Abstract
This study elucidates the anti-colorectal cancer (CRC) mechanism of Rosa rugosa cv. Plena polysaccharide RPP1 against AOM/DSS-induced carcinogenesis. Purified via water extraction and chromatography, RPP1 was characterized as an eight-monosaccharide polymer by HPLC. In CRC mice, RPP1 administration reduced colonic polyp formation and ameliorated colon shortening while normalizing elevated serum IL-1β and TNF-α levels. Integrated multi-omics analyses revealed RPP1 remodeled gut microbiota composition through suppression of pro-inflammatory Bacteroides and Desulfovibrio concurrent with enrichment of beneficial Eubacterium and Muribaculum. These microbial shifts were associated with downregulation of fecal phosphatidylcholine (PC) and lysophosphatidylcholine (LPC), perturbing ether lipid, glycerophospholipid and sphingolipid metabolism. Mechanism correlation analysis further indicated Muribaculum/Clostridium modulation mediated RPP1’s regulation of LPC levels. Transcriptomics confirmed suppression of lipid-metabolism pathways alongside activation of NOD-like receptor (NLR) and PPAR immune signaling. Collectively, RPP1 alleviates CRC through multifaceted modulation of gut microbiota, lipidomic remodeling, and immune pathway activation.
Similar content being viewed by others
Introduction
Colorectal cancer is the third most prevalent malignancy and the second leading cause of cancer-related mortality globally1. Although the incidence of CRC has declined among older adults in high-income countries, it continues to rise in emerging economies and among younger adults aged under 50 years2. The current treatment modalities for CRC include surgical resection, radiation therapy, chemotherapy, and targeted and multi-agent therapies. Although surgical intervention and pharmacological treatment can significantly enhance clinical outcomes, they don’t appreciably alter cure rates or long-term survival. Local recurrence remains a significant challenge, occurring in ∼5–10% of patients within 2 years after surgery3,4. Consequently, identifying novel therapeutic agents capable of effectively preventing and treating CRC has emerged as a pressing public health concern.
Polysaccharides are essential primary metabolites of natural organisms and play fundamental roles in biological processes such as photosynthesis and respiration5. In addition to these basic functions, polysaccharides exhibit a range of bioactivities including antioxidant6, anti-inflammatory7, antitumor8, and immunomodulatory properties9. Additionally, polysaccharides possess favorable characteristics such as biocompatibility, biodegradability, and low toxicity, which have made them promising agents for the treatment of CRC8. For example, polysaccharides obtained from Ganoderma lucidum10 have been reported to mitigate microbiota dysbiosis, boost short-chain fatty acid production, potently inhibit tumor growth, and activate antitumor immunity in CRC models. Correspondingly, hawthorn polysaccharides11 have demonstrated the capacity to rectify the intestinal weight-to-length ratio, diminish the number of colon tumors, lower inflammatory cytokine levels, and repair colonic damage in azoxymethane/dextran sulfate sodium (AOM/DSS)-induced CRC mice. These polysaccharides also maintain the abundance of intestinal microbiota, promote apoptosis of CRC cells, and increase caspase-3 levels in colonic tissues.
Polysaccharides are one of the primary bioactive constituents of roses. Rose polysaccharides exhibit diverse functions, including antioxidant, moisturizing, antiaging, immunomodulatory, antidiabetic, anti-alcoholic liver disease, and antitumor activities. Enzymatically extracted Roxburgh rose pomace polysaccharides possess high scavenging ability against 2,2-diphenyl-1-picrylhydrazyl hydroxyl radicals12. Polysaccharide–peptide complexes with antioxidant properties have been isolated from the aqueous extracts of dried rose flowers13. Rose petal polysaccharides RRRS-1 and RRRS-2 display strong free radical-scavenging capacity and moisturizing activity in vitro14. Acidic polysaccharides derived from Pingyin rose buds exhibit antioxidant and antiaging characteristics in vivo15. A novel polysaccharide WSRP-1b, isolated from bitter water rose waste, showed immunomodulatory activity in vitro and enhanced macrophage phagocytosis16. The pectin polysaccharide RP-1 from Rosa damascena petals modulates the function of the intestinal immune system and augments the production of IL-6 by macrophages17. The polysaccharides WSRP-2a and WSRP-2b, isolated and purified from the rose waste of Rosa setate × Rosa rugosa, possess both hypoglycemic and immunomodulatory activities, with WSRP-2a demonstrating more significant immunomodulatory effects16. RBPP-P, a pectin bee pollen polysaccharide isolated from roses, promotes autophagy in the liver via the AMP-activated protein kinase/mammalian target of rapamycin (AMPK/mTOR)-mediated pathway, thereby alleviating hepatic steatosis and insulin resistance18. In vivo experiments have indicated that the rosebud polysaccharide RRP effectively inhibits hepatic antioxidant activity and attenuates hepatic inflammatory response, thus ameliorating acute alcoholic liver disease in mice19. In vitro experimental investigations have revealed that the rosebud polysaccharide RRP can induce apoptosis in human cervical cancer cells via the PI3K/AKT/mTOR pathway20.
Several studies have reported that rose polysaccharides exhibit anticancer activity against CRC in vitro. Wang21 purified the total polysaccharide WRPP from rose pollen and fractionated it into three types of polysaccharides: neutral WRPP-N and acidic polysaccharides WRPP-1 and WRPP-2. In vitro experiments demonstrated that WRPP, WRPP-N, WRPP-1, and WRPP-2 possessed antiproliferative activities against human colon cancer cells HT-29 and HCT-116, with WRPP exhibiting the strongest effect. Olech22 extracted crude polysaccharides (CPL) from rose petals, leaves, and achenes and showed that CPL from petals had a potent antiproliferative effect on the colon cancer cell line SW480, whereas polysaccharides from other plant parts exhibited weaker antiproliferative activities. Despite the growing interest in the therapeutic potential of rose polysaccharides, no experimental studies have reported their in vivo anti-CRC effects. In the present study, Rosa rugosa cv. Plena was used as the experimental material. This variety is renowned for its prominent corolla morphology, vibrant coloration, rich fragrance, and ornamental, edible, and medicinal value. An AOM/DSS-induced CRC mouse model was established to systematically monitor changes in body weight, colonic morphological alterations, and serum inflammatory cytokine levels. To further elucidate the anti-CRC mechanisms of rose polysaccharides, multi-omics analysis techniques, including microbiomics, lipidomics, and transcriptomics, were integrated to comprehensively investigate the in vivo antitumor effects and underlying molecular mechanisms of rose polysaccharides.
Results
Isolation, purification, and monosaccharide composition analysis of polysaccharides from Rosa rugosa cv. Plena
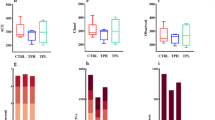
Polysaccharides were extracted from the powdered petals of Rosa rugosa cv. Plena using aqueous alcohol precipitation was used to obtain the crude polysaccharide RPP0 (extraction yield: 41.56%) and the initially purified polysaccharide RPP1 (extraction yield: 10.33%). The Sevag method was employed for protein removal, and depigmentation was achieved using a macroporous adsorbent resin. Further purification of RPP1 via DEAE-cellulose 52 anion-exchange chromatography yielded three elution peaks for RPP2-0, RPP2-1, and RPP2-3 eluted with distilled water, 0.1 M NaCl, and 0.3 M NaCl, respectively (Fig. 1a).
Plena, and analysis of its monosaccharide composition. a Elution profile of RPP1 on a DEAE-cellulose 52 column. b Mixed-label HPLC chromatograms of the composition of monosaccharide standards. c–e HPLC chromatograms, c RPP2-0. d RPP2-1. e RPP2-3. PMP: 1-phenyl-3-methyl-5-pyrazolone; 1: Man; 2: Rha; 3: GlcUA; 4: GalUA; 5: Glc; 6: Gal; 7: Xyl; 8: Ara.
