med 78, 334–340 (2012).
Noshita, T., Funayama, S., Hirakawa, T., Kidachi, Y. & Ryoyama, K. Machilin G and four neolignans from young fruits of Magnolia denudata show various degrees of inhibitory activity on Nitric Oxide (NO) production. Biosci. Biotechnol. Biochem. 72, 2775–2778 (2008).
Liu, X.-T. et al. Active components with inhibitory activities on IFN-γ/STAT1 and IL-6/STAT3 signaling pathways from Caulis Trachelospermi. Molecules 19, 11560–11571 (2014).
Masuda, I. et al. Apple procyanidins promote mitochondrial biogenesis and proteoglycan biosynthesis in chondrocytes. Sci. Rep. 8, 7229 (2018).
Forino, M. et al. 1S,3R,4S,5R)5-O-Caffeoylquinic acid: Isolation, stereo-structure characterization and biological activity. Food. Chem. 178, 306–310 (2015).
Fischer, H. O. L. & Dangschat, G. Konstitution der Chlorogensäure (3. Mitteil. über Chinasäure Derivate). Ber. Dtsch. Chem. Ges. B Ser. 65, 1037–1040 (1932).
Gupta, A., Atanasov, A. G., Li, Y., Kumar, N. & Bishayee, A. Chlorogenic acid for cancer prevention and therapy: Current status on efficacy and mechanisms of action. Pharmacol. Res. 186, 106505 (2022).
Wang, X., Qin, L., Zhou, J., Li, Y. & Fan, X. A novel design to screen chlorogenic acid-producing microbial strains from the environment. Sci. Rep. 8, 14756 (2018).
Zuo, J., Tang, W. & Xu, Y. Anti-Hepatitis B virus activity of chlorogenic acid and its related compounds. in Coffee in Health and Disease Prevention 607–613 (Elsevier, 2015). https://doi.org/10.1016/B978-0-12-409517-5.00068-1.
Chai, X. et al. Chlorogenic acid protects against myocardial ischemia–reperfusion injury in mice by inhibiting Lnc Neat1/NLRP3 inflammasome-mediated pyroptosis. Sci. Rep. 13, 17803 (2023).
Fan, X., Jiao, W., Wang, X., Cao, J. & Jiang, W. Polyphenol composition and antioxidant capacity in pulp and peel of apricot fruits of various varieties and maturity stages at harvest. Int. J. Food Sci. Technol. 53, 327–336 (2018).
Biswas, A. et al. Phytochemical content and antioxidant activity of different anatomical parts of Corchorus olitorius and C. capsularis during different phenological stages. Heliyon 9, e16494 (2023).
Kumar, N. & Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 24, e00370 (2019).
Kabtni, S. et al. Influence of climate variation on phenolic composition and antioxidant capacity of Medicago minima populations. Sci. Rep. 10, 8293 (2020).
Keffous, F. et al. Determination of the antioxidant activity of Limoniastrum feei aqueous extract by chemical and electrochemical methods. Cogent Chem. 2, 1186141 (2016).
Muhammad, H. et al. Electrochemical behavior of superoxide anion radical towards quinones: a mechanistic approach. Res. Chem. Intermed. 44, 6387–6400 (2018).
Sochor, J. et al. Electrochemistry as a tool for studying antioxidant properties. Int. J. Electrochem. Sci. 8, 8464–8489 (2013).
Iloki-Assanga, S. B. et al. Solvent effects on phytochemical constituent profiles and antioxidant activities, using four different extraction formulations for analysis of Bucida buceras L. and Phoradendron californicum. BMC Res. Notes 8, 396 (2015).
Aksoy, L., Kolay, E., Ağılönü, Y., Aslan, Z. & Kargıoğlu, M. Free radical scavenging activity, total phenolic content, total antioxidant status, and total oxidant status of endemic Thermopsis turcica. Saudi J. Biol. Sci. 20, 235–239 (2013).
Ruiz-Caro, P. et al. An electrochemical alternative for evaluating the antioxidant capacity in walnut kernel extracts. Food Chem. 393, 133417 (2022).
Osorio-Valencia, A. I. et al. Evaluation of antioxidant capacity in different food matrices through differential pulse voltammetry and its correlation with spectrophotometric methods. J. Appl. Electrochem. (2023) https://doi.org/10.1007/s10800-023-01933-9.
Gouvêa, CibeleM, C. P. & Avelar, M. Procyanidin B2 cytotoxicity to MCF-7 human breast adenocarcinoma cells. Indian J. Pharm. Sci. 74, 351 (2012).
