Abstract
Licorice (Glycyrrhiza uralensis) has traditionally been used as a food-derived herbal remedy for inflammation; however, the anti-inflammatory potential of its fermented extract in skin health is still unclear. This study investigated fermented licorice extract (FLE) for its effects against glyoxal-derived advanced glycation end products (GO-AGEs) and ultraviolet B (UVB)-induced skin inflammation in HaCaT keratinocytes. At 10 µg/mL, FLE reduced IL-6 levels by 46% and TNF-α levels by 52%, and significantly lowered PGE2 levels. Mechanistic evaluation showed that FLE suppressed inflammatory signaling pathways, particularly nuclear factor-κB (NF-κB) and mitogen-activated protein kinases (MAPKs). Untargeted metabolomics identified fermentation-enhanced bioactive metabolites, including glycyrrhetic acid-3-O-glucuronide, 18β-glycyrrhetic acid, 24-hydroxyglycyrrhetic acid, and isoliquiritigenin, which correlated with anti-inflammatory activity. Notably, 18β-glycyrrhetic acid and isoliquiritigenin exhibited potent antiglycation effects and cytokine suppression. These results suggest that fermentation enhances the bioactive profile of licorice, supporting its potential as a functional ingredient for managing skin inflammation from GO-AGEs and UVB exposure.

Similar content being viewed by others
Introduction
Skin aging is a multifaceted process characterized by histological and morphological changes in the skin barrier due to intrinsic factors such as genetics and endogenous processes, and extrinsic factors including ultraviolet radiation (UVR) and environmental contaminants1. It manifests through wrinkles, reduced elasticity, dryness, sagging, and an increased risk of benign and malignant skin conditions2. Chronic inflammation, referred to as inflammaging, is a critical driver of skin aging, characterized by persistent low-grade inflammatory responses3. Consequently, significant attention has been given to developing functional foods and dietary supplements that can regulate inflammatory processes associated with skin health4.
Advanced glycation end-products (AGEs) exacerbate oxidative stress, inflammation, and metabolic disorders, contributing to skin barrier dysfunction, psoriasis, atopic dermatitis, impaired wound healing5, and increased risk of skin cancer6,7. Our previous research demonstrated that glyoxal-derived AGEs (GO-AGEs) significantly amplify skin inflammation, particularly when combined with UVB irradiation, highlighting GO-AGEs as potential therapeutic targets for anti-inflammatory interventions8.
Recently, functional foods and dietary supplements, termed “inner beauty” ingredients such as vitamins, unsaturated fatty acids, and collagen peptides, have attracted growing interest for managing UV-induced skin inflammation due to their ability to enhance skin health via systemic circulation9. Nevertheless, a continuous demand exists for novel dietary ingredients with improved efficacy against inflammatory skin disorders.
Licorice (Glycyrrhiza uralensis) is widely used as a functional food ingredient, especially for its traditional applications in relieving cough, pain, infection, and weakened immune system10. Licorice contains several bioactive compounds, such as glycyrrhizin, liquiritin, liquiritigenin, isoliquiritin, and isoliquiritigenin, and reported to have numerous beneficial effects, including anti-inflammatory, antioxidant, antiallergic, and anti-obesity activities11. Consequently, licorice seems to be a promising agent with potential for regulating inflammation-related responses. Previous studies have reported that treatment with licorice improves fever, neuralgia, bladder and kidney pain, and skin diseases12. However, licorice consumption poses risks of adverse effects, primarily hypokalemia, hypertension, and gastrointestinal disturbances associated with glycyrrhizin. Thus, alternative processing methods are necessary to enhance its therapeutic profile and minimize these side effects10.
Nowadays, fermentation techniques, emerging as innovative strategies for product development, have been employed to enhance the anti-inflammatory capabilities of raw materials and to generate additional bioactive compounds13. Wang et al. (2023) demonstrated that licorice extract fermented by probiotics exhibited a 30% increase in antioxidant activity, along with anti-neurodegenerative and antistress effects, supporting the potential of fermentation techniques for developing new functional products14. Fermentation is known to enhance the anti-inflammatory effects of raw food sources, such as fruits and plants. Therefore, we hypothesized that fermentation might enhance the anti-inflammatory properties of licorice. However, few studies have focused on the variations in licorice metabolites during fermentation and their biological effects. Furthermore, no previous studies have identified the critical metabolites generated in licorice through fermentation. Therefore, we evaluated the anti-inflammatory effects of fermented licorice extract (FLE) and identified the components involved in such activity.
Metabolomics is a powerful analytical approach to comprehensively assess metabolic variations among small molecules (less than 1500 Da) across diverse biological systems such as biofluids, plants, microbes, and fermented foods15. Global metabolite profiling has diverse applications, such as in natural product development, dietary supplement discovery, and disease diagnosis, which rely on correlation analyses between metabolomics data and biological activities or disease severity to identify bioactive compounds and biomarkers16,17. FLE is a chemically complex matrix comprising microbial metabolites, plant-derived substances, and fermentation-induced transformation products. High-resolution mass spectrometry-based untargeted metabolomics is a useful approach for elucidating this metabolic complexity, offering high sensitivity and the detection of a wide range of compounds18.
Therefore, the objective of this study was to investigate the anti-inflammatory and antiglycation effects of fermented licorice extract (FLE) and to identify key fermentation-derived metabolites responsible for these activities. Specifically, we aimed to determine whether microbial fermentation enhances the functional properties of licorice by promoting the bioconversion of glycosylated precursors into more bioactive compounds. To this end, we employed untargeted metabolomics to characterize major metabolite transformations and assess their correlations with biological activities, including AGEs-breaking activity, inhibition of AGEs formation, and cytokine modulation. This integrative approach provides novel mechanistic insights into fermentation-enhanced licorice bioactivity and supports the potential application of FLE as a functional ingredient for alleviating skin inflammation associated with GO-AGEs and UVB exposure.
Results and discussion
FLE has antiglycation and inflammatory activities
AGEs play a vital role in various chronic diseases, including diabetes, cardiovascular diseases, neurodegenerative diseases, and skin inflammation; AGEs usually accumulate in various organs, including the epidermal layer of skin19,20. Several studies have demonstrated that dicarbonyl compounds like methylglyoxal (MGO) and GO are significant precursors for AGEs formation, contributing to AGEs accumulation from environmental sources including food, smoking, UV radiation exposure, and physical inactivity21. It was recently shown that the presence of AGEs compounds in the skin layers leads to functional changes and increases inflammation in keratinocytes and dermatitis22. Moreover, our previous study showed that GO-AGEs, in combination with UVB irradiation, enhance skin inflammation, and suggested novel anti-skin aging therapeutic targets for UVB-irradiated skin19. Thus, we first focused on the antiglycation effect of FLE and then used it to treat our GO-AGEs-treated and UVB-irradiated HaCaT cells to investigate its anti-skin inflammatory effect.
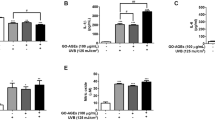
We evaluated the antiglycation effects of FLE using multiple screening assays, and the concentrations (100, 250, and 500 µg/mL) were selected based on commonly used in similar in vitro antiglycation studies23. In the GO-FLE affinity assay (Fig. 1A), FLE treatment resulted in approximately 1.5-fold higher fluorescence intensity compared to ULE (**p < 0.01) after a 6-day incubation period. This increased fluorescence is interpreted as a result of GO being trapped by FLE, indicating a carbonyl-trapping effect rather than enhanced AGEs formation. A similar concentration-dependent trend was observed with both extracts (Supplementary Fig. S1B), suggesting that fermentation enhances the antiglycation activity of licorice.