The monosaccharide compositions of RPP2-0, RPP2-1, and RPP2-3 were analyzed by PMP-HPLC, the peak area of each monosaccharide was measured, and their molar ratio was calculated. Comparison with a mixed-label chromatogram of monosaccharide standards (Fig. 1b) revealed that the major monosaccharides in RPP2-0 were Glc and Man (Fig. 1c) at a molar ratio of 0.3399:0.0109. The primary monosaccharides in RPP2-1 were Man, Rha, GlcUA, Glc, Gal, Xyl, and Ara (Fig. 1d), with a molar ratio of 0.1995:0.0276:0.0484:0.5552:0.1916:0.1941:0.1953. The main monosaccharides of RPP2-3 were Man, Rha, GlcUA, GalUA, Glc, Gal, and Ara (Fig. 1e) with a molar ratio of 0.2449:0.1392:0.0541:9.4314:0.2936:0.3608:0.3386. Collectively, the total monosaccharide composition of RPP1 was Man, Rha, GlcUA, GalUA, Glc, Gal, Xyl, and Ara with molar ratios of 0.4634:0.2668:0.1025:9.4314:1.1887:0.5524:0.1941:0.5339. The three fractions obtained, along with the extraction yield and monosaccharide composition of RPP1, are summarized in Table 1.
RPP1 attenuates AOM/DSS-induced CRC symptoms in mice
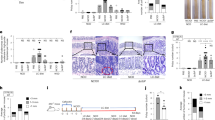
The AOM/DSS method was used to induce CRC in a mouse model (Fig. 2a) to investigate the antitumor mechanism of RPP1. As shown in Fig. 2b, during the modeling process, the weight of the mice gradually increased. Among them, AOM/DSS model mice (M group, PH group, PL group, and F group) showed a decrease in body weight during DSS water intake, and gradually increased body weight after resuming water intake. However, compared with group M, there was no significant difference in body weight among mice in groups PH, PL, and F, indicating that RPP1 intervention had no significant effect on the body weight of CRC mice. At the end of the gavage experiment, the number of colon polyps in group M was significantly higher than that in group C, with an average polyp count of 7 (Fig. 2c), confirming successful CRC modeling. After RPP1 intervention, the number of colon polyps in the PH and PL groups of mice decreased to 3.6 and 4, respectively, which were significantly lower than those in the M group. However, compared with the one polyp number in the F group, the number of polyps was still relatively high. The above experimental results indicate that RPP1 can significantly reduce the increase in the number of intestinal polyps caused by colorectal cancer in mice. Additionally, RPP1 intervention significantly alleviated AOM/DSS-induced colon shortening in mice (Fig. 2d). Statistical analysis revealed that the average colon length in group M was 80 mm, which was significantly shorter than that in group C (87 mm, p < 0.001; Fig. 2e). In contrast, the colons from group PL, PH, and F were significantly longer than those in group M, with the most pronounced increase observed in group PL (average length: 86 mm; p < 0.05).
C: control group; M: model group; PH: high-dose RPP1 group; PL: low-dose RPP1 group; F: 5-fluorouracil group. a Schematic diagram of CRC mouse model establishment and intervention protocol. b Average body weight of mice in different intervention groups. c Number of colon polyps. d Representative pictures of mouse colon. e Colon length. *p < 0.05, **p < 0.01, ***p < 0.001.
Effect of RPP1 on serum inflammatory factors and colon tissue
Serum levels of TNF-α, IL-1β, and IL-6 in mice were measured using ELISA kits. Serum TNF-α levels were significantly elevated in group M compared to group C (p < 0.05; Fig. 3a), whereas TNF-α levels in groups PH (p < 0.05) and PL (p < 0.01) were significantly reduced compared to group M, approaching the levels observed in group C. In contrast, IL-1β exhibited an inverse trend to TNF-α. Specifically, serum IL-1β levels were significantly lower in group M compared to group C (p < 0.05), while groups PH and PL exhibited significantly higher IL-1β levels (p < 0.001) compared to group M (Fig. 3b); these levels were similar to those in group C. As shown in Fig. 3c, no significant differences in serum IL-6 levels were observed among the five groups. The above experimental results indicate that CRC amplifies the inflammatory response in the tumor microenvironment of mice, whereas RPP1 intervention effectively mitigates inflammation and inhibits tumor progression in CRC mice.
Histopathological analysis revealed significant pathological changes in the colon tissues of the AOM/DSS-induced CRC mice. In group C, the intestinal mucosa exhibited intact villi, uniformly arranged epithelial cells, normal abundance of goblet cells with typical morphology and structure and no evidence of inflammatory infiltration (Fig. 3d). In contrast, group M displayed pronounced tissue damage, characterized by a marked reduction in goblet cells, thickened and irregular mucosal layer, disrupted crypt architecture with decreased crypt numbers, and increased infiltration of lymphocytes and neutrophils into the lamina propria. Compared with group M, RPP1-treated groups exhibited significantly mitigated colonic injury. Specifically, group PL showed reduced mucosal thickening, increased number of goblet cells, absence of tumor-like cellular structures, and diminished inflammatory cell infiltration. These histopathological findings suggest that RPP1 promotes colon tissue repair.
The experimental results collectively demonstrated that both low- and high-dose RPP1 alleviated AOM/DSS-induced CRC progression in mice. However, group PL exhibited a more pronounced therapeutic effect than did group PH. Therefore, group PL was selected for subsequent mechanistic investigations.
RPP1 regulates the gut microbial profile of AOM/DSS-induced CRC mice
To elucidate the effects of RPP1 on the gut microbiota of mice with AOM/DSS-induced CRC, fecal samples from each group were analyzed for microbial diversity using high-throughput sequencing. The alpha diversity of the gut microbiota was assessed using Chao (Fig. 4a) and Simpson indices (Fig. 4b). Compared with group C, CRC mice exhibited increased Chao indices and decreased Simpson indices, indicating elevated community richness and diversity. This suggests a dysregulated ratio of beneficial-to-harmful bacteria in the feces of AOM/DSS-induced CRC mice. Following low-dose rose polysaccharide intervention, the Chao and Simpson indices in CRC mice were restored to levels comparable to those in group C, indicating that polysaccharide intervention regulates the proportion of microbial communities in the feces of mice. Principal component analysis (PCA) revealed a separation between groups C and M (Fig. 4c), indicating that the intestinal microbiota of AOM/DSS-induced CRC mice was altered compared to that of normal mice. The microbiota of group PL mice was also distinctly different from that of group M mice, suggesting that RPP1 altered the intestinal microbial profile in CRC mice. Principal coordinate analysis (PCoA) revealed greater compositional differences between groups C and M (Fig. 4d), highlighting significant alterations in the fecal microbiota of mice with AOM/DSS-induced CRC. After RPP1 intervention, group PL deviated from group M, indicating the modulation of the gut microbial profile in CRC mice. The rarefaction curves demonstrated sufficient sequencing depth to capture the microbial diversity within the samples (Fig. 4e). The Venn diagram revealed 64 ASVs across all the samples, with 3, 14, and 6 unique ASVs identified in groups C, M, and PL, respectively (Fig. 4f). To analyze the effects of RPP1 on the intestinal microbiota of CRC mice, linear discriminant analysis effect size (LEfSe) was used to examine changes in the relative abundance of the dominant taxa in the intestinal microbiota of CRC mice. The results showed that Clostridia (order) and Eubacteriales (phylum) were dominant in group C, whereas Romboutsia (genus), Romboutsia_ilealis (species), and Peptostreptococcaceae (family) were dominant in group M (Fig. 4g). Low-dose RPP1 altered the fecal microbial profile of CRC mice by upregulating the relative abundance of Bacillus and Lactobacillaceae.
a Chao index for analysis of species diversity. b Simpson index for analysis of species richness. c Principal component analysis (PCA) to analyze the profile of intestinal microorganisms. d PCoA to analyze the profile of gut microorganisms. e Dilution curve. f Venn analysis of the microbial community in mouse intestinal flora. g Linear discriminant analysis effect size (LEfSe) plot. h–j Relative abundance of gut flora at the phylum (h), genus (i) species (j) level.
