Abstract
Genetically attenuated parasites (GAPs) that arrest during liver stage development have shown significant potential as malaria vaccines. Compared to Plasmodium falciparum GAPs that arrest after 24–48 h (early-arresters), parasites arresting after 6–7 days (late-arresters) have shown superior efficacy, highlighting the importance of liver stage immunity in promoting sterile protection. Here, we describe GAPs tested in humans and the pre-clinical research that led to their creation. We discuss safety and efficacy of existing GAPs with particular focus on their large-scale implementation as malaria vaccines.
Similar content being viewed by others
Main Text
Decades of malaria research have led to WHO recommendations for two malaria vaccines, RTS,S and R21, both based on the circumsporozoite protein (CSP), the most abundant protein on the surface of Plasmodium sporozoites1,2,3. The mechanism of action of these subunit vaccines is thought to be solely mediated by antibodies preventing sporozoite invasion of hepatocytes4. Hence, efficacy, defined as the percentage reduction of malaria incidence in vaccinated trial participants compared to unvaccinated controls5,6, of both RTS,S and R21, is variable (30–80%) and declines over time with waning antibody titres1,7,8. While approval of the two above-mentioned vaccines certainly represents an important step in the fight against malaria, it is insufficient to reach the WHO goal of reducing mortality by 90% in 20309.
Whole sporozoite (WSp) vaccine candidates consist of live Plasmodium falciparum (Pf) parasites that have been attenuated by either radiation, co-administration of chemoprophylaxis or genetic modification10. Protection, defined as the parasite’s ability to prevent the onset of malaria after exposure to unattenuated parasites, can be tested by means of controlled human malaria infections (CHMIs). During CHMI, live unattenuated malaria parasites are administered in a controlled setting to healthy participants11.
Though radiation-attenuated sporozoites (RAS) achieve high protection when administered in high doses to malaria-naive individuals12, in endemic areas their performance remains suboptimal13,14,15. Chloroquine chemoprophylaxis with Pf sporozoites (CPS) is the most potent WSp vaccination strategy to date, inducing 100% protection in malaria-naive individuals after mosquito bite or intravenous immunization16,17. However, the requirement for drugs and the administration of unattenuated parasites to healthy individuals makes this method unattainable for large-scale implementation.
Genetically attenuated parasites (GAPs) are another type of WSp vaccine where attenuation is achieved by gene deletion. Genetic modification allows the generation of a homogeneous parasite population that arrests at a targeted life cycle stage to induce an immune response18.
In the following, we will review the current global portfolio of GAP vaccine candidates which arrest during liver stage development with a particular focus on those that have or will soon be tested in clinical trials (Table 1, Table 2 and Table 3). Depending on which genes have been removed from the Plasmodium genome, currently existing liver stage GAPs fall into two distinct phenotypic categories: arresting either early or late during liver stage development. GAPs will be described based on this categorization and in order of creation, starting from early- and ending with late-arresting GAPs.
GAPs that did not progress to clinical testing
Double knock-out based on Δuis3Δuis4
The search for essential genes for Plasmodium liver stage development identified upregulated in infective sporozoites (uis) genes as initial targets (Table 1)19,20. These genes are upregulated in salivary gland sporozoites, yet are translationally repressed so that protein expression occurs upon infection of hepatocytes21,22. Single deletion P. berghei (Pb) mutants PbΔuis3 and PbΔuis4 showed attenuation during liver stage development19,20.
No breakthrough blood stage infections were observed for PbΔuis3 parasites when inoculated into rats in doses of up to 1 × 10⁵ sporozoites19. In C57BL/6 mice, immunization with 1 × 10⁴ PbΔuis3 plus two boosts of 2.5 × 10⁴ provided complete protection against challenge with 1 × 10⁴ wild-type (WT) parasites (Table 4). A reduced dose (twice 1 × 10⁴) still protected 70% of mice19. The P. yoelii (Py) analog also conferred 100% protection after three immunizations with 1 × 104 sporozoites (Table 4)23.
For PbΔuis4 parasites, breakthroughs were observed in 59% of C57BL/6 mice upon administration of 5 × 104 sporozoites (Table 5)20. A three-dose regimen of 1 × 10⁴ PbΔuis4 at 14-day intervals conferred 100% protection20. In Py, a threefold immunization of 1 × 10⁴ PyΔuis4 also induced protection (Table 4)23.
Due to concerns about PbΔuis4 breakthrough infections, a double knock-out PbΔuis3Δuis4 was created (Table 1)24. The parasites were completely attenuated during liver stage development. Immunizing C57BL/6 mice three times with 1 × 104 PbΔuis3Δuis4 parasites achieved sterile protection against challenge24, which was maintained even after rechallenge six months later (Table 4)24. Attempts to translate these findings to Pf failed because Pfuis3 proved refractory to deletion and the syntenic gene to uis4, Pfetramp10.3, was both functionally distinct, being expressed across multiple developmental stages, and refractory to deletion25,26. Hence, no studies have been conducted with uis3 or uis4 in the context of a Pf GAP and attempts shifted towards other candidates.