A GO-affinity assay. B GO-AGEs-breaker assay. C GO-AGEs formation assay. D Cell viability in HaCaT keratinocytes. E Cell viability in GO-AGEs-treated and UVB-irradiated HaCaT keratinocytes. F–I Inflammatory cytokine production in GO-AGE- and UVB-treated HaCaT keratinocytes, including (F) IL-1β, (G) IL-6, (H) TNF-α, and (I) PGE₂. *p < 0.05, **p < 0.01, and ***p < 0.001 vs. ULE-treated group; $p < 0.05, $$p 0.01, and $$$p < 0.001 vs. GO, GO-AGE-, or GO-AGEs/UVB-treated group; ###p < 0.001 vs. Control group (untreated cells). Results are expressed as the mean ± SEM (n = 3).
In support of this, FLE showed significantly greater AGEs-breaking activity in the GO-AGEs breaker assay, with 500 µg/mL FLE releasing 35.96 ± 2.20% free amines, compared to 23.34 ± 1.48% with ULE (**p < 0.01; Fig. 1B). Since GO-AGEs can exacerbate skin inflammation via oxidative stress and pro-inflammatory mediator production19, these results imply that high-dose FLE may mitigate such inflammatory responses. This was further confirmed by CD spectroscopy (Fig. 1C, Supplementary Fig. S1A), where FLE demonstrated superior inhibition of GO-AGE formation compared to ULE, also in a dose-dependent manner.
To further assess antiglycation efficacy, we performed an additional GO-AGEs breaker assay to evaluate the cleavage of preformed AGEs. FLE significantly enhanced the release of free amines (***p < 0.001) compared to either ULE or L. mesenteroides alone (Supplementary Fig. S1C), indicating a synergistic effect from fermentation. Collectively, these findings highlight the potential of FLE as a functional antiglycation agent capable of regulating glycotoxin formation and breaking existing AGEs cross-links, thereby offering protective effects against AGEs-mediated skin inflammation.
To investigate the anti-inflammatory effect of FLE attributed to its antiglycation activity, we performed in vitro experiments using HaCaT keratinocytes treated with GO-AGEs (100 µg/mL) and UVB-irradiated (130 mJ/cm2), as described previously19. Ascorbic acid, known for its anti-photoaging effect in UVB-irradiated keratinocytes, was used as a positive control to comparatively evaluate the anti-skin inflammatory efficacy of ULE and FLE24. As illustrated in Supplementary Data Fig. S2, combined treatment of GO-AGEs and UVB irradiation significantly decreased cell viability (###p < 0.001) and notably elevated inflammatory cytokines (IL-1β, IL-6, and TNF-α; ###p < 0.001). Our findings thus suggest that GO-AGEs and UVB irradiation may enhance inflammation processes, consistent with our prior observations19. Importantly, neither ULE nor FLE demonstrated cytotoxic effects in HaCaT cells at various concentrations (Fig. 1D), consistent with previously reported low cytotoxicity of other natural extracts25,26.
The pro-inflammatory cytokines IL-1β, IL-6, and TNF-α as well as PGE2 play critical roles in inflammation progression27. As presented in Fig. 1F–I, treatment of HaCaT cells with UVB and GO-AGEs significantly increased cytokines and PGE2 secretion by approximately 2.5–7-fold relative to the control group (Ctrl, ###p < 0.001). Treatment with FLE significantly reduced this inflammatory response in a concentration-dependent manner. Specifically, at 10 µg/mL FLE, secreted IL-1β, IL-6, TNF-α, and PGE2 levels were reduced to 71.79 ± 1.86 pg/mL ($$$p < 0.001), 144.77 ± 2.37 pg/mL ($$$p < 0.001), 52.95 ± 0.59 pg/mL ($$$p < 0.001), and 189.48 ± 1.61 pg/mL ($$$p < 0.001), respectively, compared with the UVB and GO-AGEs-treated group. Furthermore, FLE treatment more effectively suppressed cytokine release and PGE2 production compared to ULE-treated cells, exhibiting anti-inflammatory efficacy similar to that of high-dose ascorbic acid (500 µg/mL). Zhang et al. (2023) previously reported that fermented extracts reduced inflammatory cytokine levels in RAW264.7 macrophages compared to their unfermented counterparts, highlighting that fermentation enhances the cellular anti-inflammatory properties of raw extracts28. This result suggests that fermentation might similarly enhance the anti-inflammatory activity of licorice raw materials.
Effects of FLE on the related inflammatory pathways in UVB-irradiated and GO-AGEs-treated HaCaT cells
Data from in vitro experiments demonstrated that FLE exhibited significant antiglycation and anti-inflammatory effects in HaCaT keratinocytes treated with GO-AGEs and UVB irradiation. To better understand these findings, we investigated the underlying molecular mechanisms associated with skin inflammation induced by glycotoxins, particularly GO-AGEs, to identify potential therapeutic targets for skin aging. RAGE plays a crucial role in skin inflammation29. Interaction between AGEs, including GO-AGEs, and RAGE triggers inflammatory signaling pathways involving NF-κB and COX-2, subsequently inducing the release of pro-inflammatory cytokines and chemokines19. As shown in Fig. 2A, treatment with UVB and GO-AGEs significantly increased RAGE expression in HaCaT cells (#p < 0.05). Conversely, FLE treatment significantly decreased RAGE expression in a concentration-dependent manner, achieving an effect comparable to that of 10 µM ascorbic acid. COX-2 is well known to promote the production of inflammatory mediators, such as IL-1β, IL-6, TNF-α, and PGE230. Our findings indicate that FLE significantly reduced COX-2 expression and IL-1β protein levels in a concentration-dependent manner, underscoring the potential of fermentation processes to enhance the anti-inflammatory properties of licorice extracts.
A RAGE; (B) COX-2; (C) IL-1β; (D) MAPKs pathway proteins (p38, ERK, and JNK); (E) NF-κB and IκB-α protein levels. #p < 0.05, ##p < 0.01, and ###p < 0.001 vs. Control (untreated) group; *p < 0.05, **p < 0.01, and ***p < 0.001 vs. GO-AGEs/UVB-treated group. Results are expressed as the mean ± SEM (n = 3).
We further assessed the effects of FLE on MAPKs (i.e., p38, ERK, and JNK) in UVB- and GO-AGEs-treated HaCaT cells. UVB and GO-AGEs treatment strongly induced the phosphorylation of p-38, ERK, and JNK. In contrast, FLE treatment effectively reduced the phosphorylation levels of these MAPKs (Fig. 2D). NF-κB signaling, a central pathway in inflammation activated by MAPKs, was also evaluated31. Treatment with UVB and GO-AGEs significantly increased nuclear NF-κB expression (##p < 0.01) and decreased cytosolic IκB-α expression (#p < 0.05) in HaCaT cells. Conversely, FLE treatment reversed these effects in a concentration-dependent manner, reducing nuclear NF-κB expression while increasing cytosolic IκB-α levels. These results collectively suggest that UVB and GO-AGE exposure activates skin inflammation via RAGE and MAPK/NF-κB signaling pathways, while FLE treatment ameliorates inflammation by modulating these mechanisms.