To delineate specific alterations in the gut microbial profile across groups more precisely, we analyzed the relative abundance of the gut microbiota at the phylum, genus, and species levels. At the phylum level, Firmicutes, Bacteroidetes, and Proteobacteria were predominant (Fig. 4h). Compared to group C, group M exhibited a lower relative abundance of Firmicutes, Verrucomicrobia, Tenericutes, and Candidatus Melainabacteria and higher relative abundance of Bacteroidetes, Proteobacteria, Deferribacteres, and Actinobacteria, indicating that CRC induced significant shifts in the gut microbiota composition. In group PL, the relative abundances of Proteobacteria, Deferribacteres, Actinobacteria, and Verrucomicrobia was decreased, whereas those of Bacteroidetes was increased compared with those in group M, suggesting that RPP1 treatment modulated the gut microbial profile with potential benefits to the host. At the genus level, group M showed an increased relative abundance of Bacteroides, Mucispirillum, Desulfovibrio, Alistipes, and Helicobacter compared with group C, whereas these genera were reduced in group PL compared with group M (Fig. 4i). Conversely, unclassified_o_Eubacteriaceae, Muribaculum, and Eubacterium were decreased in group M but increased following low-dose RPP1 treatment, indicating that RPP1 ameliorated the gut microbial imbalance in CRC mice. At the species level, group M exhibited an increased relative abundance of unclassified_o_Bacteroidales, Desulfovibrio_desulfuricans, Bacteroides_uniformis, and unclassified_g_Alistipes, whereas beneficial bacteria such as unclassified_f_Oscillospiraceae, Muribaculum intestinale, and unclassified_o_Eubacteriaceae decreased compared to group C (Fig. 4j). RPP1 intervention mitigated these disturbances, suggesting that RPP1 regulates the gut microbial profile in AOM/DSS-induced CRC mice by inhibiting colonization by Bacteroides and Desulfovibrio and increasing the relative abundance of the beneficial bacteria Eubacterium and Muribaculum.
Effect of RPP1 on lipidomics in mice
Lipid metabolomic analysis of fecal samples from groups M and PL was performed using LC-MS. Partial least squares discriminant analysis (PLS-DA) was performed in both cationic (Fig. 5a) and anionic (Fig. 5b) modes to assess the degree of intragroup sample variability and metabolic differences between groups. The close clustering of samples within each group, indicated by the small distances between data points, suggests that the composition and concentration of metabolites were similar and not significantly different within the groups. Conversely, samples from groups M and PL exhibited a clear separation with greater dispersion, indicating significant differences in the metabolite profiles between the two groups. OPLS-DA further confirmed these findings, with significant clustering within groups and a distinct separation between groups PL and M in both cationic (Fig. 5c) and anionic (Fig. 5d) modes. These results indicate that RPP1 significantly altered fecal metabolic profiles in CRC mice. In the anionic mode, differential volcano plots were used to identify differential lipids based on the criteria of VIP > 1 and p < 0.05. In total, 204 differential lipids (91 upregulated and 115 downregulated) were identified between groups PL and M (Fig. 5e). This suggests that RPP1 modulates fecal lipid content in CRC mice. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis was conducted to elucidate the effects of RPP1 on the metabolic functions in CRC mice. The results revealed significant alterations in the lipid metabolism and cancer-related pathways involved in CRC (Fig. 5f). Topological analysis of differential lipids showed enrichment in six metabolic pathways: glycerophospholipid metabolism, sphingolipid metabolism, ether ester metabolism, linoleic acid metabolism, α-linolenic acid metabolism, and arachidonic acid metabolism (Fig. 5g). Pathways with a composite importance score > 1 were selected for detailed analysis, yielding 10 differential lipids, including four PC, five LPC, and one ceramide (Cer) (Fig. 5h). These lipids were predominantly enriched in glycerophospholipid metabolism, sphingolipid metabolism, and ether ester metabolism. Collectively, these findings suggest that RPP1 effectively reduces the fecal levels of PC and LPC in CRC mice, thereby modulating the associated metabolic pathways and attenuating the progression of AOM/DSS-induced CRC in mice.
a, b PLS-DA plot in a cationic and b anionic modes. c, d OPLS-DA plot in c cationic and d anionic modes. e Volcano plots of differential metabolites between groups PL and M. f KEGG functional annotation of differential metabolites. g Topological analysis of differential metabolites. h Abundance of differential lipids. *p < 0.05, **p < 0.01, ***p < 0.001.
Correlation analysis
To elucidate the relationship between gut microbiota and lipid metabolism, we performed clustered heatmap analyses of gut microorganisms and differential lipids involved in the three lipid metabolic pathways at both the genus and species levels. The results demonstrated that, at the genus level, Muribaculum exhibited a significant negative correlation with LPC (24:0) (p < 0.001), while Clostridium showed a significant positive correlation with LPC (20:0) (p < 0.001). Additionally, unclassified_o_Eubacteriales was significantly negatively correlated with LPC (20:0) (p < 0.001; Fig. 6a). At the species level, unclassified_f_Lachnospiraceae was significantly negatively correlated with LPC (20:0) (p < 0.01) and Cer (d18:1/24:0) (p < 0.05), whereas unclassified_g_Christensenella was significantly negatively correlated with PC (20:0/18:2) (p < 0.05; Fig. 6b). Collectively, these findings suggest that the gut microbiota, particularly Muribaculum and Clostridium, can influence the levels of key lipids such as LPC, thereby modulating CRC progression.
Effect of RPP1 on colon transcriptome profiles and key pathways
Transcriptomic analysis was conducted to elucidate the molecular mechanisms underlying the effects of low-dose RPP1 on murine CRC. Volcano plots of differentially expressed genes (DEGs) were generated using corrected p < 0.05 and fold change ≥ 2 as the screening criteria. Compared with group C, group M exhibited 1819 and 1894 significantly downregulated and upregulated DEGs, respectively (Fig. 7a). In contrast, group PL showed 1038 upregulated and 1428 downregulated DEGs relative to group M (Fig. 7b). PCA was performed for groups C, M, and PL. The samples were significantly clustered within each group, with group C distinctly separated from group M, indicating significant differences between the two groups (Fig. 7c). Group PL was significantly separated from both groups C and M but showed a closer relationship with group C, suggesting that low-dose RPP1 treatment shifted the gene expression profile of group PL away from group M and toward group C. Hierarchical clustering of DEGs revealed distinct patterns of differential gene expression in group M compared to groups C and PL, whereas groups C and PL exhibited similar expression profiles (Fig. 7d). In the KEGG enrichment analysis, DEGs between groups C and M were significantly enriched in 93 KEGG pathways (p < 0.05). These pathways include cytokine–cytokine receptor interactions and PPAR signaling pathways, which are associated with the immune system, as well as linoleic acid and arachidonic acid metabolism, which are related to lipid metabolism (Fig. 7e). DEGs between groups M and PL were significantly enriched in 117 KEGG pathways (p < 0.05). These pathways included cytokine–cytokine receptor interaction and NLR signaling pathways, which are associated with the immune system; α-linolenic acid, glycerophospholipid, and sphingolipid metabolisms, which are related to lipid metabolism; and pathways associated with inflammatory bowel disease (Fig. 7f).
a, b Volcano plot of gene expression in the colon of a group M mice compared to group C mice and b group PL mice compared to group M mice. c PCA plot of the transcriptome of mouse colon tissue. d Heat map of differential gene expression. e, f KEGG enrichment pathway of DEGs between e groups M and C and f groups PL and M. g KEGG enrichment pathway of common DEGs between groups M and C and groups PL and M. h KEGG enrichment pathway of common DEGs.