Changizi, Z., Moslehi, A., Rohani, A. & Eidi, A. Chlorogenic acid inhibits growth of 4T1 breast cancer cells through involvement in Bax/Bcl2 pathway. J. Cancer Res. Ther. 16, 1435 (2020).
Shin, A. et al. Cytochrome P450 1A1 (CYP1A1) polymorphisms and breast cancer risk in Korean women. Exp. Mol. Med. 39, 361–366 (2007).
Mukherjee, G., Nandekar, P. P. & Wade, R. C. An electron transfer competent structural ensemble of membrane-bound cytochrome P450 1A1 and cytochrome P450 oxidoreductase. Commun. Biol. 4, 55 (2021).
Rodriguez, M. & Potter, D. A. CYP1A1 regulates breast cancer proliferation and survival. Mol. Cancer Res. 11, 780–792 (2013).
Gribben, J. G. et al. Unexpected association between induction of immunity to the universal tumor antigen CYP1B1 and response to next therapy. Clin. Cancer Res. 11, 4430–4436 (2005).
Li, F., Zhu, W. & Gonzalez, F. J. Potential role of CYP1B1 in the development and treatment of metabolic diseases. Pharmacol. Ther. 178, 18–30 (2017).
D’Uva, G., Baci, D., Albini, A. & Noonan, D. M. Cancer chemoprevention revisited: Cytochrome P450 family 1B1 as a target in the tumor and the microenvironment. Cancer Treat. Rev. 63, 1–18 (2018).
Chen, C. et al. CYP1B1 inhibits ferroptosis and induces anti-PD-1 resistance by degrading ACSL4 in colorectal cancer. Cell Death Dis. 14, 271 (2023).
Chun, Y. & Kim, S. Discovery of cytochrome P450 1B1 inhibitors as new promising anti-cancer agents. Med. Res. Rev. 23, 657–668 (2003).
Dutour, R. & Poirier, D. Inhibitors of cytochrome P450 (CYP) 1B1. Eur. J. Med. Chem. 135, 296–306 (2017).
Liu, J., Sridhar, J. & Foroozesh, M. Cytochrome P450 Family 1 inhibitors and structure-activity relationships. Molecules 18, 14470–14495 (2013).
Rammali, S. et al. In vitro and computational investigation of antioxidant and anticancer properties of Streptomyces coeruleofuscus SCJ extract on MDA-MB-468 triple-negative breast cancer cells. Sci. Rep. 14, 25251 (2024).
Shahab, M. et al. Targeting human progesterone receptor (PR), through pharmacophore-based screening and molecular simulation revealed potent inhibitors against breast cancer. Sci. Rep. 14, 6768 (2024).
Stafussa, A. P. et al. Bioactive compounds of 44 traditional and exotic Brazilian fruit pulps: phenolic compounds and antioxidant activity. Int. J. Food Prop. 21, 106–118 (2018).
Kupina, S., Fields, C., Roman, M. C. & Brunelle, S. L. Determination of Total Phenolic Content Using the Folin-C Assay: Single-Laboratory Validation, First Action 2017.13. J. AOAC Int. 101, 1466–1472 (2018).
Silva, L. M. R. D. et al. Quantification of bioactive compounds in pulps and by-products of tropical fruits from Brazil. Food Chem. 143, 398–404 (2014).
Tuah, B., Asante, M., Asare, G. & Doku, D. In vitro antioxidant activity in seven selected local Ghanaian spices and an artificial spice, Shrimp Cube. World J. Nutr. Health 5, 46–52 (2017).
Chebrolu, K. K., Jayaprakasha, G. K., Yoo, K. S., Jifon, J. L. & Patil, B. S. An improved sample preparation method for quantification of ascorbic acid and dehydroascorbic acid by HPLC. LWT 47, 443–449 (2012).
Sun, H., Lin, Q., Wei, W. & Qin, G. Ultrasound-assisted extraction of resveratrol from grape leaves and its purification on mesoporous carbon. Food Sci. Biotechnol. 27, 1353–1359 (2018).
Gulati, V., Harding, I. H. & Palombo, E. A. Enzyme inhibitory and antioxidant activities of traditional medicinal plants: Potential application in the management of hyperglycemia. BMC Complement. Altern. Med. 12, 1–9 (2012).
Xiao, F., Xu, T., Lu, B. & Liu, R. Guidelines for antioxidant assays for food components. Food Front. 1, 60–69 (2020).
Re, R. et al. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 26, 1231–1237 (1999).
Benzie, I. F. F. & Strain, J. J. The Ferric Reducing Ability of Plasma (FRAP) as a measure of “Antioxidant Power”: The FRAP assay. Anal. Biochem. 239, 70–76 (1996).