Supporting our findings, a recent study showed that FLE substantially suppressed inflammatory and oxidative stress responses in an ulcerative colitis model compared to ULE32. Additionally, FLE treatment improved levels of beneficial metabolites such as gallic acid, rutin, astragalin, liquiritigenin, and quercetin, enhancing their antioxidant effects33, and displayed neuroprotective properties in a Caenorhabditis elegans Alzheimer’s model14. This evidence suggests that fermented licorice could effectively modulate multiple biological functions, including anti-inflammatory responses relevant to skin health.
Changes in licorice extract composition due to fermentation by L. mesenteroides
Microbial fermentation of plant compounds, particularly with lactic acid bacteria, is a promising approach for enhancing the bioavailability and physiological activity of diverse molecules by facilitating the bioconversion of complex phytochemicals34. To identify the bioactive compounds responsible for the enhanced antiglycation and anti-inflammatory effects observed in FLE, we conducted comparative metabolite profiling of ULE and FLE using untargeted metabolomics, followed by correlation analyses of their bioactivities. Untargeted metabolomics is an unbiased strategy that characterizes comprehensive metabolic profiles of small molecules across biological systems such as plants, microbes, and fermented foods, employing mass spectrometry for high-throughput screening18. Integrating multivariate analysis with metabolomics datasets facilitates the exploration of chemical variations among samples attributed to specific factors, such as fermentation conditions, disease states, and microbial composition35.
To elucidate the chemical transformations resulting from fermentation, multivariate analyses were performed based on metabolomics data acquired from UHPLC–Orbitrap–MS/MS. The PCA score plot clearly distinguished ULE and FLE groups along the first principal component (PC1, 43.2%), highlighting marked changes in licorice metabolite composition post-fermentation with L. mesenteroides (Fig. 3A). Furthermore, orthogonal partial least squares discriminant analysis (OPLS-DA) exhibited distinct clustering patterns, explaining 68.5% of the overall variance, with high predictive capability (Q² = 0.986), strong explanatory power (R²X = 0.685, R²Y = 0.986), and significant statistical relevance (P = 1.7 × 10⁻³) (Fig. 3C). The robustness and validity of the OPLS-DA model were confirmed through 500 permutation tests (Fig. 3D), yielding intercepts of R² and Q² values of 0.0679 and −0.447, respectively. The substantially lower R² and Q² values of randomly permuted models compared to the original model underscore its robustness and reliability.
A PCA score plot showing overall metabolic variation. B S-plot from OPLS-DA illustrating key differential metabolites contributing to the separation between ULE and FLE. C OPLS-DA score plot demonstrating group discrimination. D Cross-validation plot validating the OPLS-DA model robustness. E Heatmap of hierarchical clustering analysis of differential metabolites in ULE and FLE. Metabolite names are labeled in red to indicate increased levels and in black to indicate decreased levels after fermentation of licorice by L. mesenteroides.
Potential key biomarkers differentiating ULE and FLE were identified by screening for significant variations based on variable importance in projection (VIP > 1.0) and |p(corr)| values from the S-plot ( > 0.5) (Table 1 and Fig. 3C). In total, 42 differential metabolites were tentatively identified by comparing their mass-to-charge ratios (m/z) and MS/MS fragmentation patterns against in-house, PubChem, and HMDB databases. These included 14 flavonoids, 7 isoflavones, 8 triterpenoids and their derivatives, 6 organic acids, 3 amino acids and their derivatives, 2 sugars, and 2 other compounds (Table 1). A heatmap visualized changes in these metabolites, with rows representing normalized fold-change concentrations and columns representing individual metabolites. As illustrated in Fig. 3E, metabolite profiles changed notably following fermentation; specifically, levels of most flavonoids, isoflavones, and triterpenoid saponins decreased, whereas concentrations of triterpenoid derivatives, amino acids, and most organic acids increased significantly during fermentation.
Correlation analysis between differential metabolites and anti-inflammatory activities
To determine the specific fermentation-derived metabolites responsible for the enhanced anti-inflammatory effects of FLE, we conducted Pearson’s correlation analysis between 42 differential metabolites and their respective antiglycation and anti-inflammatory activities (Supplementary Table S1, Fig. 4). Among these, 17 metabolites showed significant positive correlations, implicating them as potential bioactive contributors.
The color scale ranges from −1 (blue, strong negative correlation) to +1 (red, strong positive correlation). Circle sizes represent the absolute correlation strengths based on each correlation coefficient. Differential metabolite names are written in red (if increased) and black (if decreased) based on their relative levels following fermentation of licorice by L. mesenteroides.
Based on statistically significant positive correlations with antiglycation and anti-inflammatory activities, 17 potential constituents included 3 triterpenoids derivatives (glycyrrhetic acid-3-O-glucuronide, 18β-glycyrrhetinic acid, 24-hydroxyglycyrrhetic acid), 2 flavonoids (isoliquiritigenin, O-desmethylangolensin), 1 isoflavone (dihydrogenistein), 1 triterpene saponin (licorice saponin G2 isomer), 4 organic acids (citric acid, succinic acid, 2-hydroxyisocaproic acid, and phenyllactic acid), 3 amino acids and their derivatives (pyroglutamic acid, N-(1-deoxy-1-fructosyl)phenylalanine, and phenylalanine), 1 sugar alcohol (mannitol), and 1 fatty acid (10-hydroxy-12(Z)-octadecenoic acid). In contrast, most flavonoids, isoflavones, and triterpene saponins were negatively correlated with anti-inflammatory activities measured in this study.
Flavonoids are secondary metabolites synthesized mainly by plants and are characterized by a basic C6-C3-C6 skeleton structure, such as isoschaftoside, violanthin, isoliquiritin, and licorice glycoside, are known to exhibit substantial biological activities, including anti-inflammatory and antioxidant effects36. Isoflavones, another subclass of flavonoids found in licorice extracts, such as ononin, daidzein, genistein, biochanin A, glyasperin C, and licoisoflavone, have demonstrated antioxidant, anti-osteoporotic, neuroprotective, and anti-inflammatory activities both in vitro and in vivo37.
In addition, licorice-derived triterpene saponins (e.g., licorice saponin G2) and triterpenoids (e.g., glycyrrhizin) have shown significant beneficial effects against inflammation, oxidative stress, cancer, and viral infections38. Interestingly, despite their known antioxidant and anti-inflammatory activities, the levels of these licorice-derived flavonoids, isoflavones, and triterpenoids significantly decreased following fermentation, correlating negatively with measured bioactivities. These results support a mechanistic link between L. mesenteroides-driven biotransformation and the generation of functionally active compounds. The elevated levels of microbial-derived metabolites with positive bioactivity correlations indicate that fermentation not only alters licorice’s phytochemical profile but enhances its physiological efficacy.
Bioconversion pathways of licorice by L. mesenteroides
Proposed bioconversion pathways activated during licorice fermentation by L. mesenteroides are depicted in Fig. 5. To elucidate these pathways, selected metabolites were compared based on their relative abundance in ULE and FLE. L. mesenteroides is a lactic acid bacterium commonly utilized in the food industry as a starter culture for fermented products such as kimchi and sauerkraut39,40. It is particularly recognized for its ability to produce exopolysaccharides, such as dextran, and is applied various medical and chemical fields. β-glucosidase activity, characteristic of lactic acid bacteria including L. mesenteroides, plays a crucial role in carbohydrate metabolism41. Although previous studies have partially explored the physicochemical properties of β-glucosidases from L. mesenteroides, their detailed mechanisms and roles in bioconversion processes remain relatively unexplored.