These results suggest that the mechanism by which low-dose RPP1 alleviates CRC may involve lipid metabolism via pathways consistent with our lipidomic findings. Based on the transcriptomic data and genomic mapping annotations, Venn diagrams were constructed to identify overlapping DEGs between groups M vs. C (upregulated) and groups PL vs. M (downregulated), as well as between groups M vs. C (downregulated) and groups PL vs. M (upregulated) (Fig. 7g). The 3327 DEGs shared between groups M vs. C and groups PL vs. M were significantly enriched in 102 pathways (p < 0.05), including the immune-related NLR pathway, PPAR pathway, and lipid metabolism-related pathways such as those involved in glycerophospholipid metabolism and ether ester metabolism (Fig. 7h). These findings indicate that low-dose RPP1 may attenuate CRC progression in mice by modulating the NLR, PPAR, GPL, and ether ester metabolic pathways.
Discussion
The biological activity of polysaccharides is intricately associated with their component monosaccharides, particularly their composition and ratio23. In this study, the crude polysaccharide RPP1 was isolated from the flowers of Rosa rugosa cv. Plena. Subsequently, three purified polysaccharide fractions, RPP2-0, RPP2-1, and RPP2-3, were obtained via DEAE-cellulose 52 column chromatography and their monosaccharide compositions were identified. It was discovered that both polysaccharides RPP1 from Rosa rugosa cv. Plena utilized in this study and the polysaccharide RRP from Rosa rugosa are composed of Glc, GalUA, Man, Rha, Gal, Ara, Xyl, and GlcUA, with GalUA and Glc as the major monosaccharides. The specific monosaccharide molar ratio of RRP was 7.78:7.59:4.23:3.22:3.15:1.65:1.0020. However, in contrast to RPP1, the Pingyin rose bud polysaccharide contains only five monosaccharide fractions lacking Man, Ara, and Rha15. Rose damask petal polysaccharide RP-1 consists of seven monosaccharides. In addition to Rha, Ara, Xyl, Man, Gal, and Glc, RP-1 contains the monosaccharide ribose, which is absent in RPP117. The rose bee pollen polysaccharide WRPP demonstrated significant antiproliferative activity against CRC cells in vitro21. Its monosaccharide composition includes Rha, GalUA, Gal, Ara, Man, Glc, and GlcUA but lacks Xyl compared to RPP1. These findings suggest that the Rosa rugosa cv. Plena polysaccharide RPP1 attenuates the progression of CRC and may be closely associated with its monosaccharide composition.
The hallmark features of AOM/DSS-induced CRC include diarrhea, weight loss, colonic shortening, and mucosal infiltration by inflammatory cells24. In this study, both groups PL and PH had a significantly reduced number of polyps, restored serum inflammatory factor levels, and alleviated colonic tissue damage compared to group M, indicating that both doses could mitigate colon cancer in mice. However, these effects were more pronounced in group PL than in group PH. Collectively, these results suggested that a saturation effect may have been reached beyond the tested dose range. Based on these findings, we focused our subsequent analyses on the effects of PL on the microbiome, lipidome, and transcriptome of mice with CRC.
To date, few studies have demonstrated the in vitro anticancer activity of rosehip polysaccharides against CRC. CRC is the most common malignant tumor of the digestive system, and the intestinal microbiota plays a crucial role in its development and treatment25. Dysbiosis in patients with CRC is characterized by a decrease in commensal bacterial species and an increase in harmful bacteria that can be used as indicators of CRC26. The effect of increased Bacteroides abundance on CRC development is complex, with both promoting and inhibitory effects. Bile salt hydrolases produced by Bacteroides spp. contribute to obesity-associated CRC progression27. Bacteroides fragilis, a common member of this genus, produces enterotoxins (BFT) that are detected in all clinical CRC samples and are abundant in advanced stages. BFT promotes tumor cell growth and inhibits the apoptosis of colonic epithelial cells28. In this study, RPP1 reduced the relative abundance of Bacteroides in CRC mice compared with that in the model group, suggesting that decreased Bacteroides abundance inhibits CRC development, which is consistent with previous literature. Desulfovibrio is a sulfate-reducing bacterium commonly found in the environment and human gastrointestinal tract. Several studies have shown a positive association between Desulfovibrio overgrowth and human diseases29. Desulfovibrio abundance was significantly higher in patients with CRC than in healthy controls30. 16S rRNA sequencing of fecal samples from CRC patients with liver metastasis revealed an abundance of Desulfovibrio, suggesting its role in colorectal barrier dysfunction and inflammation, which promote CRC development and metastasis31. In the present study, RPP1 significantly reduced Desulfovibrio abundance in CRC mice, indicating its inhibitory effect on CRC progression. Eubacterium, a core genus of intestinal microbiota, plays an important role in maintaining intestinal barrier homeostasis. Studies have shown that berberine increases the abundance of beneficial Eubacterium and decreases the abundance of Muribaculum, thereby reducing intestinal tumorigenesis32. Our study found that RPP1 increased fecal Eubacterium abundance in CRC mice, suggesting its potential to alleviate intestinal tumor development. Muribaculum, a member of the Bacteroidetes phylum, is an important component of the gut microbiota. Studies have shown that ginseng polysaccharides and fucoidan increases Muribaculum abundance in CRC mice, thus slowing disease progression33,34. Similarly, alginate increases Muribaculum abundance and attenuates CRC progression35. In our study, RPP1 increased fecal Muribaculum abundance in CRC mice, indicating its potential to mitigate CRC development.
Abnormal glycerophospholipid metabolism, exemplified by PC and phosphatidylethanolamine, has emerged as a common metabolic hallmark of CRC36. Shan37 demonstrated that grain bran peroxide exerts anti-CRC effects by reducing PC levels in colon cancer cells and downregulating the expression of GPL-related metabolic enzymes. LPC has been implicated in tumor-associated epigenetic effects38,39 and is a key GPL metabolite that elicits inflammatory responses both in vivo and in vitro. LPC levels are also regulated by gut microbiota40. Elevated LPC levels were observed in the intestinal tissues of mice with high fat diet-induced CRC, and berberine treatment effectively reversed this change41. Sphingolipids directly modulate glycerophospholipid metabolism by interfering with major phospholipases (such as PLA2, PLC, and PLD), thereby releasing lysophospholipids and fatty acids that function as signaling metabolites42. In the present study, LPC (18:0) was found to be enriched in sphingolipid metabolism. Research has shown that serum glycerophospholipid levels (such as LPC 20:2, LPC 18:0, LPC 18:4, LPE 20:2, LPC 20:1, LPC 16:1, LPC 20:0, and LPA 18:2) are significantly higher in patients with early-stage non-small cell lung cancer than in healthy individuals. KEGG enrichment analysis revealed that differential metabolites were primarily enriched in sphingolipid metabolism and sphingolipid signaling pathways, with the dysregulation of these pathways serving as potential diagnostic markers for-early stage non-small cell lung cancer43. In summary, a reduction in LPC and PC concentrations may regulate GPL and sphingolipid metabolism, thereby exerting a palliative effect on CRC or tumor development. In this study, RPP1 significantly decreased fecal LPC and PC levels in CRC mice, thereby influencing the associated GPL and sphingolipid metabolism. These findings suggest that RPP1 can downregulate key lipid levels, modulate relevant metabolic pathways, and mitigate CRC progression.