Single knockouts based on ΔFabB/F or ΔfabI
Further gene deletion candidates have been selected based on their essentiality during late liver stage development. Type II fatty acid synthesis (FASII) is crucial for Plasmodium development in hepatocytes due to the parasite’s exponential replication27,28,29. Hence, FASII-pathway members were selected for deletion, such as fabB/F and fabI (Table 1). Deletion of fabB/F in Py showed full attenuation during late liver stage development. When PyΔfabB/F were injected intravenously in BALB/c mice in doses up to 5 × 104 sporozoites, no breakthrough infections occurred. Immunization with PyΔfabB/F parasites (three times 1 × 104) achieved sterile protection27. Protection was maintained even when parasites were administered intradermally or subcutaneously, albeit at higher doses (three times 5 × 104 parasites) (Table 4)28. Immunization studies with PbΔfabI mutants were never performed as injection of 1 × 104 PbΔfabI parasites resulted in breakthrough infections in 31–100% of C57BL/6 mice (Table 5)29. The results of the PyΔFabB/F failed to translate to Pf as both PfΔfabI and PfΔfabB/F showed severe defects in sporozoite production and salivary gland invasion, making development of a PfGAP based on these genes impossible30.
Early-Arresting GAPs
Double knock-out parasites based on Δp36Δp52 (2KO)
Other studies focused on deletion of p52 (also referred to as p36p), which encodes a protein member of the 6-Cys domain family and plays an important role in the formation of the parasitophorous vacuole during liver stage development31. p52 is expressed in pre-erythrocytic stages and deletion is associated with reduced parasite infectivity31. Immunization with PbΔp52 induced up to 100% protection in mice (Table 4), yet in other experiments it led to breakthrough blood-stage infections in a subset of the animals (Table 5)32,33.
For this reason, p36 was selected as an additional candidate for deletion (Table 1). p36 belongs to the same 6-Cys domain family as p52, has an analogous function and is similarly expressed in sporozoites31. Simultaneous deletion of p36 and p52 in Py led to a fully attenuated phenotype, preventing breakthrough blood stage infections31,34. Additionally, threefold immunization with 1 × 104 PyΔp52Δp36 consistently protected against challenge in murine models (Table 4)31.
Given the safety and protection profile of PyΔp36Δp52 in mice, a double knock-out PfGAP, henceforth referred to as 2KO (PfΔp36Δp52), was generated35,36,37. PfΔp36Δp52 failed to infect livers of mice engrafted with human hepatocytes (SCID Alb-UpA humanized mice), indicating full attenuation36. These results prompted approval for clinical testing.
The administration of 2KO parasites for safety testing was the first-in-human study using GAPs and occurred in six malaria-naive participants, who were exposed to escalating numbers (five and then 200) of mosquito bites (Table 2)35. While the first exposure to five mosquito bites was well-tolerated, one participant developed malaria on day 12 after receiving 200 mosquito bites, putting a premature end to the study (Table 6 and Table 7). Sequencing revealed that the breakthrough was caused by the incomplete attenuation of the GAP rather than by reversal to WT35.
Triple knock-out parasites based on Δp36Δp52Δsap1 (3KO)
To reduce the likelihood of a breakthrough, a 3KO parasite, lacking sap1 in addition to p36 and p52, was generated (Table 1)38. sap1 (also referred to as slarp) encodes the sporozoite asparagine-rich protein 1 (SAP1) and is a post-transcriptional regulator involved in liver infectivity39. PyΔsap1 parasites are completely attenuated in highly susceptible BALB/cByJ mice in doses of up to 7.5 × 104 sporozoites38. Threefold immunization of BALB/c mice with 1 × 104 sporozoites resulted in 100% protection even when challenged 210 days after the last immunization and independently of whether unattenuated parasites where administered intravenously, by mosquito bite or as blood stages (Table 4)40. Sequential gene deletion of sap1 in the previously established PfΔp36Δp52 parasite was carried out to generate PfΔp36Δp52Δsap138. Injection with 1×106 of this GAP into humanized FRG huHep mice that had been supplemented with human erythrocytes on day 7 post-infection, did not cause a breakthrough blood stage infection38. These observations helped obtain approval for clinical testing of the PfΔp36Δp52Δsap1.
The first clinical trial with the Pf3KO GAP saw the administration of 150–200 mosquito bites to ten malaria-naive participants to test for safety (Table 2): none of the participants developed malaria in the 28-day follow-up period41. A further trial assessed the protection of Pf3KO-immunization against challenge: malaria-naive participants were immunized by either 3 × 200 or 5 × 200 Pf3KO mosquito bites, followed by CHMI four weeks later42. The protection rate was 50% in both groups after CHMI, demonstrating that additional immunizations beyond three times does not result in higher protection rates (Table 7). To investigate duration of protection, a second CHMI of protected participants was carried out six months after the last immunization. Protection was 15% in both groups after the second CHMI, indicating that immunity waned over time (Table 7)42.
Double knock-out based on Δb9Δslarp (GA1)
Another early-arresting GAP was based on genetic deletions of b9 and slarp (also referred to as sap1). b9 also encodes a member of the Plasmodium 6-Cys family (Table 1)39,43. Breakthrough blood stage infection was absent upon injection of Swiss or BALB/c mice with 5 × 104 single-deletion mutant PbΔb9. However, 20% of C57BL/6 mice became parasite-positive after inoculation with 5 × 104 PbΔb9 sporozoites (Table 5)43.
