Abstract
The removal of organic pollutants from water by advanced oxidation has been successfully achieved using iron–biochar (Fe–BC)-based material. By embedding iron particles on the biochar, the resulting Fe–BC composite possesses enhanced surface functionalities that promote electron transfer and generate reactive oxygen species (ROS). Characterizations using various analytical techniques confirm the successful formation of the Fe-based biochar and its improved catalytic features. Batch degradation experiments have demonstrated that Fe–BC exhibits significantly higher performance than unmodified biochar in the breakdown of organic contaminants, primarily through advanced oxidation processes (AOPs) facilitated by iron-induced radical (SO4•−, •OH, O2•−) formation, non-radical ROS (1O2), and electron transfer pathways. Finally, the advantages of Fe-BC in the catalytic degradation of organic pollutants are summarized, highlighting potential limitations and prompting further research to optimize Fe–BC performance and expand Fe–BC applicability.
Similar content being viewed by others
Introduction
The contamination of aquatic environments with organic pollutants is a pressing global issue that impacts ecosystems and human health. Sources such as industrial discharge, agricultural runoff, and improper waste disposal contribute to the increasing presence of hazardous substances in water bodies1,2. These contaminants, which include pharmaceuticals, pesticides, and industrial chemicals, pose complex challenges due to their persistence and bioaccumulation potential3,4. Remediating these pollutants is critical for maintaining water quality and ensuring safe water for future generations.
In recent years, SO4•−-based advanced oxidation processes (AOPs), particularly those involving peroxymonosulfate (PMS) and peroxydisulfate (PDS) which feature a reactive O–O bond in their asymmetric structure that can be activated easily, has received increasing attention for water and wastewater treatment5,6. Carbon-based materials are potential activator for PMS activation due to their environmentally friendly properties and adjustable electronic structure. The electronic structure of biochar, including surface functional groups, defect sites, and conductive characteristics, may influence catalytic activity. The presence of graphitic carbon structures with various degrees of disorder affects electron mobility and the accessibility of active sites7. The presence of oxygen-containing functional groups, including hydroxyl, carbonyl, and carboxyl, may increase the surface activity and redox properties of biochar8. Furthermore, heteroatom doping can create confined electronic states, thus enhancing catalytic efficiency3. Among carbon-based activators, biochar (BC) has the potential for persulfate (PS, such as PMS and PDS) activation activity, and biochar from waste biomass is an economical and promising material9. However, the bottleneck and challenge of BC on PMS/PDS activation still exist. It is difficult to achieve generation of reactive oxygen species (ROS) quickly and efficiently due to the high energy barrier of O−O bond in PMS. Moreover, the activation performance and service life of biochar is limited because of the finite active sites and weak electron transfer efficiency10.
Transitional metals, such as iron (Fe), play a crucial role in catalytic activities in AOPs for the degradation of organic pollutants in the environment11,12. The presence of iron, both naturally occurring and chemically doping in BC, enhances these catalytic reactions significantly. Iron is naturally present in BC, derived from the biomass feedstock, can catalyze certain chemical reactions. However, the concentration and availability of naturally occurring iron may not always be optimal for effective catalytic action. Intentionally adding Fe to BC, known as Fe-doping, increases the catalytic activity significantly11. This process involves incorporating Fe species directly into the biochar matrix, thereby enhancing its ability to generate ROS crucial for the degradation of pollutants11 (Fig. S1). Single-atom Fe incorporated biochar, characterized by each Fe atom being exposed to the reaction medium without Fe–Fe interactions, could also be one of the efficient materials for environmental remediation through the activation of PMS and PDS via electron transfer mechanism13,14,15. Moreover, a single-atom catalyst maximizes atom utilization through the total dispersion of all metal atoms on carbon and might enhance the exposure of the catalytic active site16. In the presence of H2O2 and suitable pH conditions, iron ions in biochar can engage in Fenton-like reactions, producing hydroxyl (•OH) radicals17,18. These radicals are central to degrading organic pollutants such as bisphenol A19, sulfamethoxazole (SMX)20, ciprofloxacin (CIP)11 in water, and polycyclic aromatic hydrocarbons (PAHs)21 in soil. Additionally, the high-valent iron active species (Fe(V) and Fe(IV)) exhibit enhanced selectivity. They can break down target contaminants even in substantial quantities of competing ions and natural organic matter. The high-valent iron exhibits an extended lifespan and targets contaminants as the primary non-free radical component, except for SO3•−22,23. The high-valent oxo species (Fe(IV)=O) might enable rapid cleavage of chemical bonds in target pollutants while minimizing scavenging effects often associated with conventional Fenton-based processes22,24. The interactions between Fe species and biochar, which acts as a shuttle, facilitate prolonged catalytic activity by promoting the continuous regeneration of Fe(IV)=O and extending its stability in neutral and mildly acidic conditions22.
