Abstract
Nucleoside-modified mRNA-LNP vaccines have revolutionized vaccine development against infectious pathogens due to their ability to elicit potent humoral and cellular immune responses. In this article, we present the results of the first norovirus vaccine candidate employing mRNA-LNP platform technology. The mRNA-LNP bivalent vaccine encoding the major capsid protein VP1 from GI.1 and GII.4 of human norovirus, generated high levels of neutralizing antibodies, robust cellular responses, and effectively protected human enteroids from infection by the most prevalent genotype (GII.4). These results serve as a proof of concept, demonstrating that a modified-nucleoside mRNA-LNP vaccine based on norovirus VP1 sequences can stimulate an immunogenic response in vivo and generates neutralizing antibodies capable of preventing viral infection in models of human gastrointestinal tract infection.
Similar content being viewed by others
Introduction
Human noroviruses are the leading cause of acute viral gastroenteritis globally, causing an estimated 700 million infections and 200,000 deaths annually1. Norovirus infections can affect individuals of all ages and are prevalent in countries at all income levels2. Globally, young children bear the highest disease burden, while residents of long-term care facilities are most critically affected by norovirus in upper-income countries3,4. Complications such as dehydration and malnutrition can be severe among these susceptible populations.
In response to this high disease burden worldwide, the World Health Organization has prioritized the development of a norovirus vaccine to prevent severe disease5. However, these efforts have faced challenges due to the antigenic diversity of the viruses. Noroviruses are classified into 10 designated and 2 tentative genogroups (GI-GX, GNA1, GNA2) and 48 genotypes, of which GI, GII, GIV, GVIII, and GIX contain viruses that infect humans6. The challenge of vaccine development is further complicated by the difficulty in propagating these viruses in cell lines the absence of a small-animal model for evaluating serotypes and determining correlates of protection against infection. A recent breakthrough in the field was the successful cultivation of select human norovirus genotypes in human intestinal enteroids (HIE)7. This non-transformed human intestinal organoid model, derived from surgical resections of the small intestine, mimics the human intestinal epithelium with multiple cell types and permits limited human norovirus replication. In addition, a surrogate neutralization assay based on antibody blockade of virus-like particle (VLP) binding to carbohydrate ligand is a key metric for studying norovirus vaccine responses8,9. “Blockade” antibodies, which correlate with neutralizing antibodies (nAb), have been proposed as a correlate of protection10.
Although there are currently no licensed therapeutics or vaccines for norovirus, five vaccine candidates are in clinical development11. Four are VLP vaccines administered via intramuscular injection12,13,14, while another candidate delivers the virus capsid gene intracellularly using an Adenovirus 5 vector that targets the gut mucosa15. The HilleVax 214 VLP candidate has demonstrated modest levels of short-term protection from infection in adults16,17. Efficacy data for the other candidates are not yet available. These candidate vaccines share a common approach of delivering the breadth of immunity necessary for an efficacious norovirus vaccine: a bivalent platform delivering a single immunogen from genogroup I and genogroup II, the causative agents of >95% of norovirus infections.
The major challenge in developing an effective vaccine is the wide genetic and antigenic diversity of noroviruses. There are multiple co-circulating genotypes, each containing a plethora of variants, making it difficult to create a vaccine that is broadly effective. GII.4 variants are responsible for 50–75% of norovirus infections, facilitated by cyclical dominant variant replacement events18. Although GII.4 Sydney 2012 has been the dominant variant since its emergence, between the mid-1990s and 2012, six GII.4 other variants caused pandemic levels of disease19. The emergence of early pandemic variants was primarily due to viral evolution in antigenic sites, allowing immune escape19,20,21 recent data support the hypothesis that immune imprinting has facilitated the emergence of contemporary variants with less antigenic diversification22,23. In adults, nAb responses to GII.4 exposure (infection or vaccination) are predominantly memory responses biased towards previously encountered variants8,24,25. This further complicates vaccine approaches, as memory profiles are likely to vary across different age groups due to immune imprinting.
Antibodies that compete with VLP binding to carbohydrate ligands have demonstrated a correlation with in vitro virus neutralization and protection from infection or severe disease10,19,26. Other potential correlates of protection have been suggested, including host genetics, mucosal IgA, IgG memory B cells, and serum IgA, but these are not as well characterized27. Vaccination of adults with Hillevax 214 results in a short-term increase in broad nAb8. While neutralizing antibody responses to the vaccine genotypes are longer-lasting, these responses are not increased by a booster dose at 4 weeks28. Data on neutralizing/blockade antibody responses for other genotypes and GII.4 variants outside the vaccine immunogens have not been reported for the other vaccine candidates. These findings suggest that antibodies that block ligand binding are likely to be protective. Nevertheless, as infections can occur even after infection or vaccination, it remains uncertain whether current vaccination approaches can generate sufficiently high titers of broad nAb to provide long-term protection, especially at mucosal sites and in young children with limited immunological memory to human norovirus.
In comparison, the SARS-CoV-2 mRNA vaccines stimulate robust serum nAb in both naïve and pre-exposed individuals and have been remarkably effective at reducing the severity of CoVID-1929. Furthermore, repeated vaccination or exposure leads to an increase in antibody titer and broadened cross-reactivity of both systemic and mucosal nAb30,31,32. The mRNA platform offers the advantage of easy redesign, allowing for multiplexing and updates to the vaccine formulation as new variants evolve in response to population immunity. These characteristics make the mRNA platform an attractive option for delivering the norovirus VP1 as an immunogen.
Results
mRNA constructs yield authentic-sized proteins capable of self-assembling into VLP
Our study focused on the development of a bivalent norovirus mRNA-LNP vaccine, which elicited potent and sustained neutralizing antibody responses. To elicit relevant immune responses through vaccination with the bivalent mRNA-LNP vaccine, we designed immunogens using VP1 sequences encoding Norwalk1968/GI.1 and CapeTown2012/GII.4, a strain of the globally dominant variant GII.4 Sydney. Expi293F cells were transfected with each mRNA construct and confirmed the expression of proteins with the expected sizes by western blot analysis in both cell lysates and culture supernatants (Fig. 1A, B). Western blots of cell lysates and supernatants at multiple timepoints post-transfection demonstrated consistent protein production of both constructs for at least 72 h, indicating the successful expression of vaccine antigens.