Based on these results, a double knock-out was generated, i.e. PbΔb9Δslarp. No breakthrough infection occurred after injection of 2 × 105 PbΔb9Δslarp sporozoites. Immunization of BALB/c mice with as few as 1 × 103 sporozoites induced protection against challenge with WT sporozoites. In C57BL/6 mice three immunizations with 1 × 104 sporozoites were required to induce protection, which was maintained for at least 180 days (Table 4)44.
Subsequently, a PfGAP, PfΔb9Δslarp, henceforth referred to as GA1, was generated. Survival within hepatocytes in vitro was completely abrogated by day 4 and parasites were absent at day 5 when assessed in immunodeficient SCID Alb-uPA mice engrafted with human hepatocytes44.
The promising results of the preclinical experiments warranted further assessment of GA1 in humans (Table 2). The biotechnology company Sanaria produced the GAP as an injectable cryopreserved product, referred to as PfSPZ-GA1. The PfSPZ-GA1 product was deemed safe, as no blood stage infections were observed after administering up to 9 × 105 sporozoites to humans45. Immunizations of malaria-naive participants were carried out three times at two-month intervals with either 4.5 × 105 or 9 × 105 sporozoites and followed by homologous challenge with five PfNF54-infected mosquito bites three weeks later46. Three of the 25 PfSPZ-GA1 immunized participants were protected against CHMI, one in the low-dose (4.5 × 105 sporozoites) and two in the high-dose (9 × 105 sporozoites) group (Table 7)45.
Late Arresting GAPs
It had been previously demonstrated that prolonging GAP survival in the liver could improve protection rates through increased antigenic exposure28. The knowledge that CPS, where the concomitant administration of live unattenuated sporozoites with antimalarial agents causes the parasites to die just after reaching the blood stage, achieved the highest protection (100%)16,17 of all WSp candidates to date corroborated this hypothesis by confirming the importance of prolonged antigenic exposure for protection in humans.
Single knock-out GAP based on Δmei2
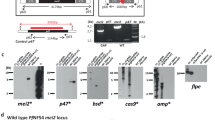
The gene meiosis inhibited 2 (mei2) was identified as a promising target for a late-arresting GAP (Table 1)47,48. mei2 encodes an RNA-binding protein expressed only during liver stage development and is involved in DNA segregation47. Removal of mei2 resulted in a late-arresting phenotype with parasites incapable of producing infectious merozoites47. A breakthrough rate of 10% and 13% was observed in BALB/cByJ mice after intravenous administration of 2 × 105 and 5 × 105 sporozoites, respectively49. Similar results were reported for Pb, with C57BL/6 mice becoming blood stage patent after administration of 2 × 105 sporozoites (Table 5)48. Immunizing BALB/c mice three times with 1 × 104 PyΔmei2 sporozoites resulted in 100% protection against challenge, showing promise as a late-arresting GAP (Table 4)47.
The PfΔmei2 was created by two different research groups and termed GA2 or late arresting replication competent 1 (LARC1) (Table 2)48,50. PfΔmei2 parasites exhibited normal infection of human hepatocytes in vitro for up to nine days48,50. When FRG huHep mice were infected with 1 × 106 GA2 or LARC1 sporozoites and supplemented with human erythrocytes, parasites were undetectable in blood from GAP-infected mice for up to 28 days post-infection48,50. The lack of parasitic genetic material in blood of GA2-infected mice and the absence of their growth in vitro suggests that either parasites are cleared in the liver stage therefore never entering the bloodstream or that aberrant, non-viable merozoites are released from the liver yet are incapable of infecting erythrocytes48,50. Based on these findings, GA2 was approved for clinical testing (Table 2).
In the first part of a phase 1/2a trial, which aimed to confirm the attenuation phenotype and safety profile of GA2, two sequential cohorts were exposed to 15 and then 50 GA2-infected mosquito bites (Table 2)51. No blood stage infections occurred, and adverse events were limited. The second part saw the three-fold immunization of ten participants with 50 GA2-infected mosquito bites at 28-day intervals with a homologous CHMI three weeks after the last immunization. Controls consisted of GA1- and placebo-immunized participants. Efficacy of GA2 was 89%, whereas only 13% of GA1- and none of the mock-immunized participants were protected (Table 7)51, demonstrating for the first time in humans that late-arresting GAPs are superior to early-arresting GAPs.
Next, GA2-immunized participants (n = 10) and placebo-exposed participants (n = 5) were exposed once to 45–55 mosquito bites and challenged in a CHMI with five 3D7-infected mosquitoes six weeks later. 90% of the GA2-exposed individuals were protected and did not develop blood stage parasitemia (Table 7), whilst all placebo-exposed participants became parasite-positive. This indicates that a single dose of GA2-parasites with 50 mosquito bites is sufficient to elicit 90% protection in a small cohort of individuals52.