Fe-modification increases the surface area and porosity of biochar in some cases, which improves its adsorption capacity and facilitates better contact between the catalyst and the pollutants11. Iron-enhanced biochar typically exhibits greater stability under various environmental conditions and can be reused in multiple cycles of pollutant degradation, making it more cost-effective and environmentally friendly25. The concern associated with Fe-doped biochar (Fe–BC) is not derived from the inherent toxicity of either iron or biochar alone, which are generally non-toxic, but rather from the potential environmental implications of iron leaching3,11. This phenomenon could disrupt local redox equilibria or precipitate undesired chemical reactions, leading to secondary environmental contamination. Such risks underscore the necessity of managing Fe–BC applications under environmentally relevant conditions to prevent adverse outcomes while exploiting its beneficial properties for contaminant remediation. Solution pH, iron oxidation state, biochar surface functionality and porosity, oxidant type and concentration, and coexisting irons are key factors influencing iron leaching26,27. Therefore, it is necessary to seek an ideal and straightforward method to simultaneously achieve a low activation barrier and rapid generation of reactive oxygen species (ROS). Moreover, doping with stabilizing agents like graphene/CNTs, encapsulation techniques, ligand functionalization of biochar, incorporating bimetallic or multi-metal strategies can be utilized to minimize iron leaching14. Therefore, it is necessary to seek appropriate catalyst to simultaneously achieve a low activation barrier and rapid generation of reactive oxygen species (ROS).
The synthesis and characterization methods of Fe-based catalysts in AOPs, their applications in activating various oxidants, and the mechanisms for organic pollutant removal within these systems have not been systematically reported in the existing peer-reviewed literature. Furthermore, systematic documentation of future research directions for improving the catalytic performance of these materials remains limited. Therefore, based on previous studies (Table 1), the objective of this paper is to bridge these gaps by providing:
-
1.
Comprehensive characterization techniques to confirm the integration and distribution of iron within the biochar matrix.
-
2.
Evaluation of the potential of Fe–BC in catalyzing surface functionalities, promoting electron transfer reactions, and generating reactive species to degrade organic pollutants in water.
-
3.
A review of the effectiveness of Fe–BC in the removal of organic contaminants, including per- and polyfluoroalkyl substances (PFAS), antibiotics, pesticides, and industrial chemicals from water, comparing its performance to that of unmodified biochar.
-
4.
Scrutiny of the long-term stability and reusability of Fe–BC over multiple cycles of use to determine its practical feasibility and economic viability for continuous operation in water treatment systems.
-
5.
Analysis of the potential for integrating Fe–BC into current water treatment infrastructures, assessing its economic viability, efficiency, and sustainability as a solution to mitigate the adverse impact of organic pollutants in aquatic environments.
-
6.
A review of the broad applications of Fe–BC in environmental settings, along with their limitations and prospects, which is highly recommended.
Organic contaminants of concern
The contamination of aquatic environments with organic contamination poses a critical challenge to environmental safety and public health due to their persistence, bioaccumulation, and often unknown ecological and health effects. This discussion investigates key organic contaminants—pesticides, pharmaceuticals (antibiotics), and industrial chemicals—their characterization, and their behavior in groundwater, wastewater, and surface waters (Table 2). These contaminants have been the focus of extensive research for decades4,28,29.
Pesticides are widely used in agriculture to control pests and disease vectors, but their extensive application leads to environmental dispersion through runoff, leaching, and drift into nearby water bodies30,31. Organochlorines, organophosphates, and carbamates are the most common types, each varying in persistence and toxicity4. These chemicals are of particular concern due to their potential to disrupt aquatic ecosystems and accumulate in the food chain, resulting in long-term ecological damage and health risks32.
Pharmaceuticals, particularly antibiotics, enter aquatic environments through various pathways including pharmaceutical manufacturing waste, hospital discharge, and improper disposal of medication33,34. These contaminants are especially worrying due to their role in promoting antibiotic resistance among aquatic and terrestrial bacteria. The environmental impact of antibiotics is complex, as they can remain active long after entering the environment, affecting microbial communities, and possibly leading to the development of resistant pathogens35.
Industrial chemicals encompass a broad category including compounds such as polychlorinated biphenyls (PCBs)36, phenolic compounds17, polycyclic aromatic hydrocarbon (PAH)37, dioxins38, and phthalates39, among others. These substances are used in various industrial processes and consumer products from which they can leach out and enter water systems through improper waste handling or accidental spills. Their high toxicity levels and resistance to natural degradation make them particularly harmful, necessitating rigorous management and remediation strategies.
The characterization of organic contaminants is critical for understanding their chemical nature, behavior, and impact in aquatic environments. Advanced analytical techniques such as liquid chromatography–mass spectrometry (LC–MS/MS), gas chromatography–mass spectrometry (GC–MS), high-performance liquid chromatography (HPLC), visible–ultraviolet (Vis-UV) spectroscopy, and nuclear magnetic resonance (NMR) spectroscopy are commonly used for the identification and quantification of organic contaminates. These methods provide crucial data on molecular structure, concentrations, and chemical properties, informing both risk assessment and the development of targeted removal strategies. Environmental scientists also employ bioassays and ecotoxicological tests to determine the biological effects of contaminants on various organisms40. These tests help in assessing the potential ecological risks and guiding regulatory frameworks aimed at minimizing environmental exposure and harm.
Organic contaminants manifest differently across various types of water bodies: (1) Groundwater, often perceived as a relatively pristine water source, can become contaminated through the infiltration of pollutants from overlying soils and surface waters41. Pesticides and industrial chemicals, due to their persistent nature, are particularly prone to accumulating in groundwater, posing risks to drinking water supplies. (2) Wastewater treatment plants are not typically designed to remove EOCs efficiently, resulting in pharmaceuticals and personal care products being routinely detected in treated effluents. Advanced treatment processes such as membrane filtration, activated carbon adsorption, and advanced oxidation are being researched and incrementally implemented to address these shortcomings34. (3) Surface waters are the most immediate recipients of EOCs through runoff, direct discharges, and atmospheric deposition. The concentration and types of contaminants in surface waters vary significantly and are influenced by local industrial activities, agricultural practices, and urban runoff patterns. The dynamic nature of surface waters complicates the behavior and fate of these contaminants, requiring comprehensive monitoring and adaptive management strategies42.