Expi293F cells were transfected with mRNA-encoding (A) norovirus GI.1 or (B) GII.4 VP1 and analyzed for protein expression by western blot as described in Methods. The supernatant was collected, clarified, ultrafiltered, and visualized via NSEM. Representative micrographs are shown from cells transfected with (C) GI.1 or (D) GII.4, with n = 5 micrograph images per group. E Experimental schematic of the study. Balb/c mice (n = 10 per group) were immunized on days 0 and 28 with 10 μg of mRNA-LNP vaccine encoding Norwalk1968/GI.1 or CapeTown2012/GII.4 VP1, or empty LNP as control at an equivalent dose. Sera were collected at various time points (4, 8, 14, 22, 26, and 34 weeks post-prime) and screened against (F) Norwalk1968/GI.1 and (G) CapeTown2012/GII.4 VLPs to assess their ability to block VLP binding to its carbohydrate ligand in a surrogate neutralization assay. The data was analyzed using GraphPad Prism, and the dilution at which 50% of VLP-ligand binding was blocked (ID50) was calculated. Sera that did not block at least 50% of VLP-ligand binding were assigned a titer of 0.5X the lower limit detection (ID50 = 25) and marked below this limit (dashed line). The nAb titer is presented as 50% inhibitory dilution (ID50) values (Mean ± SD), n = 10 per group (5 male and 5 female). The nAb titers were compared with the values from Day 56 for each strain, ∗p < 0.05 F:(D56 vs D28 p = 0.0135) G:(D56 vs D28 p = <0.0001, D56 vs D150 p = <0.0001, D56 vs D180 p = <0.0001, d56 vs d240 p = <0.0001), one-way ANOVA, Tukey’s post hoc tests. A red square symbol indicates nAb titers in a serum sample collected on Day 14 from a patient infected with the GII.4 norovirus strain. nAb titer values for empty LNP are not shown because they were below the level of detection. Schematic for Fig. 1E created with Biorender.
To further validate the capability of the mRNA constructs to deliver authentic immunogens, Expi293F cells were transfected with the GI.1 and GII.4 mRNA constructs. Subsequent examination of the clarified supernatants through negative stain electron microscopy revealed the presence of particles of approximately 40 nm in size (Fig. 1C, D) with a similar morphology to other VP1 VLPs33. These data suggest that the nucleoside-modified mRNA constructs encoding GI.1 and GII.4 VP1 successfully produced VP1 that self-assembled into VLP, indicating the potential efficacy of these constructs in vaccine development.
Norovirus bivalent mRNA-LNP vaccine elicit potent and sustained neutralizing antibody responses
To assess the antibody responses to GI.1 and GII.4 following mRNA-LNP vaccination, male and female Balb/c mice were immunized with 10 μg of each mRNA-LNP vaccine encoding norovirus VP1 (Fig. 1E), and serum nAb levels were quantified using a surrogate assay that measures antibody blocking of VLP binding to carbohydrate ligand. The ability of antibodies to block ligand binding is a key metric for evaluating norovirus vaccine responses in clinical trials as antibodies that compete for ligand binding also neutralize the virus in vitro25,26. Vaccination induced a high titer of surrogate nAb against both immunogens. The second immunization (boost) with the same dose of GI.1 and GII.4 mRNA-LNP resulted in a significant elevation of nAb responses to both immunogens, with a mean ID50 of approximately 10,000. The titers of nAb at day 100 were higher or comparable with the titers at day 60 and persisted at high levels up to day 240 post-immunization (Fig. 1F, G). Note that after a single dose (prime) with 10 μg of GII.4 mRNA-LNP vaccine, the nAb titers were higher than or comparable to those observed in the serum of a patient with GII.4 norovirus infection at day 14 (Fig. 1G). These nAb responses were not broadly cross-reactive against viruses of other genotypes (GI.3, GI.5 or GII.3) as shown in Fig. 2A, B and had significant cross-reactivity with other GII.4 variants (Fig. 2B), consistent with previously reported antigenic distances between strains in vaccinated mice34. These findings mirror the nAb responses observed in young children following their initial norovirus infections35.
Balb/c mice (n = 10 per group) were immunized on days 0 and 28 with 10 μg of mRNA-LNP vaccine encoding Norwalk1968/GI.1 or CapeTown2012/GII.4 VP1, or empty LNP as control at an equivalent dose. A nAb response titers to three genogroup I strains (GI.1, GI.3, and GI.5) and (B) against four genogroup II strains (GII.3, GII.4 Sydney 2012, GII.4 New Orleans 2009, and GII.4 Farmington Hills 2002) at day 56 post prime in a surrogate neutralization assay. The nAb titer is presented as 50% inhibitory dilution (ID50) values (Mean ± SD), n = 10 per group (5 male and 5 female). Only the cross-reactivity nAb titer values of GII.4 variants were compared, ∗p < 0.05, (2012 vs 2009 p = <0.0001, 2012 vs 2002 p = <0.0001) one way-ANOVA, Tukey’s post hoc tests.
Adverse reactions to mRNA-LNP vaccination are well-documented36,37,38, which limits the range of acceptable dosages for clinical use. In addition, multiplexing allows the inclusion of many immunogens to increase broadness39,40,41. Thus, after establishing the immunogenicity of the bivalent norovirus vaccine at high doses, we conducted a dose de-escalation study to determine the optimal mRNA-LNP dose range for nAb stimulation of the combined vaccine. Balb/c and C57BL/6 mice were immunized on days 0 and 28 via i.m. injection with doses of 0.25, 0.5, 1, and 3 μg of each mRNA-LNP (Norwalk1968/GI.1 and CapeTown2012/GII.4) vaccine per mouse, and their nAb titers were evaluated over time (Fig. 3A). After the prime, the doses of 0.25, 0.5, 1, and 3 μg of each mRNA-LNP (Norwalk1968/GI.1 and CapeTown2012/GII.4) vaccine-induced dose-dependent nAb responses in both mouse strains (Fig. 3B–E). Administering the second immunization of the bivalent mRNA-LNP vaccine at the same dose significantly boosted nAb responses to both vaccine immunogens. In C57BL/6 mice, post-boost nAb titers were high and consistent across all doses for both human norovirus genotypes (Fig. 3D, E). Similar results were observed in Balb/c mice, with only the 3 μg dosage outperforming the 0.25 μg dosage for both GI.1 and GII.4 CapeTown genotypes (*p < 0.05 in GI.1 and GII.4) (Fig. 3B, C). Thus, a total of 0.5 μg, and even 0.25 μg (10 μg/kg), for the GI.1/GII.4 mRNA-LNP bivalent vaccine with a prime/boost regimen, is sufficient to induce a robust nAb response in both genetic backgrounds.
A Experimental schematic of the study. Balb/c and C57BL/6 mice were i.m. immunized on days 0 and 28 with varying doses (0.25 μg, 0.5 μg, 1 μg, and 3 μg) of each mRNA-LNP vaccine encoding Norwalk1968/GI.1 and CapeTown2012/GII.4 VP1, or empty LNP as control. Sera were collected 4 weeks after the prime and after the boost, and then screened against (B, D) Norwalk1968/GI.1 and (C, E) Sydney2012/GII.4 VLP to evaluate their capacity to block VLP binding to their carbohydrate ligand in a surrogate neutralization assay. The nAb titer is presented as 50% inhibitory dilution (ID50) values (Mean ± SEM), n = 5. Dashed line = limit of detection. nAb titer values for empty LNP are not shown because they were below the level of detection. Only nAb titer values within Day 56 were compared, ∗p < 0.05, B:(D56 0.25 μg vs D56 3 μg p = 0.0007), C:(D56 0.25 μg vs D56 3 μg p = 0.0001) ANOVA, Tukey’s post hoc tests. Schematic for Fig. 3A created with Biorender.