A Triterpenoid derivatives: Bioconversion of glycyrrhizin and licorice saponin G2 into aglycone and hydrolyzed forms via β-glucosidase activity. B Flavonoid derivatives: Conversion of isoliquiritin apioside into isoliquiritin and isoliquiritigenin through sequential deglycosylation. Selected compounds among the differential metabolites were compared based on their abundance in ULE and FLE. The y-axis of each graph represents the peak areas (log10) of each metabolite in ULE and FLE. Differential metabolite names are displayed in red (if increased) and black (if decreased) according to their relative levels during the fermentation of licorice by L. mesenteroides. *p < 0.05, **p < 0.01, and ***p < 0.001 vs. ULE. Results are expressed as the mean ± SEM (n = 3).
We hypothesized that a β-glucosidase activity from L. mesenteroides facilitates the biotransformation of glycyrrhizin as a lysosomal hydrolase from licorice into its hydrolysis products, namely glycyrrhetic acid-3-O-glucuronide and glycyrrhetic acid, thereby significantly increasing the abundance (Fig. 5A). Supporting this hypothesis, a recent study by Zuo et al. (2025) identified a novel β-glucuronidase from the endophytic fungus Chaetomium globosum, which converts glycyrrhizin into glycyrrhetinic acid 3-O-mono-β-D-glucuronide. Therefore, a β-glucosidase from L. mesenteroides might similarly act as a biocatalyst, enhancing the formation of glycyrrhetic acid-3-O-glucuronide and 18β-glycyrrhetic acid in fermented licorice42.
Glycyrrhizin is the principal active compound in licorice, constituting approximately 10 – 25% of its total phytochemical content43. It is extensively used as an anti-inflammatory agent due to its pharmacological efficacy44. Moreover, glycyrrhizin metabolites, such as glycyrrhetic acid-3-O-glucuronide and 18β-glycyrrhetic acid, exhibit diverse beneficial properties, including anti-inflammatory, antiviral, and antioxidant effects and are frequently utilized in dietary supplements45. Consistent with these known bioactivities, our data revealed a notable increase in triterpenoid metabolites following fermentation. Specifically, the concentration of 24-hydroxyglycyrrhetic acid, a deglycosylated derivative of licorice saponin G2, increased significantly, likely due to enhanced β-glucosidase activity from L. mesenteroides during fermentation (Fig. 5A).
Additionally, isoliquiritin apioside, another licorice constituent, appeared to be converted to isoliquiritigenin by β-glucosidase activity from L. mesenteroides (Fig. 5B). Isoliquiritigenin is recognized as a bioactive compound with significant anticancer, anti-inflammatory, and antibiotic properties, and is widely applied in alternative medicine46. Our findings showed that fermentation substantially elevated isoliquiritigenin concentrations, which positively correlate with the enhanced anti-inflammatory activity observed in FLE. These results highlight the pivotal role of β-glucosidase-mediated bioconversions during fermentation with L. mesenteroides, resulting in increased levels of bioactive metabolites with enhanced anti-inflammatory potential.
Our findings aligned with previous studies demonstrated that fermentation with L. mesenteroides enhances the bioactive profiles of various plant materials47,48,49. For instance, fermentation of onion with L. mesenteroides significantly increased the levels of flavonoid aglycones, such as quercetin and isorhamnetin, leading to improved antioxidant activity48. Similarly, fermentation of ginseng residue enhanced the conversion of ginsenosides to their more bioactive aglycone forms and increased succinic acid production, contributing to augmented biological activity49. Fermentation of oregano leaves also resulted in higher total phenolic and flavonoid content and enhanced antioxidant capacity47. These studies collectively support our observation that β-glucosidase activity from L. mesenteroides effectively facilitates glycoside-to-aglycone conversions across diverse plant substrates, leading to improved bioactivity.
We further compared the key metabolites identified in our FLE including triterpenoids (e.g., glycyrrhetic acid-3-O-glucuronide, 18β-glycyrrhetic acid, and 24-hydroxyglycyrrhetic acid) and flavonoids (e.g., isoliquiritigenin) with those reported in unfermented licorice and other fermented plant extracts47,48,49,50. Glycyrrhizin, the major saponin in licorice, is known for its anti-inflammatory properties. However, its biotransformation into glycyrrhetic acid derivatives during fermentation is particularly important, as these derivatives exhibit enhanced anti-inflammatory and antiglycation activities. This pattern mirrors findings in ginseng fermentation, where levels of rare ginsenosides with improved bioactivity increased post-fermentation. Similarly, the conversion of isoliquiritin apioside into isoliquiritigenin during fermentation aligns with reports in onion fermentation, where β-glucosidase from L. mesenteroides hydrolyzed quercetin glycosides into their more bioactive aglycone forms.
While our findings highlight the anti-inflammatory potential of FLE, it is also important to consider its safety as a functional ingredient. Although fermentation can reduce glycyrrhizin levels, residual amounts may persist and potentially exert mineralocorticoid effects by inhibiting 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2), which could lead to sodium retention, hypokalemia, and hypertension51. Therefore, future studies should quantify residual glycyrrhizin and glycyrrhetinic acid levels in FLE and, if necessary, evaluate its mineralocorticoid activity to ensure safety for human consumption.
Overall, our results not only align with existing literature on L. mesenteroides–mediated fermentation of plant materials but also expand its application to licorice. By reinforcing the conserved mechanism of β-glucosidase-driven bioconversion, we emphasize the broader relevance and novelty of our findings: FLE contains significantly enhanced bioactive compounds, making it a promising functional ingredient for mitigating skin inflammation induced by glycotoxins and UV exposure.
Antiglycation and anti-inflammatory effects of 18β-glycyrrhetic acid and isoliquiritigenin
To investigate the functional roles of fermentation-enhanced metabolites such as 18β-glycyrrhetinic acid and isoliquiritigenin, we conducted glycotoxin screening and in vitro assays using GO-AGEs-treated (100 µg/mL) and UVB-irradiated (130 mJ/cm²) HaCaT keratinocytes (Figs. 6 and 7). The concentration ranges applied for both compounds were determined through preliminary optimization studies. Higher concentrations were intentionally excluded due to signal saturation and no additional anti-inflammatory effects, thereby avoiding data overestimation and plateau artifacts. This approach ensured that the selected concentrations accurately captured the bioactive ranges of both compounds. Consistent with these findings, both 18β-glycyrrhetinic acid and isoliquiritigenin exhibited concentration-dependent reactivity with GO, as evidenced by increased fluorescence intensity. Moreover, they significantly enhanced free amine release in the GO-AGEs breaker assay and inhibited AGEs formation in the GO-AGEs formation assay, indicating dual antiglycation activity.
A GO-affinity assay; (B) GO-AGEs breaker assay; (C) GO-AGEs formation assay; (D) Cell viability in GO-AGEs/UVB-treated HaCaT human keratinocytes; (E–H) Levels of proinflammatory markers including (E) IL-1β, (F) IL-6, (G) TNF-α, and (H) PGE2 in GO-AGEs/UVB-treated HaCaT cells. ###p < 0.001 vs. Control (untreated) group; *p < 0.05, **p < 0.01, and ***p < 0.001 vs. GO, GO-AGE-, or GO-AGEs/UVB-treated group. Results are expressed as the mean ± SEM (n = 3).