Herrera-Marín, P. et al. Green synthesis of silver nanoparticles using aqueous extract of the leaves of fine aroma cocoa Theobroma cacao linneu (Malvaceae): Optimization by electrochemical techniques. Electrochim. Acta 447, 142122 (2023).
Pérez-Cruz, K. et al. Synthesis and antioxidant study of new polyphenolic hybrid-coumarins. Arab. J. Chem. 11, 525–537 (2018).
Barrientos, C., Navarrete-Encina, P. & Squella, J. A. Electrochemistry and reactivity against superoxide anion radicals of Hydroxycoumarins and its derivatives. J. Electrochem. Soc. 167, 165502 (2020).
Kinyua Muthuri, L., Nagy, L. & Nagy, G. Chronopotentiometric method for assessing antioxidant activity: A reagentless measuring technique. Electrochem. Commun. 122, 106907 (2021).
Walsh, A. A., Szklarz, G. D. & Scott, E. E. Human Cytochrome P450 1A1 structure and utility in understanding drug and xenobiotic metabolism. J. Biol. Chem. 288, 12932–12943 (2013).
Wang, A., Savas, U., Stout, C. D. & Johnson, E. F. Structural characterization of the complex between α-Naphthoflavone and human Cytochrome P450 1B1. J. Biol. Chem. 286, 5736–5743 (2011).
Marciniec, K. et al. Synthesis, anti-breast cancer activity, and molecular docking study of a New Group of Acetylenic Quinolinesulfonamide Derivatives. Molecules 22, 300 (2017).
Morris, G. M. et al. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 30, 2785–2791 (2009).
Trott, O. & Olson, A. J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 31, 455–461 (2010).
Krieger, E. et al. Improving physical realism, stereochemistry, and side-chain accuracy in homology modeling: Four approaches that performed well in CASP8. Proteins Struct. Funct. Bioinforma. 77, 114–122 (2009).
Sousa Da Silva, A. W. & Vranken, W. F. ACPYPE - AnteChamber PYthon Parser interfacE. BMC Res. Notes 5, 367 (2012).
Wang, J., Wolf, R. M., Caldwell, J. W., Kollman, P. A. & Case, D. A. Development and testing of a general amber force field. J. Comput. Chem. 25, 1157–1174 (2004).
Cabrera, N. et al. Searching glycolate oxidase inhibitors based on QSAR, molecular docking, and molecular dynamic simulation approaches. Sci. Rep. 12, 19969 (2022).
Van Der Spoel, D. et al. GROMACS: Fast. Flex. Free. J. Comput. Chem. 26, 1701–1718 (2005).
Kumari, R. & Kumar, R. Open Source Drug Discovery Consortium & Lynn, A. g_mmpbsa —A GROMACS tool for high-throughput MM-PBSA calculations. J. Chem. Inf. Model. 54, 1951–1962 (2014).
Acknowledgements
The authors thank the Pontificia Universidad Católica del Ecuador for funding through the project “Chemical profile and antioxidant capacity of Pourouma cecropiifolia Mart. from the Ecuadorian Amazon” [grant number: 007-UIO-2023]. The authors also would like to thank the Central University of Ecuador for funding the purchase of reagents necessary for the development of this project.
Author information
Authors and Affiliations
Contributions
Conceived the project, P.J.E.-M.; visualization, P.J.E.-M., C.M.-D., P.A.C.-P., J.C.R.-B., and L.F.; investigation, P.J.E.-M., C.M.-D., P.A.C.-P., J.C.R.-B. and L.F.; performed the experiments, C.M.-D., R.M.-S., N.G.S.M., S.A.C., J.C.R.-B., D.R.-F. and N.B.-M.; supervision, P.J.E.-M.; data analysis, N.B.-M., N.G.S.M., P.A.C.-P., J.C.R.-B., R.M.-S., C.M.-D., D.R.-F. and L.F.; conducted and analyzed the molecular docking part, S.A.C. and J.R.M.; writing—original draft preparation, P.J.E.-M., C.M.-D., N.G.S.M., J.R.M., and P.A.C.-P.; writing—review and editing, P.J.E.-M., C.M.-D., N.G.S.M., J.R.M., N.B.-M., S.A.C., L.F., and P.A.C.-P.; project administration, P.J.E.-M., funding acquisition; P.J.E.-M.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Méndez-Durazno, C., Cisneros-Pérez, P.A., Mogollón, N.G.S. et al. Phytochemical profiling, antioxidant properties, and anticancer activity of Pourouma cecropiifolia Mart. from the Ecuadorian Amazon. npj Sci Food 9, 175 (2025). https://doi.org/10.1038/s41538-025-00503-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41538-025-00503-x