Norovirus mRNA-LNP vaccination induces strong cellular responses
Immunization with nucleoside-modified mRNA-LNP vaccines has been demonstrated to elicit elevated levels of antigen-specific T cells42. To investigate the capacity of mRNA-LNP vaccine to induce a strong cellular response, mice were immunized with 0.25 μg of CapeTown2012/GII.4 mRNA-LNP per mouse at day 0 and day 28 (Fig. 4A). Two weeks after the boost, splenocytes were stimulated with GII.4 synthetic peptide pools and the activation of T cells was analyzed by intracellular cytokine staining of splenocytes using flow cytometry (Fig. 4B) as described43. Figure 4C, D shows that the mRNA-LNP vaccine elicited strong CD4+ and CD8 + T-cell responses. CD4+ cells expressed anti-viral cytokines such as IFN-ɣ, IL-2 and TNF-α in response to GII.4 peptide stimulation in norovirus-vaccinated mice, but not in control mice (Fig. 4C). There were high levels of triple positive IFN-γ/IL-2/TNF-α co-expression by CD4 + T, highlighting the multifunctional T cell population elicited (Fig. 4E). In CD8 + T cells, IFN-γ emerged as the dominant cytokine, representing 57% of the total cytokine response, underlining its critical role in the immune response along with TNF-α (Fig. 4D). The co-expression of IFN-γ and TNF-α by CD8 + T cells further underscores the potential importance of multifunctional T cells for mediating protection and potency of the immune response (Fig. 4E).
A Experimental schematic of the study. Balb/c mice were immunized by i.m. injection with 0.25 μg of CapeTown2012/GII.4 mRNA-LNP on days 0 and 28. Two weeks post-boost, splenocytes were isolated, and a single-cell suspension of splenocytes was stimulated with the GII.4 peptide pool or control solution without peptides. B Representative T cell gating strategy. An example is shown of IFN-ɣ, TNF-α, and IL-2 production by CD8+ cells of a mouse immunized with GII.4. Cytokine production by CD4+ and CD8 + T cells was assessed by flow cytometry. C The percentages of GII.4-specific CD4+ and (D) CD8 + T cells producing IFN-ɣ, TNF-α, and IL-2 are shown, Mean ± SEM, n = 10 per group, ∗p < 0.05 (ANOVA, Tukey’s tests). E Distribution of total cytokine-positive cells according to their polyfunctionality. F Tfh and (G) B cell representative gating strategy. H Tfh- and B cells in inguinal and popliteal LNs were analyzed at day 7 post-immunization from Balb/c mice immunized by i.m. injection with 10 μg or (I) 1 μg of CapeTown2012/GII.4 mRNA-LNP vaccine and are expressed as a % of total T and B cell yield. Mean ± SEM, n = 5–10 per group, *p < 0.05, C:(IFNγ p = 0.0008, IL-2 p < 0.0001, TNFα p = 0.0005, IFNγ + IL-2+ p = 0.015, IFNγ + TNFα + p = 0.005, IL-2 + TNFα + p < 0.0001, IFNγ + IL-2 + TNFα + p < 0.0001) D:(IFNγ p < 0.0001, IL-2 p < 0.0001, TNFα p = 0.0003) unpaired t-test with Welch’s correction. Schematic for Fig. 4A created with Biorender.
T follicular helper (Tfh) cell responses are recognized as crucial for generating broad and potent antibody responses44. To evaluate the ability of CapeTown2012/GII.4 mRNA-LNP vaccine to induce strong antigen-specific Tfh- and B cell responses, mice were immunized via i.m. injection with a single dose of 1 μg or 10 μg per mouse or injected with an equal volume of sterile PBS as a control (Fig. 4A). Draining lymph nodes were examined 7 days post-prime, and the total numbers of Tfh- and B cells were identified by flow cytometry as CD4+B220−CD44hiCD62L−Bcl6+CXCR5+ and CD4-/CD8−CD19+ respectively (Fig. 4F, G). In the present study, a specific B cell antigen tag for norovirus was unavailable; therefore, we did not analyze antigen-specific GC B cells. CapeTown2012/GII.4 mRNA-LNP vaccine was able to elicit high levels of Tfh- and total B cells compared to control (Fig. 4H, I). Collectively, these data demonstrate that a single immunization of CapeTown2012/GII.4 mRNA-LNP generates a robust Tfh and B cell response in the draining lymph nodes of mice.
Sera from mice immunized with bivalent norovirus mRNA vaccine effectively neutralizes GII.4 norovirus infection of human intestinal enteroids
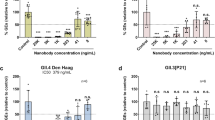
One of the major challenges for the development of effective interventions for human noroviruses has been the absence of a robust and reproducible in vitro cultivation system. However, recent developments have led to a norovirus infection model using HIE45,46. The HIE norovirus neutralization system is dependent upon availability of human norovirus-positive stool as the input, and replication is dependent on stool sample. In the present study, an in vitro replicating stool sample containing GI.1 human norovirus was unavailable; therefore, we evaluated the neutralization potency of vaccine sera against infection with a stool sample containing GII.4 2012 Sydney virus in the HIE model. On the day of infection, serum from mice immunized with the mRNA-LNP bivalent vaccine was diluted (1/100, 1:300, 1:1,000, 1:3,000, 1:10,000, and 1:30,000) and preincubated with a GII.4-Sydney positive stool sample for 1 h. Subsequently, 3D-HIEs were infected for 1 hat 37 °C. The cells were then washed and re-embedded in BME and incubated for 2 days in the presence of Ruxolitinib, (a JAK inhibitor), and the bile acid GCDCA to promote infection45.
Sera from mRNA-LNP bivalent vaccinated mice (10 μg/mouse) diluted up to 1:3000 effectively protected HIE against GII.4 infection (Fig. 5B). Similarly, sera from mice immunized with 0.25 μg/mouse of mRNA-LNP bivalent vaccine diluted up to 1:1000 protected HIE against GII.4 infections (Fig. 5C). As anticipated, sera from the control group receiving empty LNP did not block infection (Fig. 5).
A Experimental schematic of the study. Human intestinal enteroids were infected for 2 h at 37 °C with GII.4 Sydney virus (stool sample #20942) that was pre-incubated with serial dilution of sera from mice immunized by i.m. with (B) 10 μg or (C) 0.25 μg of each mRNA-LNP vaccine encoding Norwalk1968/GI.1 and CapeTown2012/GII.4 VP1 for 1 h at room temperature. Following infection, 3D HIE were washed and cultured in BME for 3 days post-infection (see Methods and Materials). Viral titers were measured by RT-qPCR. *p < 0.05 vs control (empty LNP). B, C Each dot in the graph at a given dilution represents an independent infection performed on a separate day, while each set of bars, i.e., dilutions 1:100 to 1:30,000 (B), or 1:300 to 1:10,000 (C) represents the dilution series from an individual serum sample. Schematic for Fig. 5A created with Biorender.
In summary, our data demonstrated that the bivalent mRNA vaccine consisting of GII.4 and GI.1 VP1, generates high and long-lasting titers of nAb, induces a robust T-cell response, and neutralizes GII.4 norovirus infection in the HIE model.