A GO-affinity assay; (B) GO-AGEs breaker assay; (C) GO-AGEs formation assay; (D) Cell viability in GO-AGEs/UVB-treated HaCaT human keratinocytes; (E–H) Levels of proinflammatory markers including (E) IL-1β, (F) IL-6, (G) TNF-α, and (H) PGE2 in GO-AGEs/UVB-treated HaCaT cells. ###p < 0.001 vs. Control (untreated) group; *p < 0.05, **p < 0.01, and ***p < 0.001 vs. GO, GO-AGE-, or GO-AGEs/UVB-treated group. Results are expressed as the mean ± SEM (n = 3).
Furthermore, we confirmed the anti-inflammatory effects of 18β-glycyrrhetic acid and isoliquiritigenin on keratinocytes. Each compound significantly suppressed the pro-inflammatory cytokines IL-1β, IL-6, and TNF-α as well as PGE2 production. Previous studies have reported that glycyrrhetic acid and isoliquiritigenin can inhibit NF-κB translocation and MAPKs pathways in keratinocytes, thereby suppressing the release of inflammatory cytokines52. Collectively, this evidence supports the hypothesis that FLE, enriched with these bioactive metabolites, mitigates skin inflammation by both reducing glycotoxin accumulation and modulating inflammatory signaling.
Although our findings suggest that β-glucosidase activity from L. mesenteroides plays a central role in the biotransformation of licorice metabolites, this study did not directly assess enzymatic activity or gene expression during fermentation. Future studies incorporating enzymatic assays and transcriptomic analyses are warranted to verify the role of β-glucosidase in driving these fermentation-induced metabolic changes. Moreover, while we observed marked suppression of NF-κB and MAPKs signaling pathways by treating FLE and identified 18β-glycyrrhetic acid and isoliquiritigenin as key metabolites, we did not conduct direct mechanistic studies (e.g., molecular docking or target engagement assays) to determine whether these compounds modulate upstream receptors or act directly on intracellular targets. Further investigation is necessary to establish a causal link between these metabolites and anti-inflammatory activity.
In summary, untargeted metabolomics enabled the identification of fermentation-induced metabolites contributing to the enhanced antiglycation and anti-inflammatory effects of FLE in GO-AGEs- and UVB-exposed HaCaT keratinocytes compared to ULE. Key bioactive triterpenoids (e.g., glycyrrhetic acid-3-O-glucuronide, 18β-glycyrrhetic acid, 24-hydroxyglycyrrhetic acid) and flavonoids (e.g., isoliquiritigenin) were positively correlated with the observed bioactivities. These transformations are likely facilitated by β-glucosidase-mediated deglycosylation during L. mesenteroides fermentation. Overall, our results highlight the potential of FLE as a promising functional ingredient for managing skin inflammation induced by GO-AGEs and UV exposure. Nevertheless, in vivo studies, including animal models, skin irritation testing, and bioavailability evaluations, are essential to confirm the efficacy and safety of FLE for future application in skincare products and dietary supplements.
Methods
Materials
Licorice, purchased from Handsherb (Yeoncheon-si, Korea), was used as research material. Leuconostoc mesenteroides subsp. mesenteroides (L. mesenteroides) KCTC 3505 was obtained from the Korean Collection for Type Cultures (Seoul, Korea). Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), and penicillin–streptomycin (PS) were purchased from Gibco (Invitrogen, Carlsbad, CA, USA). Enzyme-linked immunosorbent assay (ELISA) kits for the detection of cytokines and prostaglandins (PGE2, IL-1β, IL-6, and TNF-α [tumor necrosis factor-α]) were purchased from R&D Systems (Minneapolis, MN, USA). Receptor of advanced glycated end products (RAGE), cyclooxygenase-2 (COX-2), extracellular-signal-regulated kinase (ERK), p-ERK, c-Jun N-terminal kinase (JNK), p-JNK, p38, p-p38, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), IκB, p-IκB, and α-tubulin were purchased from Cell Signaling Technologies (Beverly, MA, USA). The other chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Bacterial strain and culture
L. mesenteroides subsp. KCTC 3505 (L. mesenteroides) was stored at -80 °C with 40% glycerol, and was activated on a de Man Rogosa Sharpe (Difco, MD, USA) agar plate at 30 °C. The seed culture was grown in optimized MRS broth containing sucrose (3.63%), soy peptone (2.70%), and potassium dihydrogen phosphate (0.36%) at 30 °C for 1 d.
Sample preparation
For preparing the ULE, 1 kg licorice plant was crushed and extracted with 9,000 mL of distilled water for 16 h at 85 °C with constant mixing (400 rpm). The extract was then centrifuged at 14,000 rpm for 10 min at room temperature, and the supernatant was filtered through diatomaceous earth (DE). The filtered extract was then concentrated using a low-temperature concentrator (COSMOS660-50L, Kyungseo Machines Co., Incheon, Korea) and spray-dried using a benchtop Lab-Plant SD-05 spray dryer (Lab-Plant, UK Ltd., Huddersfield, UK). To prepare the FLE, L. mesenteroides KCTC 3505 was cultured in an optimized MRS medium for 1 d, mixed with a concentrated ULE sample (L. mesenteroides KCTC 3505:5% concentrated ULE, 1:10 v/v), and cultured in a 2 L flask at 37 °C for 1 d with shaking at 150 rpm. The fermented licorice extract was collected and subsequently freeze-dried to obtain the FLE. Both ULE and FLE were dissolved in distilled water before use.
Glyoxal (GO) affinity assay
GO affinity assay was conducted to measure the interaction between GO and each sample (ULE or FLE) at different concentrations as described in our previous study with minor modification53. GO, with or without samples, was incubated in phosphate buffered saline (PBS, pH 7.4) and 0.02% sodium azide for 1 week at 37 °C. GO-sample affinities were analyzed in terms of fluorescence intensity at excitation/emission wavelengths of 355/460 nm using a multilabel plate reader (VICTOR™ X3, PerkinElmer, MA, USA).
Preparation of GO-advanced glycation end products (GO-AGEs)
For the preparation of GO-AGEs, 10 mM GO was reacted with 5 mg/mL of bovine serum albumin and 0.02% sodium azide, and dissolved in PBS (pH 7.4) for 1 week at 37 °C. The reacted solution was evaporated, filtered, and dialyzed using a ZebaTM Spin Desalting Column (7 K MWCO; Thermo Fisher Scientific, Waltham, MA, USA). The filtered GO-AGEs were stored in a freeze dryer until use.
GO-AGEs breaker assay
The GO-AGEs breaker assay was performed using 2,4,6-trinitrobenzene sulfonic acid (TNBSA) to assess the effects of FLE, according to Md Samsuzzaman et al. (2022), with minor modifications. GO-AGEs (0.5 mg/mL) were mixed with corresponding samples and reacted for 1 d at 37 °C in the dark. TNBSA within sodium bicarbonate was added to mixed samples, and then incubated once again for 2 h at 37 °C. The reactions were stopped by adding a 10% sodium dodecyl sulfate solution and 1 N hydrochloric acid. Free amines were quantified by using a microplate reader at 340 nm (Molecular Devices, CA, USA).