Double knock-out based on Δmei2Δlinup (LARC2)
Since PyΔmei2 parasites were incompletely attenuated when administered in high doses to susceptible BALB/cByJ mice (Table 5)49, a double gene deletion mutant was generated in the form of PyΔmei2Δlinup (LARC2) (Table 1). LARC2 may have similar immunogenicity as GA2, but an enhanced safety profile. linup encodes the liver stage nuclear protein, which is a conserved protein localizing to the nucleus in liver stage Plasmodium parasites53. Single deletion mutant PyΔlinup showed that the gene is essential during late liver stage development53. Injection of susceptible BALB/cByJ with 2 × 105 or 5 × 105 double deletion mutants PyLARC2 parasites did not cause breakthrough infections54. PyLARC2 elicited durable protection with 90% of BALB/cJ mice being protected when immunized twice with 5 × 104 PyLARC2 sporozoites at one-month intervals and subsequently challenged six months later54. Administration of 5 × 104 cryopreserved sporozoites at two-week intervals was equally protective. Finally, animals immunized with PyLARC2 completely cleared the infection within 12 days upon injection of blood stage parasites (Table 4)54.
In the same study, PfLARC2 was described (Table 2)54. To assess the risk of breakthrough infections in vivo, FRG NOD huHep mice were injected with one million cryopreserved PfLARC2 or WT parasites and then supplemented with human erythrocytes six and seven days after infection. Blood collected from PfLARC2-injected mice was parasite-negative up to experiment termination, in contrast to blood cultures from WT-injected mice that became parasite-positive on days 1–3 after transition54. These promising results prompted the approval for clinical testing of aseptic cryopreserved PfSPZ-LARC2. Current planning of clinical trials is underway to start testing PfLARC2 in healthy participants as well as in individuals in endemic countries (Table 3).
Possible immunological mechanisms of protection
Both humoral and cellular immune responses play a role in protection against malaria, with CD4+ and CD8+ T-cells being important for establishing liver immunity55. Late-arresting parasites may be more efficient at activating and recruiting liver-resident CD8+ T-cells through increased antigenic exposure. Rodent work has shown that immunization with late-arresting PyΔFabB/F induced a higher absolute count of CD8+ T cells that also persisted for a longer period of time compared to early arresting RAS28. Analysis of immune responses from C57BL/6 mice that had been immunized twice with 5 × 104 late-arresting PyLARC2 sporozoites showed that CD8+ T-cells and specifically CD69hiCXCR6hi CD8+ TRM cells were significantly increased in immunized mice compared to controls54.
A correlation between CD8+ T-cells and protection against malaria has been reported in human studies as well17,56,57,58. Interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α) and interleukin-2 (IL-2) producing CD4+ T cells and, to a lesser extent, CD8+ T were increased after immunization with early-arresting 2KO parasites. These responses exhibited a dose-dependent pattern, with significant increases after 200 mosquito bites compared to 5 mosquito bites35. Similar findings were reported after immunization with GA1: the frequency of IFN-γ producing CD4+ and CD8+ T cells was significantly increased after three immunizations, yet responses did not correlate with protection45.
Immunization with GA2, whether it happened once or multiple times, resulted in an increased frequency of CD4+ and Vδ2 γδ T-cells with a memory phenotype, preferentially expressing IFN-γ, TNF-α and IL-251,52,59. However, circulating CD8+ and NK cells were not increased upon immunization44,45. The difficulties in detecting CD8+ T cells may not necessarily disprove their involvement in antimalarial immunity. As Pf-specific CD8+ T-cells may exhibit liver-resident phenotypes, they are undetectable in peripheral blood and therefore difficult to identify60. Liver-residency of immune cells would also explain the superior protection of late-arresting compared to early arresting GAPs28,51, as their prolonged permanence in the liver allows for the recruitment of tissue-resident CD8+ T cells28.
Humoral immune responses also contribute to protection against malaria55. Anti-CSP antibodies are frequently measured as they are directed against the most abundant protein on the sporozoite surface61. Indeed, all reviewed studies found an increase in CSP titres in humans after immunization41,42,45,51,52,59, which however did not correlate with protection42,45,51,52. Interestingly, serum from 3KO-immunized participants was able to block in vitro hepatocyte traversal and invasion35,36, suggesting inhibitory capacity of formed antibodies in immunized participants. This was confirmed in vivo by administering serum from participants to FRG huHep mice35. Subsequent challenge with WT parasites showed an 88% reduction of liver stage burden. The serum samples with the highest functional capacity in vivo had the lowest anti-CSP antibody titers, suggesting other sporozoite proteins may be important in hepatocyte invasion and subsequent development41.
Timing of GAP arrest may also play a role in antibody production: when humoral responses were compared between GA1- and GA2-immunized participants, more and different antigens were detected in the GA2-immunized group, indicating increased antigenic exposure during late liver stage development59. The exact role of antibodies in liver immunity remains to be elucidated, although they interfere with parasite movement, traversal and hepatocyte invasion55.
Discussion
This review presents the currently existing PfGAPs, including research that was instrumental to their generation. These novel malaria vaccine candidates should be further explored, and specifically questions regarding biosafety, large scale production and implementation need to be addressed.