Effective remediation technologies and the key role of Fe
Adsorption and advanced oxidation processes
Remediation of organic contaminants is crucial, as even trace environmental concentrations (including ppt levels for PFAS) can have significant biological, ecological, and health impacts, such as increased cancer risk, reproductive disorders, immune system alterations, neurobehavioral impairment, endocrine disruption, genotoxicity, and birth defects (UN Environmental Program, 2017). Additionally, the coexistence of other hazardous compounds and their synergistic interactions can influence remediation processes, often making simultaneous removal challenging43.
Remediation can occur via physical (adsorption), chemical (catalytic AOPs), and biological (bioremediation) methods. Adsorption plays an integral role in pollution control due to its robustness in operation, and simplicity. This process involves the physical binding of contaminants onto material surfaces, such as biochar and Fe-based biochar3,44. While adsorption effectively reduces contaminant levels, it seldom achieves complete removal, especially for hydrophobic organic contaminants with lower molecular weights that are resistant to many water remediation methods. Adsorption captures pollutants on the adsorbent surface through but does not degrade the contaminant. The sorption capacities listed in Table 4 provide critical insight into the effectiveness of various iron–biochar composites (Fe–BC) in adsorbing different contaminants from water. The significant variation in surface area among the different Fe–BC materials, which is a crucial factor in catalytic performance (Table 4). A larger surface area generally provides more active sites for catalytic reactions, which can enhance the degradation rates of pollutants. For example, the magnetic mesoporous carbon (MNPs@C) demonstrates a substantial surface area, which is likely to contribute to its higher catalytic activity45, especially in complex water matrices where accessibility to active sites becomes critical.
Catalytic AOPs employ highly reactive species, typically radicals, to break down complex organic pollutants into smaller molecules. These processes often require catalysts to initiate and sustain the reaction, with common oxidants including hydrogen peroxide (H₂O₂), proxymonosulfate (HSO5−) and peroxydisulfate (S2O8²−)34. When combined with adsorption (pre-adsorption + AOP), the overall process becomes more effective as the adsorbent concentrates’ contaminants, enhancing their accessibility to oxidants46. Therefore, AOPs along or combined with adsorption are often preferred over adsorption alone, particularly for organic contaminants that require degradation.
Iron-based materials, such as zero-valent iron (ZVI) and iron-incorporated biochar (Fe–BC), are widely used as catalysts in AOPs due to their performance and cost-effectiveness27. This review focuses on AOPs, particularly the role of Fe-modified biochar (Fe–BC) as a catalyst. Fe–BC enhances AOP efficiency by facilitating the generation of reactive oxygen species (ROS), which accelerates pollutant degradation. Fe–BC effectiveness in persulfate activation is attributed to its defect-rich surface, oxygen functional groups, and excellent electron transfer properties. A comparison study of BC and Fe–BC is discussed in Table S3.
In AOPs, the iron in Fe–BC functions as a Fenton reagent, generating free radicals from hydrogen peroxide (H₂O₂) that break down organic contaminants. Under UV or visible light, Fe³⁺ can be photoreduced to Fe²⁺, further enhancing radical generation and improving photo-Fenton efficiency compared to conventional Fenton reactions26 Additionally, iron sludge generated in Fenton-based processes can often be managed or reused, improving sustainability efforts47. Iron also improves AOP performance when combined with electrochemical oxidation (Electro-Fenton), ozone-based AOPs (Fe²⁺ catalyzes O₃ decomposition), and UV/TiO₂ systems (Fe³⁺ enhances photogenerated radicals)48. The ability of Fe–BC to provide a sustained release of iron ions ensures continuous oxidant activation, leading to more efficient contaminant degradation compared to non-iron-based methods.
Integrating adsorption with AOPs leverages the strengths of both methods. In a combined pre-adsorption and AOP treatment: (1) Pollutants are concentrated on the adsorbent, reducing the volume of water that needs to be treated actively and enhancing the efficiency of the subsequent oxidative step. (2) Following adsorption, the adsorbent with bound pollutants is treated with an AOP. This step not only removes the contaminants from the adsorbent but also breaks them down chemically. (3) While adsorption alone only concentrates pollutants, the integration with AOPs ensures their breakdown into harmless compounds. This is particularly effective in scenarios where the pollutants are highly resistant to biodegradation or are in large volumes.
Synthesis methods for iron-based biochar
The iron-incorporated biochar (Fe–BC) can be synthesized using various methods, such as pyrolysis, co-reduction, co-precipitation, hydrothermal, ball milling, etc.49. The various synthesis methods for iron-based biochar are categorized and summarized in Table 3. In pyrolysis, the temperature and duration affect the Fe-based biochar’s composition. At 300–500 °C, the Fe salts are converted to Fe3O4, and above 600 °C, they reduced to Fe0 nanoparticles. The transformation of Fe salts into Fe3O4 and FeO follows thermochemical reduction kinetics, where activation energy and reaction rates influence the phase transitions. When temperature exceeds 1000 °C, the carbonaceous compounds and Fe salts in the Fe-based biochar are converted to graphene structure and Fe0/Fe3C, respectively50. Fewer polar functional groups (C–O) and higher aromatic structure (C–C) resulted when the temperature increased from 350 to 700 °C51. Higher temperatures (above 700 °C) lead to Fe particle growth and biochar graphitization, impacting Fe dispersion and biochar porosity, which can be explained using diffusion and reaction-controlled kinetics. Additionally, Fe and S loading onto biochar (increased Fe–O groups and S intermediates), enhancing the contaminant’s removal52.