Discussion
The development of mRNA-based prophylactics has spanned decades47,48,49, but their remarkable success in combating the COVID-19 pandemic has highlighted the advantages of the platform. This success has accelerated the development of vaccines for various human pathogens that were previously resistant to traditional vaccine approaches. Clinical trials for influenza, HIV, Zika, CMV, rabies, and other infections are ongoing, and an mRNA-LNP based RSV vaccine has obtained Breakthrough Therapy Designation from the FDA. The relative ease of adaptability of mRNA platforms makes them particularly attractive vaccine options for RNA viruses prone to mutation and immune escape, such as HIV, SARS-CoV-2, influenza, and human noroviruses. Here, emulating norovirus vaccine immunogens currently in clinical trials as a model system, we show that mRNA-LNP immunization yields in vivo expression of norovirus capsid protein with neutralizing epitopes, resulting in high titers of durable serum nAb that protects human intestinal cells from infection in vitro, meeting key pre-clinical milestones for a norovirus vaccine. Further, this study demonstrates that the mRNA-LNP vaccine elicits nAbs to neutralizing epitopes on icosahedral viruses, supporting further application of the technology to a wider class of human pathogens.
The primary readout of vaccine response was the titer of antibody that blocked the binding of VLPs to a carbohydrate ligand, a surrogate of nAb analysis, a proposed correlate of protection, and an essential metric in evaluating clinical studies of norovirus vaccines. Mouse vaccination with mRNA-LNP yielded very high surrogate nAb titers that persisted for at least 8 months. Although potent and durable, these nAb responses largely targeted vaccine immunogens with limited cross-reactivity to other tested genotypes but significant cross-reactivity to GII.4 variants. Within the GII.4 genotype, nAb titers for GII.4/2009 and GII.4/2002 were about ~20% and ~5%, respectively to the titer of GII.4/2012. This is in agreement with the known antigenic drift within the globally dominant GII.4 pandemic genotype33. By contrast, adult vaccination with bivalent GI.1/GII.4 VLP vaccine yields lower levels of nAb increases that decline more rapidly and were highly cross-reactive among genotypes, at least in the short-term8,9. These observations in norovirus VLP vaccinated adults are heavily weighted by the impact of norovirus immune imprinting and would not be relevant in these mouse studies, but they do illustrate a fundamental challenge to vaccination for hypervariable pathogens: namely, how to drive nAb breadth in the presence of strong type-specific pre-existing immunity. Although the full clinical impact is unclear, for SARS-CoV-2 this has been addressed by reformulation of vaccines with emergent variants of concern, primarily Omicron variants, resulting in a broadening of nAb responses and protection from severe disease. Furthermore, multiplexing of mRNA has been shown to induce breadth without sacrificing nAb titer to specific influenza antigens39,40,50. This strategy should prove effective for noroviruses as previous studies have demonstrated that multiplexing norovirus VLPs significantly broadened immunity through the inclusion of more genotypes and/or variants of the dominant genotypes51. Although limited to the immunizing genotype, the strong nAb responses to norovirus mRNA-LNP did provide cross-neutralization of GII.4 variants, supporting the feasibility of broadening a protective norovirus vaccine through multiplexing of VP1 mRNA of different strains.
This study, like all pre-clinical studies for obligate human pathogens, is limited by use of an animal model that does not support viral replication and primarily in vitro assays to measure surrogates of protection to support further studies in humans. Additionally, the mRNA-LNP platform has application challenges, including vaccine reactogenicity and limited breadth of nAb responses. Here, using a model system, optimal nAb responses were evident at a dose of 8–10 μg/kg, compared to the CoVID-19 vaccines that use 50–100 μg dose/0.8–1.5 μg/kg (mRNA -1273, NCT04470427). Future studies of alternative LNP formulations are needed to improve the safety, manufacture, and stability of LNP delivery systems52.
For SARS-CoV-2, limited breadth of nAb is compensated for by the high nAb titer, as a percentage of nAb will be directed to conserved epitopes that is boosted by single variant vaccination. For norovirus, the degree of cross-reactivity could be improved by incorporation of additional genotypes and more GII.4 variants and these studies are currently underway. Multivalent immunization with diverse norovirus GII.4 variants may also mitigate immune imprinting, as vaccination simultaneously with H1 and H3 may do for influenza vaccines53. The modest GII.4 breadth from the current vaccine formulation suggests that broader protection could be achievable with additional immunogens across age groups. As approximately 50% of norovirus outbreaks have been caused by GII.4 Sydney-like viruses for the past decade, even the current vaccine formulation may be effective in both the elderly and young children, who bear the most severe disease burden. Although protection from a divergent variant may only be achievable in adults with underlying memory B cells imprinting that supports nAb to conserved epitopes22.
The simultaneous production of multiple pro-inflammatory cytokines by T cells is considered an indicator of a robust and versatile immune response. Polyfunctional T cells, capable of simultaneously producing key Th1 cytokines such as IFN-γ, IL-2, and TNFα, have been reported following infection or immunization in numerous infectious diseases54. The magnitude of the vaccine-induced polyfunctional CD4 + T-cell response has been reported to be highly correlated with protection from infection55,56. In our study, mRNA-LNP vaccine elicited a high frequency of both triple-positive and any combination of double-positive polyfunctional CD4 + T cells, constituting ~20% of the total cytokine response, and 9% of the higher magnitude double-positive polyfunctional CD8 + T cells. This study underscores the efficacy of the mRNA vaccine in eliciting a potent and multifaceted T cell-mediated immune response against norovirus, combined with strong Tfh and B cell responses driving the high nAb titers.
In summary, our data demonstrated that the first mRNA-LNP bivalent vaccine in a mouse model successfully generated high and durable nAb titers and induced a strong polyfunctional T-cell response, which is crucial for a comprehensive and effective immune defense. The vaccine provided protection to HIE from GII.4 norovirus infection, demonstrating its potential effectiveness in preventing the spread and impact of this common and highly infectious virus. This evidence collectively underscores the vaccine’s capability to elicit a robust and lasting immune response and provides the fundamentals for moving a norovirus mRNA-LNP vaccine into phase I clinical trials.
Methods
Norovirus VP1 mRNA production and characterization
mRNA of VP1 capsid proteins of norovirus GI.1 Norwalk virus and GII.4 Sydney were produced by in vitro transcription using T7 RNA polymerase (Megascript, Ambion) on linearized plasmids encoding codon-optimized norovirus constructs. Codon-optimized sequences of Norwalk1968/GI.1 (GenBank: AGM33223.1) and CapeTown2012/GII.4 (GenBank: ALX87357.1), a strain of the GII.4 Sydney variant, were synthesized and cloned into an mRNA production plasmid as described39. Nucleoside-modified mRNAs were transcribed to contain 101 nucleotide-long poly(A) tails for optimized expression. To generate modified nucleoside-containing mRNA, 1-methylpseudouridine-5’-triphosphate (TriLink, catalog # N-1081) was used instead of UTP. In vitro transcribed mRNAs were capped co-transcriptionally using the trinucleotide cap1 analog, CleanCap (TriLink, Catalog # N-7413) as previously described57. m1Ψ-containing mRNAs were purified by cellulose purification, analyzed by agarose gel electrophoresis, and stored frozen at –20 °C. Nucleoside-modified mRNAs were produced and analyzed for dsRNA, endotoxin, and INFα levels as previously described58.
mRNA transfection in Expi293 cell line
Transfection of Expi293 cells (AATC, Catalog # CRL-1573) was performed with ExpiFectamine 293 Reagent (ThermoFisher Scientific, catalog # A14635) according to the manufacturer instructions: mRNA encoding capsid proteins of GI.1, and GII.4 were combined with ExpiFectamine 293 Reagent in Opti-MEM reduced serum medium (Thermo Scientific, Catalog # 31985-062), and the complex was incubated for 10 min at room temperature and then added to Expi293 cells. Transfected cells were incubated at 37 °C with 8% CO2 and stirred with a spinner speed of 130 rpm. Cell suspensions (2 ml) were collected at 6-, 24-, 48-, and 72-h post-transfection, centrifuged at 120 rpm for 5 min, media supernatants and cell pellets were collected and stored at −20 °C. Cell pellets were lysed in RIPA buffer (Sigma-Aldrich, Catalog # R0278) with a protease inhibitor (Sigma-Aldrich, Catalog # 11836153001) according to the manufacturer instructions. Briefly: samples were incubated on ice for 30 min, vortexed every 10 min and then centrifuged at 10,000 rpm for 10 min at 4 °C and the resulting cell-free supernatant of whole cell lysates were analyzed for protein content by BCA assay Peirce, Catalog # 23228 according to the manufacturer instructions.