Biosafety
Safety is key for the implementation of any GAP. Despite extensive preclinical testing, instances of incomplete attenuation have led to hesitancy for the large-scale use of GAPs35. Breakthrough infections in rodent malaria species may not always predict a similar phenotype in humans, as blood stage infections were detected in highly susceptible BALB/cByJ mice after administration of 2.5-5 × 105 PyΔmei2 sporozoites49, but not for PfΔmei2 in humanized mice and preliminary clinical trials48,50. Further testing in larger groups may be necessary to define the risk of breakthroughs in clinical settings. However, if the chance of such an event occurring is infinitesimal, it may not be detected in phase 2 and 3 clinical trials and still can never be proven to be absent. Hence, humanized mice injected with human erythrocytes remain the best approximation for safety predictions before clinical testing in humans.
Biosafety concerns are pertinent to the use of genetically modified organisms (GMOs) in medicine, with varying regulations worldwide. One concern is escape from the controlled conditions of the laboratory, potentially disrupting ecosystems. All GAPs created so far arrest prior to reaching the blood stage, making propagation past the liver and transmission to other humans and mosquitoes effectively impossible41,42,44,48,50. Furthermore, no transposable genetic elements have been identified in Plasmodium, meaning that DNA cannot spontaneously be exchanged between two parasites unless it happens during sexual reproduction, a process only occurring in the mosquito midgut62.
Another voiced concern is that of horizontal gene transfer from GMOs to host cells63. However, in the case of GAPs, vectors are not self-mobilizable and not present in the vaccine product. Removal of all exogenous genetic material used to guide the deletion in the GAP means that no new DNA is being introduced in the host18,44,48. GMO use in medicine is not novel, adenovirus-based vaccines for SARS-CoV-2 and Ebola are genetically engineered64. Given the proven success of such vaccines, GAPs share similar potential.
Protective capability
For any GAP to implemented, it must be safe but also confer high protection in malaria-endemic areas, where it may be hampered by vaccine hyporesponsiveness65. Most GAPs have achieved lower than desired efficacy and have therefore not proceeded to further clinical testing35,45,66. However, the advent of late-arresting GAPs (i.e. GA2 and LARC2), open the road to new scientific possibilities. The direct comparison of GA2 with GA1 unequivocally confirmed the importance of the late liver stage in achieving protection against malaria51 (Fig. 1). An important question is whether late-arresters maintain protection rates against heterologous challenge. If protection were lower than desired, one possibility would be to generate GAPs with different genetic backgrounds to match the parasites in circulation. This approach is limited, as the number of existing Pf strains is considerable and new ones continuously evolve67. Improving protection over time could require boosts, although the frequency and timing remains to be determined. Duration of protection also needs to be assessed. In this regard results from CPS studies conducted in malaria-naive individuals show potential with protection lasting up to two years after immunization68. Despite the many open interrogatives, the superior protection of GA2 compared to previously developed early-arresting GAPs is promising and makes late liver stage arresting parasites potential candidates for further vaccine development and large-scale implementation.
Implementation of GAPs
Another important question pertains to the feasibility of introducing GAPs as a large-scale vaccination strategy. The first potential obstacle is the cost of manufacturing of cryopreserved sporozoites, as it still requires rearing of aseptic mosquitoes, manual dissection of salivary glands and extraction of sporozoites. However, dissection-independent production of sporozoites69 and in vitro culturing of sporozoites has been reported70, allowing a complete bypass of the dissection step. Storage of sporozoites at −150 °C could pose logistical challenges in endemic areas, but liquid nitrogen systems already exist in Africa and require minimal infrastructure. The additional advantage of a liquid nitrogen-based cold chain is that, except for the generation of liquid nitrogen, it is independent of electricity supply71. While logistical hurdles exist, recent technological advances offer viable solutions.
Future Directions: Remaining Problems and Potential Solutions
Despite the promise of efficacy and deliverability of GAPs, some questions remain open. The route of administration of GAPs is one such discussion point. Intravenous injection of sporozoites is more efficacious as large proportions of sporozoites reach the liver72, yet intradermal and/or intramuscular administration would be more practical73. Intradermal or intramuscular injections could also provide the advantage of early immune system activation74,75, as skin-draining lymph nodes play an important role in the initiation of the immune response against malaria76.
Another question is whether approved malaria vaccines may act synergistically or antagonistically with GAP vaccine candidates. If part of the African population is immunized with RTS,S and R21, could efficacy of GAPs be decreased due to pre-existing immunity? The two subunit-based malaria vaccines induce mostly humoral immunity targeted to CSP. However, since antibodies do not distinguish between GAPs or WT parasites, GAPs may be intercepted before reaching the liver where important immune responses occur. Further clinical studies involving GAPs in combination with RTS,S or R21 are necessary to understand the interactions between the existing vaccines.
Conclusion
In conclusion, GAPs have shown to be safe, well-tolerated and highly efficacious. What emerges from this review is that any future research should focus on late-arresting GAPs, as they have achieved the highest protection rates in malaria-naive individuals (Table 3, Fig. 1). While the results of phase 1/2 clinical trials are promising, some aspects remain to be investigated: efficacy of all late-arresting GAPs must be confirmed in larger trials, against heterologous challenge and in pre-exposed populations; duration of protection needs to be examined also. Despite these questions being unanswered to date, this field of research is just beginning to evolve. Many of the current hurdles may be easily overcome soon, making GAPs increasingly promising vaccine candidates against malaria.