Correlation clustering heatmap analysis revealed that, at the genus level, Muribaculum was significantly negatively correlated with LPC (24:0) (p < 0.001), while Clostridium exhibited a significant positive correlation with LPC (20:0) (p < 0.001). Additionally, unclassified_o_Eubacteriales were significantly negatively correlated with PC (18:0/16:0) (p < 0.001). Shen44 reported elevated levels of phosphatidylglycerol, Cer, LPC, and phosphatidylethanolamine in the plasma lipid studies of patients with CRC, suggesting that these metabolites may serve as potential biomarkers of CRC. The reduced LPC concentrations in mice with high fat diet-induced CRC following berberine administration is consistent with our findings. This study also identified a significant negative correlation between LPC and the genus Akkermansia, leading to the conclusion, based on previous studies, that Akkermansia may play a crucial role in regulating LPC concentration within the GPL pathway45,46. Collectively, these results indicate that Muribaculum and Clostridium regulate LPC levels, thereby modulating the associated glycerophospholipid metabolism.
In this study, we used transcriptomics to elucidate the mechanisms by which RPP1 exerts its anti-CRC effects via the intestinal genes. Specifically, RPP1 modulates ether ester and glycerophospholipid metabolism, both of which are integral to lipid metabolism. Additionally, RPP1 regulates the NLR and PPAR signaling pathways, both of which are associated with inflammation, thereby interfering with CRC progression in mice. The lipid-related metabolic pathways identified in our transcriptomic analysis are consistent with the lipidomic results. In humans, NLR are a family of intracellular pattern recognition receptors that play a crucial role in innate immunity47. Dysregulation of the NLR pathway leads to chronic inflammation and contributes to the pathogenesis of various diseases48. Li49 conducted a transcriptomic analysis of colon tissues from patients with CRC, and KEGG enrichment analysis revealed DEGs were associated with the NLR pathway. However, dysregulation of the NLR signaling pathway results in excessive inflammation through genes that are overexpressed in advanced CRC and correlates with poor patient prognosis50. PPAR is a receptor for endogenous lipid molecules that regulates the expression of a network of genes involved in adipogenesis, lipid metabolism, inflammation, and maintenance of metabolic homeostasis. Therefore, it is a novel target for the treatment and prevention of inflammatory diseases51,52. Numerous studies have demonstrated that PPAR activation inhibits CRC cell proliferation, promotes CRC cell differentiation and apoptosis, and is a key receptor for ameliorating CRC53. Additionally, treatments such as umeboshi pills can treat colitis-associated CRC by modulating the PPAR signaling pathway54. Consequently, downregulation of the NLR and PPAR signaling pathways has been implicated in the inhibition of CRC progression. In the present study, both the NLR and PPAR signaling pathways were significantly downregulated in the colonic tissues of mice in the RPP1-treated group, suggesting that a low dose of RPP1 can effectively attenuate CRC progression.
In the present study, RPP1, which is a polysaccharide extracted from Rosa rugosa cv. Plena, significantly delays the progression of AOM/DSS-induced CRC. The underlying mechanisms are primarily attributed to the modulation of the gut microbiota profile, which is characterized by increased relative abundance of beneficial bacteria and concomitantly reduced abundance of harmful bacteria, thereby exerting a beneficial impact on intestinal microecology. Additionally, RPP1 significantly influenced lipid metabolism pathways, including glycerophospholipid, sphingolipid, and ether lipid metabolism, by downregulating fecal LPC and PC levels. Transcriptomic analysis further corroborated these findings, revealing that RPP1 regulates lipid metabolism and immune system-related signaling pathways such as the NLR and PPAR pathways, thereby exerting antitumor effects. Although this study preliminarily elucidated the potential mechanisms of action of RPP1 in CRC and provided a theoretical basis for its clinical application, further validation of its safety and efficacy through clinical trials is essential. In particular, the dose-dependent efficacy of RPP1 and its potential applications in CRC prevention and treatment warrant further in-depth investigation. Moreover, exploring the combined application of RPP1 and other anticancer drugs or therapies may offer novel strategies for CRC treatment.
Methods
Materials
Rosa rugosa cv
Plena were purchased from Shaanxi Bolin Biotechnology Co. (Shaanxi, China). DEAE-cellulose 52 (catalog code C8930), glucuronic acid (GlcUA, catalog code SG8820), anhydrous glucose (Glc, catalog code SG8510), galactose (Gal,catalog code SG8010), mannose (Man, catalog code SD8420), arabinose (Ara,catalog code SA9090), rhamnose (Rha, catalog code SR8090) and xylose (Xyl, catalog code SX8030) were purchased from Beijing Solepol Technology Co. (Beijing, China,). n-Butanol (catalog code B802037) and chloroform (catalog code C805849) were purchased from Shanghai McLean Biochemical Technology Co. Ltd. (Shanghai, China). Galacturonic acid (GalA, catalog code 111646) was purchased from the China Institute for Food and Drug Control (Beijing, China). 1-Phenyl-3-methyl-5-pyrazolone (PMP, catalog code B25309) was purchased from Shanghai Yuanye Biotechnology Co. AOM was purchased from Sigma-Aldrich (St. Louis, MO, USA, catalog code A5486). DSS was purchased from MP Biomedicals (San Diego, CA, USA, MDL code MFCD00081551). Enzyme-linked immunosorbent assay (ELISA) kits were purchased from the Nanjing Jianjian Bioengineering Institute (Nanjing, China).
Extraction and preliminary purification of polysaccharides from Rosa rugosa cv. Plena
Polysaccharides from Rosa rugosa cv. Plena were extracted using aqueous alcoholic precipitation55. Dried Rosa rugosa cv. Plena petals were crushed and passed through an 80-mesh sieve to obtain a fine powder. The powder was extracted using distilled water at a solid-to-liquid ratio of 1:30 (w/v) at 90°C for 3 h. This process was repeated thrice, and the combined extracts were concentrated under reduced pressure. Anhydrous ethanol was added to the concentrated extract to achieve a final ethanol concentration of 80%, and the mixture was left undisturbed at 4°C overnight. The precipitated polysaccharides were collected by centrifugation, dried, and stored as crude polysaccharides (RPP0). The extraction yield was calculated based on the initial dry weight of plant material. The crude polysaccharide precipitate was subsequently deproteinized using the Sevag method (chloroform:n-butanol = 4:1, repeated 5–8 times)56 and depigmented using macroporous adsorbent resin AB-857. The purified polysaccharides were obtained by vacuum freeze-drying and designated as RPP1, and the extraction yield was calculated. Further separation and purification were performed using DEAE-cellulose 52 chromatography58. Specifically, 20 mg/mL polysaccharide solution was prepared in deionized water and loaded onto a DEAE-cellulose 52 column (Φ2.6 mm × 30 cm). The column was eluted sequentially with distilled water followed by 0.1, 0.2, 0.3, 0.4, and 0.5 M NaCl solutions at a flow rate of 1.3 mL/min. The eluate was collected in 10 mL centrifuge tubes, and the polysaccharide content was monitored using the phenol-sulfuric acid method. Elution curves were plotted with the tube number on the x-axis and the absorbance at 490 nm on the y-axis. Under the elution conditions involving 0, 0.1, and 0.3 M NaCl solutions (fractions eluted with 0.2 and 0.5 M NaCl were not collected due to their relatively small peak areas), three distinct polysaccharide fractions were obtained and designated as RPP2-0, RPP2-1, and RPP2-3, respectively. The extraction yield for each fraction was calculated.