GO-AGEs formation assay
The GO-AGEs formation assay was performed according to our previous study54. Briefly, the samples were incubated in PBS (pH 7.4) containing 10 mM GO, 5 mg/mL BSA, and 0.02% sodium azide for 1 week at 37 °C in the dark. GO-AGEs formation was measured in terms of fluorescence intensity at excitation/emission wavelengths of 355/460 nm using a VICTOR™ X3 multilabel plate reader (PerkinElmer).
Circular dichroism (CD) spectroscopy analysis
The secondary structure of reacted solution from the GO-AGEs formation assay was analyzed using CD spectroscopy (Supplementary Data Fig. S1A). The spectra of the reacted samples were corrected based on those of the buffer and chemical controls, and the thermal denaturation of GO-AGEs was monitored by recording CD signals between wavelengths of 200 and 260 nm. The temperature ramping speed was set to 1 °C/min.
Cell cultures
Immortalized keratinocyte cells (HaCaT) were obtained from the Korean Cell Line Bank (Seoul National University, Seoul, Korea) and cultured in DMEM (Gibco) with 10% FBS (Gibco) and 1% PS (Gibco). Ultraviolet B (UVB) irradiation assays were performed as previously reported at 130 mJ/cm2 UVB using a UVB irradiation machine (Bio-Link Crosslinker BLX-312; Vilber Lourmat GmbH, Marne-la-Vallée, France)19.
Cell viability assay
For measuring the cell viability, HaCaT cells were seeded into 96-well plates (4 × 105 cells/well) and incubated for 1 d at 37 °C in a 5% CO2 incubator. HaCaT cells were then treated with 100 µg/mL GO-AGEs and either ULE or FLE samples in the presence or absence of UVB irradiation (130 mJ/cm2) for 1 d. An MTT solution (0.5 mg/mL) was then added to the wells; after 1 h, the solution was removed, and dimethyl sulfoxide was added. The absorbance was measured at 340 nm using a microplate reader (Molecular Devices, CA, USA).
ELISA
To measure the levels of proinflammatory cytokines, such as IL-1β, IL-6, and TNF-α, and PGE2 production, HaCaT cells were seeded into 6-well plates (4 × 105 cells/well) and incubated for 1 d at 37 °C in a 5% CO2 incubator. HaCaT cells were then treated as for the cell viability assay described above. The supernatant obtained from the treated plates was collected, and the secreted cytokine levels and PGE2 production were analyzed via ELISA kits, following the manufacturer’s guidelines.
Western blot assay
HaCaT cells were harvested, washed with cold PBS, and lysed in PRO-PREP™ lysis buffer (iNtRON Biotechnology) containing protease and phosphatase inhibitors. Cytosolic and nuclear fractions were extracted using reagents from Thermo Fisher Scientific. Protein concentrations were measured using the Bradford assay. Equal protein amounts (30 – 40 µg) were separated on 8–10% SDS-PAGE gels and transferred onto PVDF membranes. Membranes were blocked in 5% skim milk in TBST (0.1% Tween 20) for 2 h, incubated overnight (4 °C) with primary antibodies (1:1000; Cell Signaling Technology) against p-ERK, ERK, p-JNK, JNK, p-p38, p38, NF-κB, IκB-α, COX-2, IL-1β, histamine-H3, RAGE, and α-tubulin, followed by incubation with horseradish peroxidase-linked secondary antibodies. Bands were visualized using chemiluminescence (ECL) and analyzed with Image Master™ 2D Elite software.
Untargeted metabolomics analysis
For metabolite extraction, three biological replicates were analyzed for each sample. ULE and FLE (40 mg each) were dissolved in 1 mL methanol containing 2-chlorophenylalanine (10 µg/mL) as an internal standard. Each sample mixture was homogenized using a mixer mill for 10 min. After homogenization, the suspension was incubated at 4 °C for 40 min, and then centrifuged at 15,000 × g and 4 °C for 10 min. The supernatant was filtered using a 0.2 μm syringe filter. The filtered supernatants (800 μL each) were dried under vacuum and resuspended in 1.8 mL methanol for UHPLC-LTQ-Orbitrap-MS/MS analysis. Quality control (QC) samples were prepared by pooling 50 μL aliquots from each supernatant into a pooled solution. QC and blank samples were injected into each of the three test samples.
Metabolite analysis of ULE and FLE was performed using a Vanquish binary pump C system (Thermo Fisher Scientific) equipped with a Waters ACQUITY UPLC HSS T3 column (150 mm × 2.1 mm, 1.8 μm particle size; Waters) and an Orbitrap Exploris™ 120 mass spectrometer (Thermo Fisher Scientific). Chromatographic separation was achieved using a Waters ACQUITY UPLC HSS T3 column (150 mm × 2.1 mm, 1.8 μm particle size; Waters), using the following mobile phases: (A) 0.1% (v/v) formic acid in water and (B) 0.1% (v/v) formic acid in acetonitrile. The following gradient conditions were employed: 0–1 min, 5% B; 1–10 min, 5–100% B; 10–11 min, 100% B; 11–13 min, 100–5% B; and 13–15 min, 5% B, at a flow rate of 0.3 mL/min and a column temperature of 40 °C. The injection volume was 5 μL. The following settings were established: sheath gas flow rate = 50 Arb; auxiliary gas = 15 Arb; ion transfer tube temperature = 325 °C; vaporizer temperature = 300 °C; spray voltage = -3.4 kV (negative); full scan resolution = 60,000; scan range (m/z) = 100–1500; MS/MS resolution = 30,000; and collision energy (%) = 30.
Data processing and statistical analysis
The raw data files obtained from UHPLC–Orbitrap–MS/MS were processed for retention time correction, peak detection, and alignment using MS-DIAL software (version 5.4). The raw files were converted to the ABF file format using the Analysis Base File Converter and MSConvert. The parameters were set as follows: MS1 tolerance = 0.01 Da; MS2 tolerance = 0.025 Da; retention time range = 0 –15 min; MS1 mass range and MS/MS mass range = 100 – 1500; minimum peak height = 1000 amplitude; and mass slice width = 0.1 Da. Analytical stability and reproducibility were assessed by periodic injection of QC samples throughout the analytical run. The tight clustering of these QC samples in a principal component analysis (PCA) confirmed minimal analytical variation during the measurement.
To investigate the metabolic differences between ULE and FLE, multivariate statistical analyses, including PCA and orthogonal partial least squares discriminant analysis (OPLS-DA), were conducted using SIMCA-P+ (version 15.0.2; Umetrics, Umea, Sweden). Differential metabolites were selected based on their Variable Importance in Projection (VIP) values greater than 1.0 obtained from the OPLS-DA models and |p(corr)| ( > 0.5) of the S-plot. Metabolite identification followed the Metabolite Standards Initiative (MSI) criteria. Compounds classified as Level 1 were confirmed by comparing with authentic standards analyzed under identical LC-MS/MS conditions, using in house spectral library. Both retention times (within ± 0.2 min) and MS/MS fragmentation pattern were matched, with a dot product (DP) score ≥ 0.8. Level 2 compounds were tentatively assigned based on similarity of their MS/MS spectra (DP score ≥ 0.8) to known reference spectra from public resources such as GNPS, HMDB, and curated databased of alkaloid and polyphenol libraries obtained from MoNA server (https://mona.fiehnlab.ucdavis.edu/). Further annotations were supported by manual comparison with fragmentation data found in the reported literature55. Correlation analyses were performed between the differential metabolites and their antiglycation and anti-inflammatory activities to identify potential compounds. Pearson’s correlation coefficients and statistical significance were calculated using R software with the corrplot and ggcorrplot packages. Differential metabolites resulting from the biotransformation of licorice-derived compounds were analyzed for significant differences in their relative contents between ULE and FLE using an unpaired t-test. All p-values were subsequently adjusted using the Benjamini-Hochberg procedure to control the false discovery rate (FDR) using the MetaboAnalyst 6.0 web server (https://www.metaboanalyst.ca/). Metabolites with an adjusted p-value below 0.05 were considered statistically significant.