Immunoblots
Media supernatants (equal volume/well) and whole cell lysates (equal protein/well) were assayed for norovirus VP1 by denaturing SDS-PAGE 4–12% Bis-Tris followed by western blot analysis. Samples were transferred to PVDF membrane using an iBlot 2 dry apparatus (Thermofisher). The membranes were blocked with 10% of non-fat dry milk in TBS buffer containing 0.1% Tween-20. GI.1, and GII.4 VP1 were detected using custom primary rabbit anti-VLP polyclonal sera (Cocalico, Stevens, PA.) at 1:2,500 overnight at 4 °C, followed by a secondary goat anti-rabbit IgG HRP at 1:10,000 for 1 h at room temperature. Membranes were stripped and re-probed with anti-GAPDH (Cell Signaling Technology, Catalog # 2118S) for 1 h at room temperature at a 1:1000 dilution, and a secondary goat anti-rabbit IgG HRP (Bio-Rad, Catalog # 1706515) at a 1:10,000 for 1 h at room temperature. All antibody incubations were performed in a 1% of non-fat dry milk in TBS buffer containing 0.1% Tween-20. Membranes were developed using ECL Prime (Amersham Biosciences, Amersham, UK, Catalog # RPN2232) prior to visualization on an Amersham ImageQuant 800 (Cytiva). The levels of protein expression were quantitated by densitometry using ImageJ and normalized to the GAPDH protein level for each genotype. Full blots are available in Supplemental Fig. 1.
Norovirus mRNA-LNP formulation
Purified mRNAs were encapsulated in LNP using a self-assembly process as previously described59. Briefly, an aqueous solution of mRNA at pH 4.0 was rapidly mixed with an ethanolic lipid mixture of ionizable cationic lipid, phosphatidylcholine, cholesterol, and polyethylene glycol-lipid (50:10:38.5:1.5 mol/mol). The LNP formulation used in this study is proprietary to Acuitas Therapeutics (Vancouver, Canada), and LNP compositions are described in the patent application WO 2017/004143. The formulated mRNA-LNP were characterized and subsequently stored at –80 °C at an RNA concentration of 1 mg/ml and a total lipid concentration of 30 mg/ml. The mean hydrodynamic diameter of mRNA-LNP, measured by dynamic light scattering using a Zetasizer Nano ZS (Malvern Instruments Ltd, Malvern, UK) was ~80 nm with a polydispersity index of 0.02–0.06 and encapsulation efficiency of 95%. The two mRNAs encoding GI.1 Norwalk virus or GII.4 CapeTown were combined in equal concentrations based on mass prior to encapsulation in LNP by Acuitas. The vaccine formulations were stored at −80 °C and not reused once thawed.
mRNA vaccine in vivo studies
For all immunizations, mice were anesthetized with isoflurane (3–4% in oxygen for induction, 1–2% in oxygen for maintenance) and received intramuscular (i.m.) injections of mRNA-LNP (0.25 μg, 0.5 μg, 1 μg, 3 μg or 10 μg in 50 μl PBS into the upper hind leg). After vaccination, blood samples were obtained four weeks following each immunization from retro-orbital bleed and sera were isolated for analyses. After reaching endpoints, mice were euthanized by carbon dioxide inhalation with death following euthanasia ensured by confirmation of loss of vital signs and absence of corneal reflex. All mouse experiments were approved by the Institutional Animal Care and Use Committees of the University of Pennsylvania. All experimentation adhered to the Guide for the Care and Use of Laboratory Animals by the National Research Council. Mice were housed and cared for in the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC)-accredited facilities.
For the initial evaluation of the mRNA-LNP vaccines, 6–8-week-old male and female Balb/c or C57Bl/6 mice were purchased from Charles River Laboratories (Wilmington, MA). Mice were immunized with indicated doses of Norwalk1968/GI.1 and/or CapeTown2012/GII.4 mRNA-LNP vaccine. Mice immunization schedules and timings of collection of blood, lymph nodes (LN), and spleens in various experiments are shown in Figs. 1E, 3A, 4A and 5A.
Polyfunctional T-Cell analysis
The induction of antigen-specific T-cells was analyzed upon peptide stimulation using intracellular cytokine staining. Balb/c mice (male and female), aged 6–8 wks, were immunized by i.m. injection with 0.25 μg of CapeTown2012/GII.4 mRNA-LNP per mouse on days 0 (prime) and 28 (boost). Control mice were injected with an equal volume of sterile PBS. Splenocytes were collected 14 days after boost, counted using a Vi-Cell automated cell counter and then one million splenocytes in 100 μl of RPMI-1640 medium were added per well. Splenocytes were stimulated with 80 μl of a synthetic peptide pool of CapeTown/GII.4 VP1 (150 peptides, each with 15 AA length and 11 AA overlap, a purity level between 80 and 90%, synthesized by GenScript) at a final concentration of 1.5 μg/mL, and 1 μg/mL of CD28 (co-stimulatory signal) for 1 h at 37 °C. To inhibit the cytokine secretion, 1-h post-stimulation splenocytes were treated with 20 μl containing brefeldin-A (BD Biosciences, Catalog # 51-2301 ZK) and monensin (BD Biosciences, Catalog # 51-2092KZ) at final concentrations of 0.2 μM and 0.14 μM, respectively, for 5 h at 37 °C. Then splenocytes were washed twice in FACS buffer (PBS with 2% FBS) and stained for live-dead cells using the Aqua LD Dye (Invitrogen, Catalog # L34966) for 10 min in the dark at room temperature. Then cells were blocked with anti‐CD16/CD32, TruStain FcX, (BioLegend, Catalog # 101320) for 15 min at 4 °C, and then cells were surface stained with CD4, and CD8 in FACS buffer for 30 min in the dark at 4 °C. Subsequently, cells were washed with FACS buffer and permeabilized using the Cytofix/Cytoperm kit (BD Biosciences, Catalog # 51-2090KZ) according to the manufacturer’s instructions. Finally, intracellular cytokine staining for induction of interferon-gamma (IFN-γ), interleukin-2 (IL-2), and tumor necrosis factor-alpha (TNF-α) was performed at 4 °C for 30 min. For the FACS analysis, splenocytes were resuspended in 1% paraformaldehyde in PBS, and stored at 4 °C until analysis. Splenocytes were analyzed on a modified Cytek Aurora spectral analyzer. Five hundred thousand events were collected per sample. Flow cytometry data were analyzed using FlowJo software (Tree Star, Inc., Ashland, OR, USA). Dimethyl Sulfoxide (DMSO) served as the solvent for dissolving the overlapping peptide pools and was included as a negative control to assess cytokine stimulation in CD4+ and CD8 + T-cells in the absence of peptides. Gate coordinates were set based on the DMSO-treated cells and then applied to the peptide-treated cells for each animal in the study. This approach enhances the reliability and accuracy of the data regarding the activation and functionality of CD4+ and CD8 + T-cells in response to the vaccine antigens. PMA- (10 ng/ml, Millipore Sigma, Catalog # P1585-1M6), ionomycin- (250 ng/ml, StemCell, Catalog # 73722) stimulated samples were used as positive controls. Splenocytes were stained as per the antibody panel in Supplementary Table 1. The gating strategy is provided in Fig. 4B.