Determination of monosaccharide composition
The monosaccharide composition and molar ratios of the rose polysaccharides were determined by performing 1-phenyl-3-methyl-5-pyrazolone–high-performance liquid chromatography (PMP-HPLC)59. Briefly, 10 mg of each polysaccharide sample (RPP2-0, RPP2-1, and RPP2-3) was weighed into a test tube and 2 M trifluoroacetic acid was added. The samples were sealed in nitrogen and hydrolyzed in an oven at 110 °C for 5 h. The hydrolyzed samples were then dried under a stream of nitrogen. Methanol was added to dissolve the residues, which were then dried in nitrogen. This procedure was repeated three times to ensure the complete removal of residual trifluoroacetic acid. The samples were reconstituted in distilled water to a final volume of 3 mL. Next, 500 μL of the reconstituted solution was mixed with 500 μL of 0.3 M NaOH and 500 μL of 0.5 M PMP-methanol solution. The mixture was vortexed thoroughly and placed in a water bath at 70 °C for 60 min. After cooling, 0.3 M HCl was added to neutralize NaOH. The mixture was then extracted with chloroform, and the organic layer was discarded after centrifugation. This procedure was repeated thrice to ensure complete removal of the organic phase. The aqueous phase was filtered through a 0.22 μm membrane filter and stored for HPLC analysis. The monosaccharide standards were processed using the same procedure.
The monosaccharide standards and polysaccharide samples were analyzed using a Shimadzu LC-2030C 3D Plus HPLC system (Shimadzu Corporation, Kyoto, Japan), equipped with an Agilent Eclipse XDB-C18 column (Φ 4.6 × 150 mm; Agilent Technologies, Santa Clara, CA, USA). The chromatographic conditions were as follows: detection wavelength, 250 nm; injection volume, 10 µL; flow rate, 1.0 mL/min; column temperature, 30 °C; and mobile phase, pH 6.7 phosphate buffer (0.025 mol/L KH2PO4 and NaOH) and acetonitrile in a ratio of 83:17 (v/v).
Animals and experimental design
All experimental protocols and animal welfare procedures were conducted in accordance with the relevant ethical guidelines and approved by the Animal Ethics Committee of Beijing Pony Testing International Group Co., Ltd. (Spectrum Testing Animal, PONY-2023-FL-16; Beijing, China). Forty healthy male C57BL/6 N mice were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China) and housed in specific-pathogen-free animal facilities at Beijing Pony Testing International Group Co., Ltd., maintained at 22 ± 2 °C, 50–60% relative humidity, and a 12 h light/12 h dark cycle. Polysaccharide RPP1 was used in animal experiments.
The animal experimental design was based on previously reported methods60, with the gavage dosage of RPP1 referenced in Ji61. During the first week of the experiment, the mice were acclimated to the laboratory environment and provided free access to food and water. They were randomly divided into five groups (n = 8 per group) according to their body weight: control group (group C), model group (group M), high-dose polysaccharide group (200 mg/kg bw, group PH), low-dose polysaccharide group (100 mg/kg bw, group PL), and positive control group treated with 5-fluorouracil (20 mg/kg bw, group F). Starting from the second week, all groups except group C were injected intraperitoneally with 10 mg/kg bw AOM. One week later, the mice were administered 2% DSS solution instead of drinking water for 5 days, followed by drinking water again for 14 days. This cycle was repeated twice, with each recovery period lasting two weeks. Concurrently, mice in group F received intraperitoneal injections of 5-fluorouracil (20 mg/kg bw) every 3 days, whereas mice in groups PH and PL were administered 200 and 100 mg/kg bw RPP1 solutions, respectively, via oral gavage daily. The mice in groups C and M were administered 0.9% NaCl via oral gavage. Body weights were recorded every three days throughout the experimental period. On day 71, mice were euthanized by cervical dislocation following orbital blood sampling. Fecal samples were collected in sterile tubes and stored at −80 °C. Colon tissues were excised and rinsed with phosphate-buffered saline, and the length and number of polyps were measured. The colon was then longitudinally divided into two segments: one was stored at −80 °C, and the other was fixed in 4% paraformaldehyde for subsequent histological analysis.
ELISA
Mouse blood was centrifuged at 5000 × g for 15 min at 4 °C, and the serum was collected for subsequent analysis. Serum levels of inflammatory cytokines were measured using ELISA kits, with at least five replicates per group. Concentrations of IL-1β, IL-6, and TNF-α were determined according to the manufacturer’s instructions.
Histological analysis of the colon
Colon tissues were fixed in 4% paraformaldehyde for at least 24 h, processed using graded ethanol dehydration (50, 70, 95, and 100% ethanol), and embedded in paraffin wax. Paraffin blocks were sectioned at 4–5 µm thickness, stained with hematoxylin and eosin, and examined under a microscope to analyze histological changes in the colon62.
Microbiome analysis
In conjunction with the pathological and histological results, fecal samples from groups C, M, and PL were selected for microbiome analysis, with six biological replicates per group. The analysis was conducted by Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China) according to the protocol described in a previous study63. Briefly, total DNA was extracted from the samples and used as a template to amplify the 16S rRNA V3-V4 region using specific primers. The amplicons were sequenced on an Illumina NovaSeq platform (Illumina, San Diego, CA, USA). The raw sequencing data were processed using the DADA2_CCS sequence noise reduction method to generate representative sequences of amplicon sequence variants (ASVs) and abundance information. Subsequent statistical and visualization analyses were performed using the Majorbio Cloud Platform (https://cloud.majorbio.com).
Lipidomic analysis
Mouse fecal samples from groups M and PL were selected for lipidomic analysis, with six biological replicates per group. Lipidomic analysis was conducted by Majorbio Bio-Pharm Technology Co. Ltd. following the protocol described in a previous study64. Briefly, metabolites were extracted from the samples by adding a methanol solution, followed by ultrasonication and protein precipitation at −20 °C. The supernatant was collected via centrifugation and transferred to a sample vial. Liquid chromatography-mass spectrometry (LC-MS) was performed in both positive and negative ion modes to acquire MS and MS/MS data for the metabolites. Peak extraction, alignment, and identification were performed using LipidSearch software (Thermo Fisher Scientific, Waltham, MA, USA). A metabolite list and data matrix were generated and differential lipid metabolites were screened using a combination of t tests and variable importance in projection (VIP) values from an orthogonal partial least squares discriminant analysis (OPLS-DA). Biological information on these differential lipids was further mined using the Majorbio Cloud Platform (https://cloud.majorbio.com).
Transcriptomic analysis
Mouse colon tissue samples from groups M and PL were selected for transcriptomic analysis, with four biological replicates per group. Transcriptomic analysis was conducted by Majorbio Bio-Pharm Technology Co., Ltd. following the protocol described in a previous study65. Briefly, total RNA was extracted and purified using lithium chloride to remove the residual DSS. The concentration and purity of the extracted RNA were assessed using a Nanodrop 2000 spectrophotometer(Thermo Fisher Scientific, Waltham, MA, USA), and RNA integrity was evaluated using agarose gel electrophoresis. RNA quality number was determined using an Agilent 5300 Bioanalyzer (Agilent Technologies), and oligo (dT)-containing magnetic beads were used to isolate mRNA from total RNA. The isolated mRNA was randomly fragmented into approximately 300 bp fragments using a fragmentation buffer under optimized conditions. Single-stranded cDNA was synthesized from fragmented mRNA using random primers and reverse transcriptase, followed by second-strand synthesis to generate a stable double-stranded cDNA structure. The synthesized cDNA was subjected to end repair, purification, and size selection. The selected fragments were amplified using PCR to construct a final sequencing library. Libraries were sequenced on an Illumina NovaSeq X Plus platform (Illumina). Raw sequencing data were processed and analyzed using the Majorbio Cloud Platform (https://cloud.majorbio.com).