The physicochemical properties of Fe–BC, which are pivotal in the removal of organic contaminants, are systematically summarized in Table 4. The co-precipitation method allows for controlling the Fe species, biochar matrix, reactivity, and mobility of Fe-based biochar products. These factors can be regulated by adjusting the factors like pH, temperature, and the Fe2+/Fe3+ ratio. Generally, the Fe-based biochar synthesized by co-precipitation was predominantly magnetic iron oxides such as Fe3O4 and Fe2O353. Also, magnetic nanoparticles (MNPs), such as CoFe2O4 and CuFe2O4, which possess high catalytic activity, lower toxicity, and are cost-efficient54. The magnetic mesoporous carbon (MNPs@C) can be recovered from the supernatant by using external magnet due to its high magnetic response45. The co-reduction predominantly produces zero-valent iron species (ZVIs) by reducing Fe species by potassium borohydride (KBH4) or sodium borohydride (NaBH4). These ZVIs are powerful reductants for removing inorganic contaminants55. Moreover, the introduction of carboxymethyl cellulose (CMC) stabilized the nZVI56. The nZVI loading onto biochar matrix also decreased the surface area from 491.15 to 382.58 m2 g−1, indicating its dispersion on the biochar surface57. Higher pyrolysis temperatures (800/900 °C) typically result in a higher degree of carbonization, leading to increased porosity and specific surface area, which enhance the adsorption capacity of the biochar3. The temperature also affects the size and distribution of iron particles embedded within the biochar matrix. Smaller iron particles with a more uniform distribution are often more catalytically active due to increased surface area and better interaction with pollutants. Carrying out pyrolysis in inert or reductive atmospheres can affect the chemical structure of the biochar. An inert atmosphere tends to preserve more organic functional groups, while a reductive atmosphere can enhance the reduction of iron oxides to more reactive forms3,11. The presence of a reductive atmosphere during pyrolysis can lead to the formation of zero-valent iron or lower oxide states, which are highly effective for Fenton-like reactions in pollutant degradation11.
The kinetic behavior of ZVI formation under different reduction conditions can provide insights into the surface chemistry and long-term stability of Fe-based biochar. Compared to pyrolysis, co-precipitation, and co-reduction, the hydrothermal carbonization method is more efficient, cost-effective, less energy consumption, simple58. Moreover, ball milling is a recent, simple and efficient technique for preparing the Fe-based biochars59. By combining ball milling and pyrolysis at 700 °C, the functional groups of the formed Fe0–biochar were exposed, enhancing the Cr(VI) sorption60. However, the ball milling technology is limited by the high-energy consumption61.
Fe-based biochar can be modified using various techniques such as cation introduction followed by pyrolysis, hydrothermal technique, etc. The most common technique, pyrolysis, is simple and low-cost. The Fe@HC-800 developed at 800 °C showed an impressive performance in removing phenol and reused for three cycles62. The Fe/Zn@PB9 synthesized at 900 °C, was effective in activating PDS for imidacloprid removal63.The successful embedded-Fe in the magnetic corn stalk biochar was indicated by the presence of Fe–O, Fe2C, FeFe2O4, C=O, C–O, C=C, and C–C bonds, where micropores and mesopores were formed64.
Yang et al.65 developed a Fe3O4-based biochar using hydrothermal technique to create specific cavities using a template molecule, known as molecularly imprinted polymer (MIP). high specific surface area, functional groups, and specific imprinted cavities governed its large adsorption potential. In this study, the successful imprinting was identified by the presence of ester C–O, O=C–O, C=O, –CH2, and –CH3 bonds55,66, where the pyridinic N, pyrrolic N, and graphitic N responsible for generating the ROS were also observed67. The imprinted layer was located on the biochar’s surface as indicated by the weaker diffraction peaks of and γ-Fe2O3 and Fe3O4. The bonding between Fe2O3, Fe3O4, and biochar surface was strongly indicated by the Fe–O bonds68. This Fe3O4-based biochar was effective in removing salicylic acid through sorption and advanced oxidation mechanism. In terms of physical/chemical properties, the highly stable MIP is excellent for contaminant removal using adsorption technique due to its selectivity and high affinity. Moreover, the high selectivity coefficient of 11.67 enhanced the sorption selectivity in both single and binary systems. A study by Meischl et al. 69 also showed that the molecular imprinting on the polymer sorbent increased the adsorption capacity of acetylsalicylic acid by 7.79-fold compared to unmodified polymer sorbent. The chemisorption, such as π–π and hydrogen-bond interactions, and monolayer-multilayer adsorption dominated the adsorption mechanism of salicylic onto the Fe3O4-based biochar due to the specific pores of imprinted graphite structure in the Fe3O4-based biochar and the presence of aromatic rings70,71.