Flow cytometric phenotyping of T follicular helper and B cell responses
For immunophenotyping of murine T follicular helper (Tfh) cells and germinal center B (GC B) cells, draining bilateral popliteal and inguinal lymph nodes from immunized mice 7 days after the first immunization were processed using 40 μm cell strainers in DMEM to obtain single-cell suspensions. One million cells were suspended in 100 ml of FACS buffer. All cells were stained for live-dead (eBiosciences Fixable viability dye eFluor780, Thermo Fisher, Catalog # 65-0865-14) for 10 min in the dark at room temperature, incubated with Fc block (BioLegend, Catalog # 101320) for 15 min at 4 °C, washed with FACS buffer, and stained for 1 h using the antibody panel in Supplementary Table 2. Following staining, cells were washed twice, fixed with 200 μl of 1% paraformaldehyde and data acquired on a modified LSR II flow cytometer (BD Biosciences). Flow cytometry data were analyzed using FlowJo software (Tree Star, Inc., Ashland, OR, USA). The gating strategy is provided in Fig. 4E.
Virus-like particles and surrogate antibody neutralization assay
Norovirus VLPs of GI.1 (AFS33555.1), GI.3 (AFK851.1), GI.5 (AHW998831.1), GII.3 (AFK75854.1), GII.4 Sydney (AGJ52172.1), and GII.4 FH (AFJ04708.1) were produced by insertion of the capsid gene into Venezuelan equine encephalitis virus replicons as described previously59,60,61. For antibody blockade of ligand binding assays, VLP (0.25 μg/ml) were pre-treated with 2 or 3-fold serial dilutions of sera before transfer to plates coated with pig gastric mucin (10 μg/ml) (Sigma Aldrich, Catalog # H4784). Bound VLP was visualized by rabbit anti-VLP sera (custom sera, Cocalico) followed by antirabbit IgG-HRP (Cytiva, Catalog # NA934) and color development with TMB ultra (Thermo Fisher, Catalog # 34029). The percentage of VLP binding with serum pretreatment compared to the amount with no serum pretreatment was determined and the ID50 titers (ID50) were calculated from dose-response sigmoidal curve fit of normalized data in Graphpad Prism v9.5.1. Samples that did not block ≥50% of control binding at the lowest dilution tested62 were assigned a titer of 20 for statistical comparisons23,25.
Neutralization assay in human intestinal enteroids
HIEs possess the multicellular complexity and organization of the intestinal epithelium. They are physiologically active, containing all epithelial cell types of the normal gastrointestinal tract and retaining segment-specific properties. HIEs derived from fetal ileum, HT124 (obtained from University of Michigan Medical School, Translational Tissue Modeling Laboratory), were maintained at 37 °C with 5% CO2 in basement membrane extract (BME) (MatriGel, Corning, Catalog # 354234 or Cultrex Ultimatrix, Catalog # BME001-05) and maintenance media, CMGF+ (Advanced DMEM-F12, GlutaMAX, PenStrep, B27, N2, mrEGF, N-acetyl cysteine, Leu15-Gastrin, A-83-01, and SB202190), that was replaced every other day, for 3–4 days after splitting. For this study, HIE differentiation was triggered by Wnt3a removal with differentiation media (advanced DMEM-F12, Noggin, B27, N2, mrEGF, N-acetyl cysteine, Leu15-Gastrin, and A-83-01) for 6 days with medium changes every other day. Stool sample # 20942 positive for norovirus GII.4 Sydney was used for infection at multiplicity of infection (MOI) of 1 as previously described45. We estimated the MOI based on the number of spheroids per condition with an average of 300 cells/spheroid, as measured by immune fluorescence.
On the day of infection, the amount of a 10% (w/v) filtered human norovirus positive stool in PBS that corresponded at MOI of 1 was pre-incubated 1 h at room temperature with 2x of the selected dilution of sera from immunized mice in CMGF+ medium with 500 μM glycochenodeoxycholic acid ((GCDCA), Cayman Chemicals, Catalog # 16942). 3D-HIE were collected in a 15-mL falcon tube and BME was diluted out with CMGF– medium. After pelleting (100 × g for 3 min at 4 °C), 3D-HIEs were resuspended in differentiation media and transferred into a 1.5-mL Eppendorf tube. 3D-HIEs were then incubated with a mixture of a 10% (w/v) human norovirus filtrate and the dilution of the sera from immunized mice at 37 °C for 2 h. The final concentration of the sera corresponds to the dilutions indicated in Fig. 5. Following incubation, the 3D-HIE were washed twice with CMGF-, resuspended in BME and transferred into a 24-well plate (one well per condition). Infected 3D-HIEs were incubated for 2 days at 37 °C with infection media (differentiation media with 500 μM of GCDCA and 2 µM of the JAK inhibitor Ruxolitinib, Cayman Chemical Catalog # 11609). 3D-HIEs were then harvested in TRI Reagent (Zymo Research, Cat # R2050-1) and viral RNA was quantified by RT-qPCR as described previously45,62.
Statistical analysis
Statistical analyses were completed using GraphPad Prism version 9 or 10. Results are reported as Means ± SEM. Data were tested for normal distribution using a Shapiro-Wilks test. If normally distributed, statistical significance for multiple group comparisons was determined using a one-way ANOVA with Tukey’s post-hoc test. If not normally distributed, statistical significance for multiple comparisons was determined using a Kruskal-Walli’s test. For parametric single comparisons, statistical significance was determined using an unpaired t-test with Welch’s correction compared to control groups as indicated in figure legends. If single comparisons were nonparametric, statistical significance was determined using a Mann–Whitney U test. All P < 0.05 were considered statistically significant. All statistical tests were conducted using a 5% significance level. n = 5–10 animals/group and is further indicated in figure legends.
Data availability
The data that support the findings of this study are included in the manuscript. All materials used in this manuscript are available from the corresponding author upon reasonable request.
References
Lopman, B. A., Steele, D., Kirkwood, C. D. & Parashar, U. D. The vast and varied global burden of norovirus: prospects for prevention and control. PLoS Med. 13, e1001999 (2016).
Lindsay, L., Wolter, J., De Coster, I., Van Damme, P. & Verstraeten, T. A decade of norovirus disease risk among older adults in upper-middle and high income countries: a systematic review. BMC Infect. Dis. 15, 425 (2015).