Data analysis
All data are presented as mean ± standard deviation, with significant differences at p < 0.05. One-way analysis of variance (ANOVA) was performed using SPSS Statistics 23 (IBM Corporation, Redmond, WA, USA) and Origin 2021 (OriginLab Corporation, Northampton, MA, USA), and post hoc analyses were performed using LSD, Tukey, and Tamhane T2 tests.
Data Availability
All data generated or analyzed during this study are included in this article, and can be provided by the corresponding author upon reasonable request. All raw sequencing data of Microbiome (SRA15490396) and Transcriptome (SRA15490736) generated in this study are available in the NCBI Sequence Read Archive (SRA) under BioProject accession PRJNA1297128. The raw sequencing data of Lipidomic (OMIX011179) is deposited in China National Center for Bioinformation (CNCB) under BioProject accession PRJCA043779.
References
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74, 229–263 (2024).
Eng, C. et al. Colorectal cancer. Lancet 404, 294–310 (2024).
Kumar, R. A comprehensive overview of colon cancer: a grim reaper of the 21st century. Curr. Med. Chem. 28, 2657–2696 (2021).
Din, F. V. N. & Dunlop, M. G. Colorectal cancer: management. Medicine 52, 263–267 (2024).
Ruan, J. et al. Colorectal cancer inhibitory properties of polysaccharides and their molecular mechanisms: a review. Int. J. Biol. Macromol. 238, 124165 (2023).
Wang, S. L. et al. Isolation, purification, structural analysis and in vitro activity of polysaccharides from Amelanchier alnifolia Nutt. Process Biochem 136, 229–236 (2024).
Jiang, S., Xu, H., Zhao, C., Zhong, F. & Li, D. Oyster polysaccharides relieve DSS-induced colitis via anti-inflammatory and maintaining the physiological hypoxia. Int. J. Biol. Macromol. 238, 124150 (2023).
Li, H. et al. Purification, structural characterization, and antitumor activity of a polysaccharide from perilla seeds. Int. J. Mol. Sci. 24, 15904 (2023).
Choi, J. et al. Korean ginseng berry polysaccharide enhances immunomodulation activities of peritoneal macrophages in mice with cyclophosphamide-induced immunosuppression. J. Microbiol. Biotechnol. 33, 840–847 (2023).
Li, W. et al. Ganoderma lucidum polysaccharide supplementation significantly activates T-cell-mediated antitumor immunity and enhances anti-PD-1 immunotherapy efficacy in colorectal cancer. J. Agric. Food Chem. 72, 12072–12082 (2024).
Ma, L. et al. Polysaccharides extracted from hawthorn (Crataegus pinnatifida) exhibiting protective effects against DSS/AOM-induced colorectal cancer in vivo. J. Funct. Foods 107, 105618 (2023).
Zhou, X., Zhu, G., Yang, Y., Du, B. & Lin, D. Optimization of enzymatic-assisted extraction of polysaccharides from Roxburgh rose pomace and its antioxidant activity. E3S Web Conf. 78, 02014 (2019).
Ng, T. B. et al. A polysaccharopeptide complex and a condensed tannin with antioxidant activity from dried rose (Rosa rugosa) flowers. J. Pharm. Pharmacol. 58, 529–534 (2006).
Zhang, C. et al. Purification, characterization, antioxidant and moisture-preserving activities of polysaccharides from Rosa rugosa petals. Int. J. Biol. Macromol. 124, 938–945 (2019).
Tang, Y. et al. The chemical structure and anti-aging bioactivity of an acid polysaccharide obtained from rose buds. Food Funct. 9, 2300–2312 (2018).
Wu, M. et al. Structural elucidation and immunomodulatory activity of a neutral polysaccharide from the Kushui rose (Rosa setate x Rosa rugosa) waste. Carbohydr. Polym. 232, 115804 (2020).
Slavov, A., Kiyohara, H. & Yamada, H. Immunomodulating pectic polysaccharides from waste rose petals of Rosa damascena Mill. Int. J. Biol. Macromol. 59, 192–200 (2013).
Li, X. et al. Pectic bee pollen polysaccharide from Rosa rugosa alleviates diet-induced hepatic steatosis and insulin resistance via induction of AMPK/mTOR-mediated autophagy. Molecules 22, 699 (2017).
Li, H. et al. Rosa rugosa polysaccharide attenuates alcoholic liver disease in mice through the gut-liver axis. Food Biosci. 44, 101385 (2021).
Liu, Y. et al. Rosa rugosa polysaccharide induces autophagy-mediated apoptosis in human cervical cancer cells via the PI3K/AKT/mTOR pathway. Int. J. Biol. Macromol. 212, 257–274 (2022).
Wang, B. et al. Antitumor activity of bee pollen polysaccharides from Rosa rugosa. Mol. Med. Rep. 7, 1555–1558 (2013).
Olech, M. et al. Polysaccharide-rich fractions from Rosa rugosa thunb-composition and chemopreventive potential. Molecules 24, 1354 (2019).
Fan, Y. et al. Bee pollen polysaccharide from Rosa rugosa Thunb. (Rosaceae) promotes pancreatic β-cell proliferation and insulin secretion. Front. Pharmacol. 12, 688073 (2021).
Glauben, R. & Siegmund, B. Inhibition of histone deacetylases in inflammatory bowel diseases. Mol. Med. 17, 426–433 (2011).
Wang, L. et al. Gut microbiome in tumorigenesis and therapy of colorectal cancer. J. Cell. Physiol. 238, 94–108 (2022).
Kumar, A. et al. Implication of gut microbes and its metabolites in colorectal cancer. J. Cancer Res. Clin. Oncol. 149, 441–465 (2022).
Sun, L. et al. Bile salt hydrolase in non-enterotoxigenic bacteroides potentiates colorectal cancer. Nat. Commun. 14, 755 (2023).
Goodwin, A. C. et al. Polyamine catabolism contributes to enterotoxigenic bacteroides fragilis-induced colon tumorigenesis. Proc. Natl. Acad. Sci. USA 108, 15354–15359 (2011).
Singh, S. B., Carroll-Portillo, A. & Lin, H. C. Desulfovibrio in the gut: the enemy within? Microorganisms 11, 1772 (2023).
Senthakumaran, T. et al. Microbial dynamics with CRC progression: a study of the mucosal microbiota at multiple sites in cancers, adenomatous polyps, and healthy controls. Eur. J. Clin. Microbiol. Infect. Dis. 42, 305–322 (2023).
Yu, Y. et al. High-fat diet enhances the liver metastasis potential of colorectal cancer through microbiota dysbiosis. Cancers 14, 2573 (2022).
Chen, H. et al. Berberine inhibits intestinal carcinogenesis by suppressing intestinal pro-inflammatory genes and oncogenic factors through modulating gut microbiota. BMC Cancer 22, 566 (2022).
Huang, J. et al. Ginseng polysaccharides alter the gut microbiota and kynurenine/tryptophan ratio, potentiating the antitumour effect of anti-programmed cell death 1/programmed cell death ligand 1 (anti-PD-1/PD-L1) immunotherapy. Gut 71, 734–745 (2021).
Liu, X. et al. Fucoidan ameliorated dextran sulfate sodium-induced ulcerative colitis by modulating gut microbiota and bile acid metabolism. J. Agric. Food Chem. 70, 14864–14876 (2022).
Pan, D. et al. Phycocyanin ameliorates colitis-associated colorectal cancer by regulating the gut microbiota and the IL-17 signaling pathway. Mar. Drugs 20, 260 (2022).