Data Availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Shin, S. H., Lee, Y. H., Rho, N. K. & Park, K. Y. Skin aging from mechanisms to interventions: focusing on dermal aging. Front Physiol. 14, 1195272 (2023).
Wong, Q. Y. A. & Chew, F. T. Defining skin aging and its risk factors: a systematic review and meta-analysis. Sci. Rep. 11, 22075 (2021).
Zhuang, Y. & Lyga, J. Inflammaging in skin and other tissues - the roles of complement system and macrophage. Inflamm. Allergy Drug Targets 13, 153–161 (2014).
Finnegan, D., Tocmo, R. & Loscher, C. Targeted Application of Functional Foods as Immune Fitness Boosters in the Defense against Viral Infection. Nutrients 15, https://doi.org/10.3390/nu15153371 (2023).
Wang, L., Jiang, Y. & Zhao, C. The effects of advanced glycation end-products on skin and potential anti-glycation strategies. Exp. Dermatol. 33, e15065 (2024).
Gkogkolou, P. & Böhm, M. Advanced glycation end products: Key players in skin aging?. Derm.-Endocrinol. 4, 259–270 (2012).
Birukov, A., Cuadrat, R., Polemiti, E., Eichelmann, F. & Schulze, M. B. Advanced glycation end-products, measured as skin autofluorescence, associate with vascular stiffness in diabetic, pre-diabetic and normoglycemic individuals: a cross-sectional study. Cardiovascular Diabetol. 20, 1–11 (2021).
Sultana, R., Parveen, A., Kang, M.-C., Hong, S.-M. & Kim, S. Y. Glyoxal-derived advanced glycation end products (GO-AGEs) with UVB critically induce skin inflammaging: in vitro and in silico approaches. Sci. Rep. 14, 1843 (2024).
Fam, V. W. et al. Plant-Based Foods for Skin Health: A Narrative Review. J. Acad. Nutr. Dietetics 122, 614–629 (2022).
Wahab, S. et al. Glycyrrhiza glabra (Licorice): A Comprehensive Review on Its Phytochemistry, Biological Activities, Clinical Evidence and Toxicology. Plants (Basel) 10, https://doi.org/10.3390/plants10122751 (2021).
Shaikh, S. et al. Biological insights and therapeutic potential of Glycyrrhiza uralensis and its bioactive compounds: an updated review. Arch Pharm Res, https://doi.org/10.1007/s12272-024-01522-0 (2024).
Fiore, C., Eisenhut, M., Ragazzi, E., Zanchin, G. & Armanini, D. A history of the therapeutic use of liquorice in Europe. J. Ethnopharmacol. 99, 317–324 (2005).
Luo, X. et al. Fermentation: improvement of pharmacological effects and applications of botanical drugs. Front Pharm. 15, 1430238 (2024).
Wang, X., Liu, Y., Kang, N. & Xu, G. Wide identification of chemical constituents in fermented licorice and explore its efficacy of anti-neurodegeneration by combining quasi-targeted metabolomics and in-depth bioinformatics. Front Neurosci. 17, 1156037 (2023).
Schrimpe-Rutledge, A. C., Codreanu, S. G., Sherrod, S. D. & McLean, J. A. Untargeted Metabolomics Strategies-Challenges and Emerging Directions. J. Am. Soc. Mass Spectrom. 27, 1897–1905 (2016).
Marques, C. F. & Justino, G. C. An optimised MS-based versatile untargeted metabolomics protocol. Separations 10, 314 (2023).
Rinschen, M. M., Ivanisevic, J., Giera, M. & Siuzdak, G. Identification of bioactive metabolites using activity metabolomics. Nat. Rev. Mol. cell Biol. 20, 353–367 (2019).
Beccaria, M. & Cabooter, D. Current developments in LC-MS for pharmaceutical analysis. Analyst 145, 1129–1157 (2020).
Sultana, R., Parveen, A., Kang, M. C., Hong, S. M. & Kim, S. Y. Glyoxal-derived advanced glycation end products (GO-AGEs) with UVB critically induce skin inflammaging: in vitro and in silico approaches. Sci. Rep. 14, 1843 (2024).
Prasad, C., Davis, K. E., Imrhan, V., Juma, S. & Vijayagopal, P. Advanced Glycation End Products and Risks for Chronic Diseases: Intervening Through Lifestyle Modification. Am. J. Lifestyle Med 13, 384–404 (2019).
Zgutka, K., Tkacz, M., Tomasiak, P. & Tarnowski, M. A Role for Advanced Glycation End Products in Molecular Ageing. Int J Mol Sci 24, https://doi.org/10.3390/ijms24129881 (2023).
Chen, C. Y. et al. Advanced Glycation End Products in the Skin: Molecular Mechanisms, Methods of Measurement, and Inhibitory Pathways. Front Med (Lausanne) 9, 837222 (2022).
Kim, J. S., Lee, J. H., Hong, S. M., Cho, K. H. & Kim, S. Y. Salvia miltiorrhiza prevents methylglyoxal-induced glucotoxicity via the regulation of apoptosis-related pathways and the glyoxalase system in human umbilical vein endothelial cells. Biol. Pharm. Bull. 45, 51–62 (2022).
Savini, I., D’Angelo, I., Ranalli, M., Melino, G. & Avigliano, L. Ascorbic acid maintenance in HaCaT cells prevents radical formation and apoptosis by UV-B. Free Radic. Biol. Med 26, 1172–1180 (1999).
Im, A.-R. et al. Protective effect of fermented Cyclopia intermedia against UVB-induced damage in HaCaT human keratinocytes. BMC Complementary Alternative Med. 16, 1–10 (2016).
Vostálová, J., Zdařilová, A. & Svobodová, A. Prunella vulgaris extract and rosmarinic acid prevent UVB-induced DNA damage and oxidative stress in HaCaT keratinocytes. Arch. dermatological Res. 302, 171–181 (2010).
Wautier, J. L. & Wautier, M. P. Pro- and Anti-Inflammatory Prostaglandins and Cytokines in Humans: A Mini Review. Int J Mol Sci 24, https://doi.org/10.3390/ijms24119647 (2023).
Zhang, H. et al. Antioxidant and anti-inflammatory activities of rape bee pollen after fermentation and their correlation with chemical components by ultra-performance liquid chromatography-quadrupole time of flight mass spectrometry-based untargeted metabolomics. Food Chem. 409, 135342 (2023).
Leibold, J. S. et al. Keratinocyte-specific deletion of the receptor RAGE modulates the kinetics of skin inflammation in vivo. J. Invest Dermatol 133, 2400–2406 (2013).