Iron-based biochar materials in AOPs
In AOPs, oxidants such as persulfate (including peroxymonosulfate, PMS and peroxydisulfate, PDS), hydrogen peroxide (H₂O₂), and periodate play a crucial role in generating highly reactive species that degrade organic pollutants. As shown in Table 5, the different Fe–BC materials are organized and explained in terms of their roles and how they work in AOPs. The key function of these oxidants in AOPs is their ability to generate radicals upon activation. These radicals are highly reactive and attack organic pollutants, breaking chemical bonds and leading to the degradation or mineralization of the contaminants. In many AOPs, catalysts (such as Fe²⁺, Fe-modified biochar, or UV light) are used to activate these oxidants, increasing the production of radicals, and enhancing the degradation process. The generated radicals have strong oxidation potentials, enabling them to break down a wide range of organic pollutants, including those that are resistant to conventional treatment methods.
In the case of AOPs technique, the Fe3O4-based biochar produces SO4•− and •OH from the PS activation as the predominant radical species. In comparison, previous AOPs study showed that the MIP imprinted in the metal-organic frameworks in the form of Fe–MOF-74/MIP was effective in degrading dimethyl phthalate within 120 min66. However, the Fe3O4-based biochar imprinted by the MIP was superior in term of its selectivity towards contaminants compared to other MIP-modified biochar, such as biochar functionalized with VBTAC-MIP72, GO-MWCNTs/Fe3O4/SiO2-MIP73, Fe3O4@BC-MIP74, MIPs@Fe3O475, Fe3O4@Chitosan-MIP76, VTTS-MGO@mSiO2@MIP77, and carbon microsphere-MIP78. The outstanding selectivity of Fe3O4-based biochar imprinted by MIP was proven during the removal of salicylic acid and benzoic acid in the binary system. The salicylic acid was adsorbed more selectively than the benzoic acid by the Fe3O4-based biochar imprinted by MIP due to its selective recognition sites, increasing the degradation efficiency by SO4•−.
Zhang et al. (2019)79 reported that Fe0, Fe2+, and Fe3+ present on the Fe-doped graphitic biochar (Fe@GBC)’s surface played an important role for PS activation, where SO4•− and •OH were both responsible for 17β-estradiol (E2) degradation. Rong, X. et al. (2019)80 synthesized magnetic biochar (γ-Fe2O3@BC) by doping nitrogen atom and iron oxides into biochar. The γ-Fe2O3@BC owned more active sites such as OFGs. The intensity of ROS from PDS activation by γ-Fe2O3@BC was higher than those produced by PDS activation by pristine biochar. The γ-Fe2O3@BC/PDS system was effective for BPA removal within 20 min, where 90% of BPA was mineralized.
Initial pH affects the degradation efficiency where acidic condition enhances the degradation more than the neutral and alkali condition. This is because the SO4•− and •OH reacts with OH− under alkaline conditions instead of the organic contaminants. At the same time, the Fe2+ reacts with OH− and biochar to form precipitate, inhibiting the oxidant activation. This condition caused the decrease of degradation efficiency81. The presence of anions and humic acid also have implications to the degradation efficiency depending on the organic contaminants. Liu et al. (2020)82 reported that HCO3− and Cl− inhibited the peroxodisulfate (PDS) activation, but HCO3−, Cl−, and PO43− facilitated the triclosan (TCS) removal76. Meanwhile, the study by Wang, J. et al. (2017)83 reported that HCO3−, CO32−, H2PO4−, SO42−, NO3− suppressed the AO7 removal, but Cl− and Br− enhance the removal of AO7 dye. This is due to the SO4•− and •OH adsorption and reduction by anions on the catalyst’s surface84. Moreover, the anions also occupied the active site of the biochar, leading to the decrease of its catalytic activity85. The humic acid can also react with SO4•− and •OH, and be adsorbed on the biochar’s active surface, inhibiting the biochar’s ability to activate the oxidant82.
Fe-doped biochar study by Wang et al. (2020)86 reported the excellence performance of magnetic Fe/N co-doped carbon catalysts (UBC-x) for phenolic contaminant removal. The N and Fe loaded onto the biochar enhanced the catalytic activities producing the ROS, where the 1O2 was the dominant ROS for complete phenolic contaminant removal. On the graphite surface, the successful N doping was indicated by the disappearance of O–C=O band and the appearance of N=C–N2, where the C–O/C–N portion increased in the biochar. Meanwhile, the successful Fe doping was indicated by the decrease of Fe3+ to Fe2+ ratio, where the dominant Fe species was Fe3O4. Another Fe,N-codoped biochar study by87 also reported the success peroxodisulfate (PDS) activation for tetracycline hydrochloride (TCH) removal. The doping product, known as Fe/N-PABC, consisted of graphite N, pyridine N, Fe0, and Fe2+, where the functional groups were Fe-O, C–N, C=O attributed to carbonyl group stretching, C–O, –OH, and P-containing functional groups such as P–O and P=O88. also reported the synthesis of N,S,P co-doped biochar. The successful doping was shown by the graphitic-C/C=C/C–C, C=O/C=N, C–OH/C–O–C/C–N/C–P, and shaking up of π–π* bonds in the biochar, pyrrole N (400.2 eV), graphitic N, and pyridine N bonds for N doping, C–SOx–C and C–S–C for S doping, and P–C and P–O bonds for P doping.
The Fe-CB600 synthesized from maize straw and cob biochar at temperature 600 °C was modified using liquid phase reduction89. The reduction using NaBH4 created a well-dispersed nZVI with high degradation reactivity and large surface area of biochar, where the biochar’s porous structure prevented the irreversible aggregation. The ZVI was the major crystal phase of iron species as indicated by the presence of α-Fe0. Meanwhile, the biochar composite contained carbonyl/carboxyl (C=O) with the CO– stretching vibration modes, hydroxyl groups (OH–), C–C, CO=, and Fe–O, confirming the Fe attachment to the biochar’s surface facilitated by oxygen-containing groups.