Data availability
No datasets were generated or analysed during the current study.
References
Tinto, H. et al. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet 386, 31–45 (2015).
Organization, W. H. Malaria vaccines (RTS,S and R21), https://www.who.int/news-room/questions-and-answers/item/q-a-on-rts-s-malaria-vaccine (2024).
Birkett, A., Miller, R. S. & Soisson, L. A. The Importance of Exercising Caution When Comparing Results from Malaria Vaccines Administered on the EPI Schedule and on a Seasonal Schedule. Am. J. Trop. Med Hyg. 107, 1356–1356 (2022).
Kurtovic, L. et al. Antibody mechanisms of protection against malaria in RTS,S-vaccinated children: a post-hoc serological analysis of phase 2 trial. Lancet Microbe 5, 100898 (2024).
Shim, E. & Galvani, A. P. Distinguishing vaccine efficacy and effectiveness. Vaccine 30, 6700–6705 (2012).
Vaccine efficacy, effectiveness and protection, https://www.who.int/news-room/feature-stories/detail/vaccine-efficacy-effectiveness-and-protection (2025).
Datoo, M. S. et al. Safety and efficacy of malaria vaccine candidate R21/Matrix-M in African children: a multicentre, double-blind, randomised, phase 3 trial. Lancet 403, 533–544 (2024).
Datoo, M. S. et al. Efficacy of a low-dose candidate malaria vaccine, R21 in adjuvant Matrix-M, with seasonal administration to children in Burkina Faso: a randomised controlled trial. Lancet 397, 1809–1818 (2021).
WHO. (2021).
Moita, D. & Prudencio, M. Whole-sporozoite malaria vaccines: where we are, where we are going. EMBO Mol. Med 16, 2279–2289 (2024).
Stanisic, D. I., McCarthy, J. S. & Good, M. F. Controlled Human Malaria Infection: Applications, Advances, and Challenges. Infect Immun 86 https://doi.org/10.1128/IAI.00479-17 (2018).
Epstein, J. E. et al. Protection against Plasmodium falciparum malaria by PfSPZ Vaccine. JCI Insight 2, 1–14 (2017).
Jongo, S. A. et al. Increase of Dose Associated With Decrease in Protection Against Controlled Human Malaria Infection by PfSPZ Vaccine in Tanzanian Adults. Clin. Infect. Dis. 71, 2849–2857 (2020).
Jongo, S. A. et al. Multi-Dose Priming Regimens of PfSPZ Vaccine: Safety and Efficacy against Controlled Human Malaria Infection in Equatoguinean Adults. Am. J. Trop. Med Hyg. 106, 1215–1226 (2022).
Jongo, S. A. et al. Safety, Immunogenicity, and Protective Efficacy against Controlled Human Malaria Infection of Plasmodium falciparum Sporozoite Vaccine in Tanzanian Adults. Am. J. Trop. Med Hyg. 99, 338–349 (2018).
Roestenberg, M. et al. Protection against a Malaria Challenge by Sporozoite Inoculation. N. Engl. J. Med 361, 468–477 (2009).
Mordmuller, B. et al. Sterile protection against human malaria by chemoattenuated PfSPZ vaccine. Nature 542, 445–449 (2017).
Khan, S. M., Janse, C. J., Kappe, S. H. & Mikolajczak, S. A. Genetic engineering of attenuated malaria parasites for vaccination. Curr. Opin. Biotechnol. 23, 908–916 (2012).
Mueller, A. K., Labaied, M., Kappe, S. H. I. & Matuschewski, K. Genetically modified parasites as a protective experimental malaria vaccine. Nature 433, 164–167 (2005).
Mueller, A. K. et al. Liver stage developmental arrest by depletion of a protein at the parasite-host interface. P Natl Acad. Sci. USA 102, 3022–3027 (2005).
Kaiser, K., Matuschewski, K., Camargo, N., Ross, J. & Kappe, S. H. Differential transcriptome profiling identifies Plasmodium genes encoding pre-erythrocytic stage-specific proteins. Mol. Microbiol 51, 1221–1232 (2004).
Lindner, S. E. et al. Transcriptomics and proteomics reveal two waves of translational repression during the maturation of malaria parasite sporozoites. Nat. Commun. 10, 4964 (2019).
Tarun, A. S. et al. Protracted sterile protection with Plasmodium yoelii pre-erythrocytic genetically attenuated parasite malaria vaccines is independent of significant liver-stage persistence and is mediated by CD8+ T cells. J. Infect. Dis. 196, 608–616 (2007).
Jobe, O. et al. Genetically-attenuated liver-stages induce sterile protracted protection that is mediated by MHC class I-dependent IFN-γ producing CD8+ T cells. Am. J. Trop. Med Hyg. 77, 90–90 (2007).
Mackellar, D. C. et al. Plasmodium falciparum PF10_0164 (ETRAMP10.3) is an essential parasitophorous vacuole and exported protein in blood stages. Eukaryot. Cell 9, 784–794 (2010).
Goswami, D. et al. A Plasmodium falciparum ATP-binding cassette transporter is essential for liver stage entry into schizogony. Iscience 25, 104224 (2022).
Vaughan, A. M. et al. Type II fatty acid synthesis is essential only for malaria parasite late liver stage development. Cell Microbiol 11, 506–520 (2009).