Kulesza, A., Paczek, L. & Burdzinska, A. The Role of COX-2 and PGE2 in the Regulation of Immunomodulation and Other Functions of Mesenchymal Stromal Cells. Biomedicines 11, https://doi.org/10.3390/biomedicines11020445 (2023).
Eberhardt, W., Huwiler, A., Beck, K. F., Walpen, S. & Pfeilschifter, J. Amplification of IL-1 beta-induced matrix metalloproteinase-9 expression by superoxide in rat glomerular mesangial cells is mediated by increased activities of NF-kappa B and activating protein-1 and involves activation of the mitogen-activated protein kinase pathways. J. Immunol. 165, 5788–5797 (2000).
Hu, F. et al. Fermented licorice extract alleviates ulcerative colitis by inhibiting the TLR4/NF-κB pathway and rebuilding intestinal microbiota in mice. Food Biosci. 61, 104918 (2024).
Xiong, R.-G. et al. Preparation and evaluation of liquorice (Glycyrrhiza uralensis) and ginger (Zingiber officinale) kombucha beverage based on antioxidant capacities, phenolic compounds and sensory qualities. Int. J. Gastronomy Food Sci. 35, 100869 (2024).
Ayar-Sümer, E. N., Verheust, Y., Özçelik, B. & Raes, K. Impact of lactic acid bacteria fermentation based on biotransformation of phenolic compounds and antioxidant capacity of mushrooms. Foods 13, 1616 (2024).
Jacyna, J., Kordalewska, M., Wiczling, P. & Markuszewski, M. J. Evaluation of robustness in untargeted metabolomics: application of multivariate analysis, linear regression and hierarchical modeling. J. Chromatogr. Open 2, 100035 (2022).
Roy, A. et al. Flavonoids a Bioactive Compound from Medicinal Plants and Its Therapeutic Applications. Biomed. Res Int 2022, 5445291 (2022).
Yang, S. E., Lien, J. C., Tsai, C. W. & Wu, C. R. Therapeutic Potential and Mechanisms of Novel Simple O-Substituted Isoflavones against Cerebral Ischemia Reperfusion. Int J Mol Sci 23, https://doi.org/10.3390/ijms231810394 (2022).
Li, F. et al. Review of Constituents and Biological Activities of Triterpene Saponins from Glycyrrhizae Radix et Rhizoma and Its Solubilization Characteristics. Molecules 25, https://doi.org/10.3390/molecules25173904 (2020).
Jung, J. Y. et al. Effects of Leuconostoc mesenteroides starter cultures on microbial communities and metabolites during kimchi fermentation. Int. J. Food Microbiol. 153, 378–387 (2012).
Nag, S. et al. Survey of brachytherapy practice in the United States: a report of the Clinical Research Committee of the American Endocurietherapy Society. Int J. Radiat. Oncol. Biol. Phys. 31, 103–107 (1995).
Pino-García, R. D., Porrelli, A., Rus-Fernández, P., Segura-Carretero, A. & Curiel, J. A. Identification, purification and characterization of a novel glycosidase (BgLm1) from Leuconostoc mesenteroides. LWT 122, 108829 (2020).
Zuo, X., Xu, Y., Ren, G., Jiang, D. & Liu, C. Licorice endophytes activate glycyrrhizin synthesis metabolic flux through feedback of β-glucuronidase conversion activity. Int. J. Biol. Macromolecules 302, 140484 (2025).
Sontia, B., Mooney, J., Gaudet, L. & Touyz, R. M. Pseudohyperaldosteronism, liquorice, and hypertension. J. Clin. Hypertens. (Greenwich) 10, 153–157 (2008).
Selyutina, O. Y. & Polyakov, N. E. Glycyrrhizic acid as a multifunctional drug carrier - From physicochemical properties to biomedical applications: A modern insight on the ancient drug. Int J. Pharm. 559, 271–279 (2019).
Wang, L., Yang, R., Yuan, B., Liu, Y. & Liu, C. The antiviral and antimicrobial activities of licorice, a widely-used Chinese herb. Acta Pharm. Sin. B 5, 310–315 (2015).
Peng, F. et al. A Review: The Pharmacology of Isoliquiritigenin. Phytother. Res 29, 969–977 (2015).
Bautista-Hernández, I. et al. Solid-state fermentation for phenolic compounds recovery from mexican oregano (Lippia graveolens Kunth) residual leaves applying a lactic acid bacteria (Leuconostoc mesenteroides). Agriculture 14, 1342 (2024).
Lee, Y. G., Cho, J. Y., Kim, Y. M. & Moon, J. H. Change in flavonoid composition and antioxidative activity during fermentation of onion (Allium cepa L.) by Leuconostoc mesenteroides with different salt concentrations. J. food Sci. 81, C1385–C1393 (2016).
Su, X. et al. Co-production of polysaccharides, ginsenosides and succinic acid from Panax ginseng residue: A typical industrial herbal waste. Bioresour. Technol. 331, 125073 (2021).
Chen, L., Gong, J., Yong, X., Li, Y. & Wang, S. A review of typical biological activities of glycyrrhetinic acid and its derivatives. RSC Adv. 14, 6557–6597 (2024).
Awad, N., Makar, G., Burroughs, V., Ravi, P. & Burroughs, S. R. Licorice-induced apparent mineralocorticoid excess causing persistent hypertension and hypokalemia. Acta Endocrinol. (Buchar.) 16, 508–510 (2020).
Wu, Y. et al. Pharmacological Effects and Underlying Mechanisms of Licorice-Derived Flavonoids. Evid. Based Complement Altern. Med 2022, 9523071 (2022).
Md, S. et al. Depression like-behavior and memory loss induced by methylglyoxal is associated with tryptophan depletion and oxidative stress: a new in vivo model of neurodegeneration. Biol. Res 57, 87 (2024).
Lee, J. H., Samsuzzaman, M., Park, M. G., Park, S. J. & Kim, S. Y. Methylglyoxal-derived hemoglobin advanced glycation end products induce apoptosis and oxidative stress in human umbilical vein endothelial cells. Int. J. Biol. Macromolecules 187, 409–421 (2021).
Lan, X. F. et al. Pharmacokinetics-based identification of pseudoaldosterogenic compounds originating from Glycyrrhiza uralensis roots (Gancao) after dosing LianhuaQingwen capsule. Acta Pharm. Sin. 42, 2155–2172 (2021).
Acknowledgements
This research was supported by grants from the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (RS-2023-NR076984 and RS-2023-007607). Also, this work was supported by a grant from the National Institute of Biological Resources (NIBR), funded by the Ministry of Environment (MOE) of the Republic of Korea (NIBR202518101).
Author information
Authors and Affiliations
Contributions
S.-M.H., D.-E.K., and S.-H.K. wrote the main manuscript text and processed all the data; C.-H.L., S.L., and S.L. prepared figures 3-5 and table 1; C.-H.L. and M.K.L. offered suggestions and references for the manuscript; S.-M.H., S.-H.K., Y.K.S. and S.Y.K. conducted a full-text analysis and review and made revisions and corrections. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hong, SM., Kim, DE., Kim, SH. et al. Biotransformation by beta glucosidase enhances anti inflammatory metabolites in licorice using untargeted metabolomics. npj Sci Food 9, 166 (2025). https://doi.org/10.1038/s41538-025-00533-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41538-025-00533-5