Kumar et al. (2017)90 invented the g-C3N4/FeVO4/Fe@NH2-biochar and g-C3N4/FeVO4 by combining the graphitic carbon nitride (g-C3N4), a cost-effective metal free polymer, with semiconductor materials, such as FeVO4, BiOBr, and Bi2WO6. Both g-C3N4/FeVO4/Fe@NH2-biochar and g-C3N4/FeVO4 have superior physicochemical characteristic, including stability, high specific surface area, nanosheet porous structure, broad solar spectrum, and satisfying photocatalytic activity. The successful modification was indicated by the presence of C–NH, N–C, aromatic rings (N–C = N), and amino group (N–H). The Fe-based biochars prepared by various methods have their own characteristics and benefits. For example, the Fe-based biochar synthesized by co-precipitation sorbed the arsenic better than that by pyrolysis91,92. In another study, the Fe-based biochar prepared by hydrothermal carbonization has more OFGs than that by pyrolysis method93. Cai et al. (2019)94 reported that the magnetic carbonaceous adsorbent (MCA) synthesized by hydrothermal carbonization method showed better performance for Cr(VI) sorption (142.86 mg g–1) than that by the reductive codeposition (58.82 mg g–1)95, coprecipitation (23.85 ± 0.23 mg g–1)96, and impregnation-pyrolysis (43.122 mg g–1)97. The study conducted by80 compared the effectivity of oxidant activation where the γ-Fe2O3@BC for PDS activation produced more ROS than the γ-Fe2O3@BC for PS activation and pristine biochar in activating PDS. In the case of reusability, the UBC-x showed excellent stability and performance as a catalyst for PMS activation, where it was used for five recycling runs for BPA degradation86. Similarly, the MNPs@C composites also showed an outstanding efficiency, reaching five recycling runs in degrading ACT45. After five runs, the MNPs@C’s ability decreased due to decrease of active surface sites, mass loss, blocking of pores by the byproducts, competition between contaminant and byproduct interacting with the MNPs@C’s active sites, and consumption of reactive oxidizing species by the organic matters. However, the low removal efficiency of ROS-based oxidation can produce toxic byproducts67. Besides, the ROS are easily quenched by the presence of the other substances in the reaction system65. The ROS lifetimes are also short.
Characterization of Fe–BC surface functionalities
Various characterization techniques can be employed to identify the chemical composition and morphology of Fe–BC composite materials. The X-ray diffraction (XRD), Brunauer–Emmett–Teller (BET), elemental analysis (EA), energy dispersive spectrometry (EDS), Fourier transform infrared spectrometry (FTIR), scanning electron microscopy (SEM), transmission electron microscopy (TEM), Raman spectroscopy, solid-state nuclear magnetic resonance (NMR), X-ray photoelectron spectroscopy (XPS), magnetic hysteresis loop analysis, and many more analytical techniques could be employed to characterize Fe–BC. Several methods for extracting Fe could also be used to find out the types of Fe present in the Fe–BC sample, such as the soluble, reducible, and extractable Fe98.
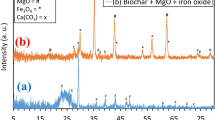
The crystal structure of the BC and Fe–BC composites can be analyzed using XRD patterns. The (111) and (100) crystallographic planes of carbon are represented by the 26.6° and 43.8° diffraction peaks in the BC sample, respectively, in the XRD pattern. The peak intensity of carbon will become weaker and disrupt the internal structure of BC during the loading of Fe-species. The new peak can be appeared in the Fe–BC sample at 44.5°, 38.7°, 35.6° and 51.9° due to the presence of Fe, Fe2O3, Fe3O4, and Fe3C, respectively99 (Fig. 1a). Moreover, the different Fe species content in the Fe–BC composites can be assessed using quantitative X-ray diffraction (Q-XRD), where samples are mixed with 20–25 wt% of CaF2 as the internal standard100 and typical Fe minerals as quality control.
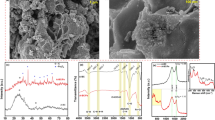
a–d XRD, N2 adsorption-desorption isotherms for BET surface area and pore distribution measurement, TEM images and TEM image with lattice spacing of Fe–BC (adapted from the ref. 99). e–g SEM image with EDS, FTIR and XPS survey spectra (adapted from the ref. 109). h Fe Magnetic hysteresis loops of Fe–BC (adapted from the ref. 119).
Surface area, pore size, and pore volume of the Fe–BC sample could be determined by using the BET analyzer by the multilayer adsorption capacity under different nitrogen partial pressures. The total pore volume and the surface area of Fe–BC could be lower than BC were lower in BET analysis49,52,57,101,102. This could be attributed to the presence of Fe species/compounds in the pores of the BC sample and the accumulation of BC flakes around these compounds99. However, some researchers also showed that the co-precipitation of FeCl3 and granular BC can effectively increase the surface area and pore volume of Fe–BC than the BC (Fig. 1b)103.