Butler, N. S. et al. Superior antimalarial immunity after vaccination with late liver stage-arresting genetically attenuated parasites. Cell Host Microbe 9, 451–462 (2011).
Yu, M. et al. The fatty acid biosynthesis enzyme FabI plays a key role in the development of liver-stage malarial parasites. Cell Host Microbe 4, 567–578 (2008).
van Schaijk, B. C. et al. Type II fatty acid biosynthesis is essential for Plasmodium falciparum sporozoite development in the midgut of Anopheles mosquitoes. Eukaryot. Cell 13, 550–559 (2014).
Labaied, M. et al. Plasmodium yoelii sporozoites with simultaneous deletion of P52 and P36 are completely attenuated and confer sterile immunity against infection. Infect. Immun. 75, 3758–3768 (2007).
van Schaijk, B. C. et al. Gene disruption of Plasmodium falciparum p52 results in attenuation of malaria liver stage development in cultured primary human hepatocytes. PLoS One 3, e3549 (2008).
van Dijk, M. R. et al. Genetically attenuated, P36p-deficient malarial sporozoites induce protective immunity and apoptosis of infected liver cells. Proc. Natl Acad. Sci. USA 102, 12194–12199 (2005).
Annoura, T. et al. Assessing the adequacy of attenuation of genetically modified malaria parasite vaccine candidates. Vaccine 30, 2662–2670 (2012).
Spring, M. et al. First-in-human evaluation of genetically attenuated Plasmodium falciparum sporozoites administered by bite of Anopheles mosquitoes to adult volunteers. Vaccine 31, 4975–4983 (2013).
VanBuskirk, K. M. et al. Preerythrocytic, live-attenuated Plasmodium falciparum vaccine candidates by design. Proc. Natl Acad. Sci. USA 106, 13004–13009 (2009).
O’Neill, M. T., Phuong, T., Healer, J., Richard, D. & Cowman, A. F. Gene deletion from Plasmodium falciparum using FLP and Cre recombinases: implications for applied site-specific recombination. Int J. Parasitol. 41, 117–123 (2011).
Mikolajczak, S. A. et al. A next-generation genetically attenuated Plasmodium falciparum parasite created by triple gene deletion. Mol. Ther. 22, 1707–1715 (2014).
Aly, A. S. I., Lindner, S. E., MacKellar, D. C., Peng, X. X. & Kappe, S. H. I. SAP1 is a critical post-transcriptional regulator of infectivity in malaria parasite sporozoite stages. Mol. Microbiol 79, 929–939 (2011).
Aly, A. S. I. et al. Targeted deletion of SAP1 abolishes the expression of infectivity factors necessary for successful malaria parasite liver infection. Mol. Microbiol 69, 152–163 (2008).
Kublin, J. G. et al. Complete attenuation of genetically engineered Plasmodium falciparum sporozoites in human subjects. Sci Transl Med 9 https://doi.org/10.1126/scitranslmed.aad9099 (2017).
Murphy, S. C. et al. A genetically engineered Plasmodium falciparum parasite vaccine provides protection from controlled human malaria infection. Sci Transl Med 14 https://doi.org/10.1126/scitranslmed.abn9709 (2022).
Annoura, T. et al. Two Plasmodium 6-Cys family-related proteins have distinct and critical roles in liver-stage development. Faseb J. 28, 2158–2170 (2014).
van Schaijk, B. C. et al. A genetically attenuated malaria vaccine candidate based on P. falciparum b9/slarp gene-deficient sporozoites. Elife 3, 1–18 (2014).
Roestenberg, M. et al. A double-blind, placebo-controlled phase 1/2a trial of the genetically attenuated malaria vaccine PfSPZ-GA1. Sci. Transl. Med 12, 1–9 (2020).
Langenberg, M. C. C., Dekkers, O. M. & Roestenberg, M. Are placebo controls necessary in controlled human infection trials for vaccines?. Lancet Infect. Dis. 20, e69–e74 (2020).
Dankwa, D. A., Davis, M. J., Kappe, S. H. I. & Vaughan, A. M. A Plasmodium yoelii Mei2-Like RNA Binding Protein Is Essential for Completion of Liver Stage Schizogony. Infect. Immun. 84, 1336–1345 (2016).
Franke-Fayard, B. et al. Creation and preclinical evaluation of genetically attenuated malaria parasites arresting growth late in the liver. Npj Vaccines 7, 1–17 (2022).
Vaughan, A. M. et al. A Plasmodium Parasite with Complete Late Liver Stage Arrest Protects against Preerythrocytic and Erythrocytic Stage Infection in Mice. Infect. Immun. 86, 1–18 (2018).
Goswami, D. et al. A replication-competent late liver stage-attenuated human malaria parasite. JCI Insight 5, 1–19 (2020).
Lamers, O. A. C. et al. Safety and Efficacy of Immunization with a Late-Liver-Stage Attenuated Malaria Parasite. N. Engl. J. Med 391, 1913–1923 (2024).
Roozen, G. V. T. et al. Single immunization with genetically attenuated Pf∆mei2 (GA2) parasites by mosquito bite in controlled human malaria infection: a placebo-controlled randomized trial. Nat. Med 31, 218–222 (2025).