Bartsch, S. M., Lopman, B. A., Ozawa, S., Hall, A. J. & Lee, B. Y. Global economic burden of norovirus gastroenteritis. PLoS ONE 11, e0151219 (2016).
Black, R. E. et al. Estimated global and regional causes of deaths from diarrhoea in children younger than 5 years during 2000-21: a systematic review and Bayesian multinomial analysis. Lancet Glob. Health 12, e919–e928 (2024).
Giersing, B. K., Modjarrad, K., Kaslow, D. C. & Moorthy, V. S. Committee WHOPDfVA, Committee WHOPDfVPDA. Report from the World Health Organization’s Product Development for Vaccines Advisory Committee (PDVAC) meeting, Geneva, 7-9th Sep 2015. Vaccine 34, 2865–2869 (2016).
Chhabra, P. et al. Updated classification of norovirus genogroups and genotypes. J. Gen. Virol. 100, 1393–1406 (2019).
Ettayebi, K. et al. New insights and enhanced human norovirus cultivation in human intestinal enteroids. Msphere. 2021;6:10–1128.
Lindesmith, L. C. et al. Broad blockade antibody responses in human volunteers after immunization with a multivalent norovirus VLP candidate vaccine: immunological analyses from a phase I clinical trial. PLoS Med. 12, e1001807 (2015).
Haynes, J. et al. In depth breadth analyses of human blockade responses to norovirus and response to vaccination. Viruses. 2019;11:392.
Reeck, A. et al. Serological correlate of protection against norovirus-induced gastroenteritis. J. Infect. Dis. 202, 1212–1218 (2010).
Armah, G. et al. Vaccine value profile for norovirus. Vaccine 41, S134–S152 (2023).
Leroux-Roels, I. et al. A randomized, double-blind, placebo-controlled, dose-escalating phase I trial to evaluate safety and immunogenicity of a plant-produced, bivalent, recombinant norovirus-like particle vaccine. Front. Immunol. 13, 1021500 (2022).
Treanor, J. et al. A phase 2 study of the bivalent VLP norovirus vaccine candidate in older adults; impact of MPL adjuvant or a second dose. Vaccine 38, 5842–5850 (2020).
Lopez, P. et al. Immunogenicity and tolerability of a bivalent virus-like particle norovirus vaccine candidate in children from 6 months up to 4 years of age: a phase 2 randomized, double-blind trial. Hum. Vaccin Immunother. 19, 2204787 (2023).
Kim, L. et al. Safety and immunogenicity of an oral tablet norovirus vaccine, a phase I randomized, placebo-controlled trial. JCI Insight. 2018;3:e121077.
Atmar, R. L. et al. Norovirus vaccine against experimental human Norwalk Virus illness. N. Engl. J. Med. 365, 2178–2187 (2011).
Sherwood, J. et al. Efficacy of an intramuscular bivalent norovirus GI.1/GII.4 virus-like particle vaccine candidate in healthy US adults. Vaccine 38, 6442–6449 (2020).
Kendra, J. A., Tohma, K. & Parra, G. I. Global and regional circulation trends of norovirus genotypes and recombinants, 1995-2019: a comprehensive review of sequences from public databases. Rev. Med. Virol. 32, e2354 (2022).
Tohma, K., Lepore, C. J., Gao, Y., Ford-Siltz, L. A., Parra, G. I. Population genomics of GII.4 noroviruses reveal complex diversification and new antigenic sites involved in the emergence of pandemic strains. mBio. 2019;10:10–1128.
Lindesmith, L. C. et al. Mechanisms of GII.4 norovirus persistence in human populations. PLoS Med. 5, e31 (2008).
Lindesmith, L. C. et al. Immunogenetic mechanisms driving norovirus GII.4 antigenic variation. PLoS Pathog. 8, e1002705 (2012).
Lindesmith, L. C. et al. Immune imprinting drives human norovirus potential for global spread. mBio 13, e0186122 (2022).
Lindesmith, L. C. et al. Emergent variant modeling of the serological repertoire to norovirus in young children. Cell Rep. Med. 4, 100954 (2023).
Lindesmith, L. C. et al. Impact of pre-exposure history and host genetics on antibody avidity following norovirus vaccination. J. Infect. Dis. 215, 984–991 (2017).
Lindesmith, L. C. et al. Sera antibody repertoire analyses reveal mechanisms of broad and pandemic strain neutralizing responses after human norovirus vaccination. Immunity 50, 1530–41.e8 (2019).
Atmar, R. L. et al. Comparison of microneutralization and histo-blood group antigen-blocking assays for functional norovirus antibody detection. J. Infect. Dis. 221, 739–743 (2020).
Ramani, S., Estes, M. K. & Atmar, R. L. Correlates of protection against norovirus infection and disease-where are we now, where do we go? PLoS Pathog. 12, e1005334 (2016).
Atmar, R. L. et al. Persistence of antibodies to 2 virus-like particle norovirus vaccine candidate formulations in healthy adults: 1-year follow-up with memory probe vaccination. J. Infect. Dis. 220, 603–614 (2019).
Narowski, T. M. et al. SARS-CoV-2 mRNA vaccine induces robust specific and cross-reactive IgG and unequal neutralizing antibodies in naive and previously infected people. Cell Rep. 38, 110336 (2022).
Stolovich-Rain, M. et al. Intramuscular mRNA BNT162b2 vaccine against SARS-CoV-2 induces neutralizing salivary IgA. Front. Immunol. 13, 933347 (2022).
Liu, S. et al. Comparison of the mucosal and systemic antibody responses in Covid-19 recovered patients with one dose of mRNA vaccine and unexposed subjects with three doses of mRNA vaccines. Front. Immunol. 14, 1127401 (2023).
Mancuso, R. et al. Systemic and mucosal humoral immune response induced by three doses of the BNT162b2 SARS-CoV-2 mRNA vaccines. Vaccines 10, 1649 (2022).
Devant, J. M., Hofhaus, G., Bhella, D. & Hansman, G. S. Heterologous expression of human norovirus GII.4 VP1 leads to assembly of T=4 virus-like particles. Antivir. Res. 168, 175–182 (2019).
Kendra, J. A., Tohma, K., Ford-Siltz, L. A., Lepore, C. J. & Parra, G. I. Antigenic cartography reveals complexities of genetic determinants that lead to antigenic differences among pandemic GII.4 noroviruses. Proc. Natl Acad. Sci. USA 118, e2015874118 (2021).
Brewer-Jensen, P. D. et al. Norovirus infection in young nicaraguan children induces durable and genotype-specific antibody immunity. Viruses 14, 2053 (2022).
Broudic, K. et al. Nonclinical safety evaluation of a novel ionizable lipid for mRNA delivery. Toxicol. Appl. Pharm. 451, 116143 (2022).
Granot, Y. & Peer, D. Delivering the right message: challenges and opportunities in lipid nanoparticles-mediated modified mRNA therapeutics-An innate immune system standpoint. Semin. Immunol. 34, 68–77 (2017).
Moghimi, S. M. & Simberg, D. Pro-inflammatory concerns with lipid nanoparticles. Mol. Ther. 30, 2109–2110 (2022).