The microscopic morphology, structural features, pore characteristics, and pore distribution of prepared Fe–BC can be examined by SEM and TEM (Fig. 1c–e). The smoothness and conspicuous and well-developed pore structures of Fe–BC compared to BC might be attributed to the chemical bonding of Fe materials with carbonaceous substances on the outer surfaces and internal pore structures of Fe–BC104,105,106,107. TEM images confirm the conjecture and dispersed granules at a low magnification of 500 nm (Fig. 1c). In the TEM images, the lattice spacing of (320) at 0.215 nm, (110) at, 0.244 nm, and (311) at 0.250 nm for Fe2O3, Fe and Fe3O4, respectively99 (Fig. 1d). Moreover, the lattice fringe of Fe3C (221) at 0.304 nm and fringe of FeS at 0.294 and 0.263 nm corresponds to the (100) and (101) crystal planes, respectively108. The Fe in the prepared Fe–BC could also be shown by the elemental mapping pictures or SEM/TEM EDS (Fig. 1e).
The introduction of Fe in BC could also be analyzed by Raman spectroscopy by measuring the disordered structure of the Fe–BC sample. The Raman results might indicate that Fe–BC is more distorted than BC, and low graphitization is also shown105.
The loading of Fe in BC could confirm by the FTIR results, the bonding of Fe with surface oxygen functional groups. The FTIR peak ~557 or 530 cm−1 could be shown for the bond Fe–OH/Fe–O109 (Fig. 1f), confirming the presence of amorphous Fe-oxides or high content of Fe. Moreover, the FTIR peaks could also be shown in the regions of 1150–1050 and 620–580 cm−1, respectively, for the Fe–O bond of Fe3O4105.
The survey XPS spectra could confirm the presence of Fe in Fe–BC. The characteristic peaks for Fe at binding energy of 711 and 724 eV could be due to the Fe(III) and Fe(II)109,110 (Fig. 1g). Moreover, the deconvoluted peak could also confirm the presence of different Fe-species. The EA could be used to measure the carbon (C), hydrogen (H), nitrogen (N), oxygen (O) and iron (Fe) content in the Fe–BC by mass balance. The aromaticity and polarity of BC can be evaluated by the calculations of mole ratios of O/C, H/C, (O + N)/C111. Moreover, the polarity and hydrophilic properties of Fe–BC is higher due to the higher ratio of O/C and (O + N)/C (0.27 and 0.39, respectively for BC) and (0.35 and 0.48, respectively, for Fe–BC)112.
In Fe-BC composites, C/Fe mass ratio has a great impact on the structure and pore distribution113. The increase of Fe content may lead to excessive accumulation of Fe-species on the BC surface, or block the active sites, and the shape could be irregular. However, it is difficult for BC to provide more adsorption sites114. Therefore, it is important to measure the appropriate C/Fe mass ratio which could optimize the properties of BC113. Moreover, the TGA data could also be used to determine the Fe-loaded percentage in the Fe–BC sample115.
The content of aromatic and phenolic carbon groups in Fe–BC could be measured by the use of quantitative 13C NMR spectra105. Moreover, Boehm titration is widely used to quantify acidic and basic functional groups such as phenolic, carboxylic, and lactonic functional groups present on the surface of Fe–BC105,116.
In real wastewater treatment, the magnetic properties of Fe–BC are necessary for showing recyclability and avoiding secondary contamination49,117,118,119. The saturation magnetization (Ms) response could be great in the presence of a permanent magnet. For example, the Fe–BC catalyst was 40.6 emu/g, and oxidizing Fe–BC showed a great magnetic response in the presence of a permanent magnet (Fig. 1h)119. Overall, the above characterization could be used to confirm the synthesis of Fe–BC for Fe loaded on the surface of BC.
Fe–BC composites have already proven their effectiveness and affordability as catalysts for the elimination of redox-sensitive organic contaminants49,117,118,120. The loading of Fe species in BC would change properties like surface area, pore volume, pore distribution, etc. Masud et al. (2023) reported the removal % of CIP improved from 63.37% in the KBC800/PMS system to 97.48% in the Fe-KBC800/PMS system, confirming that PMS activation was promoted by Fe-doping. Moreover, the addition of Fe species can provide BC with a high electron-transfer capability and high reduction reactivity. Consequently, the mechanisms might also be dependent upon the properties of the contaminants. Even though the removal mechanism of the contaminants is complicated with any catalyst, especially by Fe–BC composites118,121.
There are several species of Fe present in Fe-BC composites, such as Feo, Fe2+, Fe3+, and Fe3O449. For example, the presence of Fe0, Fe2+, and S2+, in Fe–BC improves the removal of tetrabromobisphenol A from wastewater122. Fe0-coated BC can also be removed from nitro explosives and halogenated phenols from wastewater123. Here, the active species Fe0 acted as an electron transfer mediator in the reductive transformation and the surface of the Fe–BC composites49.
In the catalytic process, Fe–BC materials may also activate the oxidants. The loading of Fe species in BC improves the catalytic activity by creating defects, improve functionality and Fe acted as an electron transfer mediator between oxidant and contaminants124. Moreover, Fe0 could be converted to Fe2+ by interaction with water and oxygen113. Fe–BC composites degraded the aniline in the presence of H2O2 by generating ROS by Fe3O449. Therefore, Fe–BC composites can be employed as very effective and economical catalysts for eliminating redox-sensitive contaminants.