Goswami, D. et al. A conserved nuclear protein is critical for late liver stage development. Commun Biol 7 https://doi.org/10.1038/s42003-024-07063-y (2024).
Goswami, D. et al. A replication competent Plasmodium falciparum parasite completely attenuated by dual gene deletion. EMBO Mol. Med 16, 723–754 (2024).
Cockburn, I. A. & Seder, R. A. Malaria prevention: from immunological concepts to effective vaccines and protective antibodies. Nat. Immunol. 19, 1199–1211 (2018).
Mura, M. et al. Immunoprofiling Identifies Functional B and T Cell Subsets Induced by an Attenuated Whole Parasite Malaria Vaccine as Correlates of Sterile Immunity. Vaccines (Basel) 10 https://doi.org/10.3390/vaccines10010124 (2022).
Murphy, S. C. et al. PfSPZ-CVac efficacy against malaria increases from 0% to 75% when administered in the absence of erythrocyte stage parasitemia: A randomized, placebo-controlled trial with controlled human malaria infection. PLoS Pathog. 17, e1009594 (2021).
Bijker, E. M. et al. Cytotoxic Markers Associate With Protection Against Malaria in Human Volunteers Immunized With Plasmodium falciparum Sporozoites. J. Infect. Dis. 210, 1605–1615 (2014).
Colstrup, E. et al. Correlative humoral and cellular immunity to genetically attenuated malaria parasites in humans. iScience 28, 112589 (2025).
Rogers, K. J., Vijay, R. & Butler, N. S. Anti-malarial humoral immunity: the long and short of it. Microbes Infect. 4-5, 104807 (2021).
Singer, M., Kanatani, S., Castillo, S. G., Frischknecht, F. & Sinnis, P. The Plasmodium circumsporozoite protein. Trends Parasitol. 40, 1124–1134 (2024).
Guttery, D. S., Zeeshan, M., Holder, A. A., Tromer, E. C. & Tewari, R. Meiosis in Plasmodium: how does it work?. Trends Parasitol. 39, 812–821 (2023).
Midtvedt, T. Antibiotic resistance and genetically modified plants. Microb Ecol Health Dis 25 https://doi.org/10.3402/mehd.v25.25918 (2014).
Sakurai, F., Tachibana, M. & Mizuguchi, H. Adenovirus vector-based vaccine for infectious diseases. Drug Metab. Pharmacokinet. 42, 100432 (2022).
van Dorst, M. et al. Immunological factors linked to geographical variation in vaccine responses. Nat Rev Immunol https://doi.org/10.1038/s41577-023-00941-2 (2023).
Reuling, I. J. et al. An open-label phase 1/2a trial of a genetically modified rodent malaria parasite for immunization against Plasmodium falciparum malaria. Sci Transl Med 12 https://doi.org/10.1126/scitranslmed.aay2 (2020).
Rich, S. M. & Ayala, F. J. Population structure and recent evolution of Plasmodium falciparum. Proc. Natl Acad. Sci. USA 97, 6994–7001 (2000).
Roestenberg, M. et al. Long-term protection against malaria after experimental sporozoite inoculation: an open-label follow-up study. Lancet 377, 1770–1776 (2011).
Blight, J. et al. Dissection-independent production of Plasmodium sporozoites from whole mosquitoes. Life Sci Alliance 4 https://doi.org/10.26508/lsa.202101094 (2021).
Eappen, A. G. et al. In vitro production of infectious Plasmodium falciparum sporozoites. Nature 612, 534–539 (2022).
Richie, T. L. et al. Sporozoite immunization: Innovative Translational Science to Support the Fight against malaria. Expert Rev. Vaccines 22, 964–1007 (2023).
Ishizuka, A. S. et al. Protection against malaria at 1 year and immune correlates following PfSPZ vaccination. Nat. Med 22, 614–623 (2016).
Organization, W. H. (ed World Health Organization) (Geneva, 2022).
Pohl, K. & Cockburn, I. A. Innate immunity to malaria: The good, the bad and the unknown. Front Immunol. 13, 914598 (2022).
Sinnis, P. & Zavala, F. The skin: where malaria infection and the host immune response begin. Semin Immunopathol. 34, 787–792 (2012).
Chakravarty, S. et al. CD8+ T lymphocytes protective against malaria liver stages are primed in skin-draining lymph nodes. Nat. Med 13, 1035–1041 (2007).
Acknowledgements
This research has been funded by the Dutch research Council ZonMw under a VIDI grant (No 09150172010035).
Author information
Authors and Affiliations
Contributions
O.A.C.L. conceived and conceptualized the idea and wrote the original draft of the manuscript. J.M.M.K. supervised, revised, edited and significantly contributed to the content and original draft of the manuscript. B.M.D.F. and M.R. revised and edited the draft and final manuscript. M.R. acquired the funding. All authors have read, revised and approved the submitted manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lamers, O., Franke-Fayard, B., Roestenberg, M. et al. The path from early- to late-liver stage arresting genetically attenuated parasites as a malaria vaccination strategy. npj Vaccines 10, 218 (2025). https://doi.org/10.1038/s41541-025-01265-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41541-025-01265-z