Martinez, D. R. et al. Chimeric spike mRNA vaccines protect against Sarbecovirus challenge in mice. Science 373, 991–998 (2021).
Arevalo, C. P. et al. A multivalent nucleoside-modified mRNA vaccine against all known influenza virus subtypes. Science 378, 899–904 (2022).
Matias, J. et al. mRNA vaccination of rabbits alters the fecundity, but not the attachment, of adult Ixodes scapularis. Sci. Rep. 14, 496 (2024).
Laczko, D. et al. A single immunization with nucleoside-modified mRNA vaccines elicits strong cellular and humoral immune responses against SARS-CoV-2 in mice. Immunity 53, 724–32.e7 (2020).
Egan, K. P. et al. A trivalent HSV-2 gC2, gD2, gE2 nucleoside-modified mRNA-LNP vaccine provides outstanding protection in mice against genital and non-genital HSV-1 infection, comparable to the same antigens derived from HSV-1. Viruses 15, 1483 (2023).
Lederer, K. et al. SARS-CoV-2 mRNA vaccines foster potent antigen-specific germinal center responses associated with neutralizing antibody generation. Immunity 53, 1281–95.e5 (2020).
Mirabelli, C. et al. Human norovirus efficiently replicates in differentiated 3D-human intestinal enteroids. J. Virol. 96, e0085522 (2022).
Ettayebi, K. et al. Replication of human noroviruses in stem cell-derived human enteroids. Science 353, 1387–1393 (2016).
Kramps, T. & Probst, J. Messenger RNA-based vaccines: progress, challenges, applications. Wiley Interdiscip. Rev. RNA 4, 737–749 (2013).
Lutz, J. et al. Unmodified mRNA in LNPs constitutes a competitive technology for prophylactic vaccines. NPJ Vaccines 2, 29 (2017).
Zhang, G., Tang, T., Chen, Y., Huang, X. & Liang, T. mRNA vaccines in disease prevention and treatment. Signal. Transduct. Target Ther. 8, 365 (2023).
Chivukula, S. et al. Development of multivalent mRNA vaccine candidates for seasonal or pandemic influenza. NPJ Vaccines 6, 153 (2021).
LoBue, A. D. et al. Multivalent norovirus vaccines induce strong mucosal and systemic blocking antibodies against multiple strains. Vaccine 24, 5220–5234 (2006).
Meo, S. A., Bukhari, I. A., Akram, J., Meo, A. S. & Klonoff, D. C. COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines. Eur. Rev. Med. Pharm. Sci. 25, 1663–1669 (2021).
Allen, J. D. & Ross, T. M. Bivalent H1 and H3 COBRA recombinant hemagglutinin vaccines elicit seroprotective antibodies against H1N1 and H3N2 influenza viruses from 2009 to 2019. J. Virol. 96, e0165221 (2022).
Burel, J. G., Apte, S. H., Groves, P. L., McCarthy, J. S. & Doolan, D. L. Polyfunctional and IFN-gamma monofunctional human CD4(+) T cell populations are molecularly distinct. JCI Insight 2, e87499 (2017).
Darrah, P. A. et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat. Med. 13, 843–850 (2007).
Parhiz, H., Atochina-Vasserman, E. N. & Weissman, D. mRNA-based therapeutics: looking beyond COVID-19 vaccines. Lancet 403, 1192–1204 (2024).
Freyn, A. W. et al. Antigen modifications improve nucleoside-modified mRNA-based influenza virus vaccines in mice. Mol. Ther. Methods Clin. Dev. 22, 84–95 (2021).
Baiersdorfer, M. et al. A facile method for the removal of dsRNA contaminant from in vitro-transcribed mRNA. Mol. Ther. Nucleic Acids 15, 26–35 (2019).
Maier, M. A. et al. Biodegradable lipids enabling rapidly eliminated lipid nanoparticles for systemic delivery of RNAi therapeutics. Mol. Ther. 21, 1570–1578 (2013).
Agnihothram, S. et al. Development of a broadly accessible Venezuelan Equine Encephalitis virus replicon particle vaccine platform. J. Virol. 92, e00027–18 (2018).
Debbink, K. et al. Human norovirus detection and production, quantification, and storage of virus-like particles. Curr. Protoc. Microbiol. 31, 15K 1 1–K 1 45 (2013).
Kolawole, A. O. et al. Astrovirus replication in human intestinal enteroids reveals multi-cellular tropism and an intricate host innate immune landscape. PLoS Pathog. 15, e1008057 (2019).
Acknowledgements
We thank Matt Collins from Emory University for providing human sera from patients. We gratefully acknowledge the technical and administrative support of Florin Tuluc and Jennifer B. Murray (CHOP Flow Cytometry Core) and the Microscopy Services Laboratory, Department of Pathology and Laboratory Medicine, University of North Carolina Chapel Hill. The authors would like to thank Houping Ni for technical assistance. The research was supported by BioNTech (DW, CW) and NIH R01 AI148260 (RSB).
Author information
Authors and Affiliations
Contributions
E.N.A-V. and D.W. conceived the concept. E.N.A-V., L.C.L., C.M., N.A.O., E.K.R., P.D.B-J., X.M.L., H.S., J.A.M., I.B., M.L.M., M.R.Z., S.R.M., B.L.M., Y.K.T., and C.E.W. performed the experiments and analyzed the data. E.N.A-V. drafted the manuscript and supervised all other activities; E.N.A-V., L.C.L., C.E.W., R.S.B., and D.W. reviewed and edited the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
In accordance with the University of Pennsylvania policies and procedures and our ethical obligations as researchers, we report that D.W. and ENA-V. are named on patents that describe the use of a nucleoside-modified mRNA vaccine for norovirus. D.W. is also named on a patent that described the use of nucleoside-modified mRNA as a platform to deliver therapeutic proteins and vaccines. We have disclosed those interests fully to the University of Pennsylvania, and we have in place an approved plan for managing any potential conflicts arising from the licensing of our patents. L.C.L. and R.S.B. hold patents on norovirus vaccine design and have ongoing collaborations with GIVax, VaxArt, MERK and Takeda Vaccines, and HilleVax that are unrelated and do not pose conflicts of interest with this report. RSB is a member of the advisory committee for VaxArt, Takeda, and Invivyd. B.L.M. and Y.K.T. are employees of Acuitas Therapeutics, a company focused on the development of lipid nanoparticulate nucleic acid delivery systems for therapeutic applications. All other authors, C.M., N.A.O, E.K.R., P.D.B-J., X.M-L., H.S., J.A.M., I.B., M.L.M., M.R.Z., S.R.M., B.L.M., Y.K.T., and C.E.W. declare no completing interests.
Inclusion and Diversity
We worked to ensure sex balance in the selection of non-human subjects. One or more of the authors of this paper self-identifies as a gender minority in their field of research. We support the inclusive, diverse, and equitable conduct of scientific research. While citing references scientifically relevant to this work, we also actively worked to promote gender balance in our reference list.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Atochina-Vasserman, E.N., Lindesmith, L.C., Mirabelli, C. et al. Bivalent norovirus mRNA vaccine elicits cellular and humoral responses protecting human enteroids from GII.4 infection. npj Vaccines 9, 182 (2024). https://doi.org/10.1038/s41541-024-00976-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41541-024-00976-z