Liang, Z. Q. et al. Exploring the anti-metastatic effects of astragalus mongholicus Bunge-Curcuma aromatica Salisb. on colorectal cancer: a network-based metabolomics and pharmacology approach. Phytomedicine 114, 154772 (2023).
Shan, S. et al. Inhibitory effects of peroxidase from foxtail millet bran on colitis-associated colorectal carcinogenesis by the blockage of glycerophospholipid metabolism. J. Agric. Food Chem. 68, 8295–8307 (2020).
Shimizu, R. et al. Cholangiocyte senescence caused by lysophosphatidylcholine as a potential implication in carcinogenesis. J. Hepatobiliary Pancreat. Sci. 22, 675–682 (2015).
Gao, F., Chen, J., Zhang, T. & Liu, N. LPCAT1 functions as an oncogene in cervical cancer through mediating JAK2/STAT3 signaling. Exp. Cell Res. 421, 113360 (2022).
Tang, X. et al. Gut microbiota-mediated lysophosphatidylcholine generation promotes colitis in intestinal epithelium-specific Fut2 deficiency. J. Biomed. Sci. 28, 20 (2021).
Chen, H. et al. Berberine inhibits high fat diet-associated colorectal cancer through modulation of the gut microbiota-mediated lysophosphatidylcholine. Int. J. Biol. Sci. 19, 2097–2113 (2023).
Rodriguez-Cuenca, S., Pellegrinelli, V., Campbell, M., Oresic, M. & Vidal-Puig, A. Sphingolipids and glycerophospholipids - The “ying and yang” of lipotoxicity in metabolic diseases. Prog. Lipid Res. 66, 14–29 (2017).
Ni, B. et al. Combined analysis of gut microbiome and serum metabolomics reveals novel biomarkers in patients with early-stage non-small cell lung cancer. Front. Cell Infect. Microbiol. 13, 1091825 (2023).
Shen, S. et al. A plasma lipidomics strategy reveals perturbed lipid metabolic pathways and potential lipid biomarkers of human colorectal cancer. J. Chromatogr. B 1068, 106941–48 (2017).
Wen, X. et al. Fecal microbiota transplantation ameliorates experimental colitis via gut microbiota and T-cell modulation. World J. Gastroenterol. 27, 2834–2849 (2021).
Zhang, N. et al. Integrated analysis of the alterations in gut microbiota and metabolites of mice induced after long-term intervention with different antibiotics. Front. Microbiol. 13, 832915 (2022).
Ting, J. P.-Y., Willingham, S. B. & Bergstralh, D. T. NLRs at the intersection of cell death and immunity. Nat. Rev. Immunol. 8, 372–379 (2008).
Giambra, V. et al. Gut microbiota, inflammatory bowel disease, and cancer: the role of guardians of innate immunity. Cells 12, 2654 (2023).
Li, C., Liu, Q., Song, Y., Wang, W. & Zhang, X. Construction of a prognostic model of colon cancer patients based on metabolism-related lncRNAs. Front. Oncol. 12, 944476 (2022).
Wang, B., Li, H., Wang, X. & Zhu, X. The association of aberrant expression of NLRP3 and p-S6K1 in colorectal cancer. Pathol. Res. Pract. 216, 152737 (2020).
Chen, Y. et al. Lactobacillus plantarum ameliorates colorectal cancer by ameliorating the intestinal barrier through the CLA-PPAR-γ axis. J. Agric. Food Chem. 72, 19766–19785 (2024).
Straus, D. S. & Glass, C. K. Anti-inflammatory actions of PPAR ligands: new insights on cellular and molecular mechanisms. Trends Immunol. 28, 551–558 (2007).
Sabatino, L. et al. Chiral phenoxyacetic acid analogues inhibit colon cancer cell proliferation acting as PPARγ partial agonists. Sci. Rep. 9, 5434 (2019).
Ma, L. et al. Mechanic evaluation of Wu-Mei-Pill on colitis-associated colorectal cancer: an integrated transcriptomics, metabolomics, and experimental validation study. Phytomedicine 128, 155509 (2024).
An, Q. et al. Structure analysis of polysaccharides purified from cyclocarya paliurus with DEAE-cellulose and its antioxidant activity in RAW264.7 cells. Int. J. Biol. Macromol. 157, 604–615 (2020).
Li, Y. et al. Apple polysaccharide protects ICR mice against colitis-associated colorectal cancer through the regulation of microbial dysbiosis. Carbohydr. Polym. 230, 115726 (2020).
Ren, H. et al. Rehmannia glutinosa polysaccharides: optimization of the decolorization process and antioxidant and anti-inflammatory effects in LPS-stimulated porcine intestinal epithelial cells. Antioxidants 12, 914 (2023).
Wu, Y. T. et al. Purification, characterization and antioxidant activity of polysaccharides from Porphyra haitanensis. Int. J. Biol. Macromol. 165, 2116–2125 (2020).
Chen, Z. et al. Extraction and characterization of polysaccharides from semen cassiae by microwave-assisted aqueous two-phase extraction coupled with spectroscopy and HPLC. Carbohydr. Polym. 144, 263–270 (2016).
Zhu, J. et al. Lactobacillus plantarum Zhang-LL inhibits colitis-related tumorigenesis by regulating arachidonic acid metabolism and CD22-mediated B-cell receptor regulation. Nutrients 15, 4512 (2023).
Ji, X. et al. Microbiome-metabolomic analysis of the impact of Zizyphus jujuba cv. Muzao polysaccharides consumption on colorectal cancer mice fecal microbiota and metabolites. Int. J. Biol. Macromol. 131, 1067–1076 (2019).
Lu, S. Y. et al. Gracilaria lemaneiformis polysaccharides alleviate colitis by modulating the gut microbiota and intestinal barrier in mice. Food Chem. X 13, 100197 (2022).
Gong, H. et al. Structural characteristics of steamed Polygonatum cyrtonema polysaccharide and its bioactivity on colitis via improving the intestinal barrier and modifying the gut microbiota. Carbohydr. Polym. 327, 121669 (2024).
Yang, Y., Yu, P., Lu, Y., Gao, C. & Sun, Q. Disturbed rhythmicity of intestinal hydrogen peroxide alters gut microbial oscillations in BMAL1-deficient monkeys. Cell Rep. 42, 112183 (2023).
Liu, C. et al. Cohousing-mediated microbiota transfer from milk bioactive components-dosed mice ameliorates colitis by remodeling colonic mucus barrier and lamina propria macrophages. Gut Microbes 13, 1903826 (2021).
Acknowledgements
We would like to thank for the English language editing services provided by Editage (www.editage.cn). This research was funded by Classification Development of Beijing Municipal Universities-Construction of Urban Agriculture and Forestry Characteristic Teacher Team, grant number 11000024T000002961733.
Author information
Authors and Affiliations
Contributions
C.D.(first author): Methodology, Data Curation, Formal Analysis, Visualization, Writing - Original Draft; X.Z.: Data Curation, Formal Analysis, Visualization; J.Z.: Methodology, Visualization; H.Z.: Writing - Review & Editing; J.J.: Resources, Supervision; X.P.: Resources, Writing - Review & Editing; X.L.(Corresponding Author): Conceptualization, Funding Acquisition, Resources, Writing - Review & Editing; Y.X.(Corresponding Author): Conceptualization, Resources, Supervision, Writing - Review & Editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Dai, C., Zheng, X., Zhu, J. et al. Polysaccharides derived from Rosa rugosa cv. Plena ameliorate colorectal cancer by regulating intestinal microbiota composition and lipid metabolism pathway. npj Sci Food 9, 176 (2025). https://doi.org/10.1038/s41538-025-00544-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41538-025-00544-2