The incorporation of any metal with the Fe–BC can improve the cyclic stability and reactivity of the materials by receiving electrons from the metal. Furthermore, the PMS catalytic system may also be enhanced by the interaction between the metal and BC substrate125. Metal interaction may lower the redox potentials of the metal redox pair and enhance the rate of PMS activations because of the synergetic effects between bimetal encapsulation in BC125. For example, the process of removing TC by using FeMn@BC to activate PMS and improve electron transport between PMS and TC. The surface reaction can occur in the following manners:
Mechanisms for contaminant removal using Fe–BC
Mechanisms involved in the contaminant removal
The Fe-based biochar was used for catalyzing the removal of various organic contaminants, such as 4-nonylphenol, 2,4,6-trichlorophenol, and decabromodiphenyl ether, etc. via adsorption, reduction, advanced oxidation, and degradation etc. The removal mechanism using AOPs was initiated by the attachment of organic contaminants on the surface of inner pores of Fe-based biochar (known as “adsorption”), followed by the degradation by the ROS11.
The incorporation of Fe into the biochar (Fe–BC) might improve the efficiency of organic contaminant remediation in various ways like catalysis, adsorption, etc.99,109. The adsorption is the preliminary process involved in the contaminant removal attributed to the Fe-based biochar’s large specific surface area, where the mechanisms include hydrogen bonds, hydrophobic interaction, electrostatic interaction, and π–π interaction126,127. In the BPA removal using AOPs, the BPA was initially sorbed onto the Fe-contained sludge biochar followed by the complete degradation by the activated PDS, where the Fe-contained sludge biochar alone adsorbed 22.1% of BPA128. Also, the adsorbents, Fe0@C-700 and Fe0@C-700/PS alone adsorbed 11.8% and 24.2% of 4-chlorophenol, respectively, prior to complete degradation55. These findings suggest the important contribution of adsorption prior to the AOPs.
The synergistic mechanism of iron–carbon in Fe–BC plays a crucial role in catalyzing organic contaminant removal through multiple pathways. The iron species in Fe–BC facilitate the generation of reactive oxygen species (ROS), including hydroxyl radicals (•OH) and sulfate radicals (SO4•–), which enhance pollutant degradation. The Fe–BC structure provides a stable and sustainable release of Fe ions, ensuring continuous activation of oxidants such as peroxymonosulfate (PMS) and persulfate (PDS). Furthermore, the carbon matrix of Fe–BC acts as an electron shuttle, promoting electron transfer and improving the redox cycling between Fe(II) and Fe(III), which is essential for Fenton and photo-Fenton reactions129. The defect-rich surface and abundant oxygen functional groups in biochar enhance catalytic efficiency by promoting electron transfer and surface interactions130. Additionally, Fe–BC contributes to nonradical pathways through singlet oxygen (1O2) production, enabling selective degradation of contaminants with minimal scavenger interference131. These synergistic effects make Fe–BC an effective and sustainable catalyst for AOPs, surpassing the efficiency of non-iron-based methods.
The degradation mechanism is generally divided into two pathways: radical and nonradical pathways. In the case of the radical pathway, persistent free radicals (PFRs) generated in the pyrolysis process, such as semiquinone-type radicals, phenol-type radicals, and cyclopentadienyl-type radicals, contribute to the SO4•− and •OH formation132. Furthermore, the PDS can be adsorbed on the aromatic graphite-like surface of biochar, creating metastable complex. On the other hand, the quinoid C = O group, phenolic –OH groups, and defects of the biochar catalyze the PDS to form O2•−, and 1O2 which are responsible for nonradical degradation pathway133. The biochar pyrolyzed at 700 °C (BC/700) was also effective in activating PS for phenanthrene (PHE) removal134. The BC/700 facilitated the Fe3+ reduction to Fe2+. The SO4•− and •OH dominated the radical species pathway, whereas the nonradical pathway contributed 56.3% of the removal efficiency. Moreover, Wang et al. (2022)63 showed that radical pathway involving SO4•− and •OH, and nonradical pathway with 1O2 as a dominant ROS facilitated in degrading imidacloprid using Fe/Zn@PB9. Similar to the Fe/Zn@PB9 synthesized at 900 °C, MCB-900 created radical and nonradical pathways during the degradation of norfloxacin (NOF) using PDS, involving SO4•−, •OH, and 1O264. Initially, Fe2+ reacted with S2O82– to produce SO42– and SO4•−, causing the Fe2+ decrease and the Fe3+ increase. Then, the produced SO4•− generated the formation of •OH and H+. The dissolved oxygen played an important role in the O2•− formation, where the O2•− contributed to the 1O2 production. Besides, the C=O functional group facilitated the electron extraction from the surrounding carbon, donating the electron to break the O–O bond of PDS.
The organic and inorganic contaminant reduction in the Fe-based biochar systems occurred due to the redox properties of Fe0, Fe(II), and S(II). Several studies have reported the crucial roles of Fe and S in the Fe-based biochar for enhancing the reduction. Tetrabromobisphenol A (TBBPA) was reduced to bisphenol A and monobromobisphenol A135. Another study, 2,4-Dinitrotoluene (DNT) and 2,4-dichlorophenol (DCP) was successfully reduced using Fe0-containing biochar136. Meanwhile, nitrobenzene (NB) was reduced to aniline by using S-nZVI@HCl–biochar, where the O–C–O, C–O–C, and C–O were responsible for NB reduction56. The Fe-based biochar can also reduce Cr(VI) and trichloroethylene (TCE) due to the Fe-based biochar’s aliphatic carbon and carboxyl groups106,137. These reduction reactions were facilitated by the PFRs and Fe species, where the PFRs reduced Fe(III) to Fe(II) to facilitate electron transfer during the contaminant reduction138. Besides, the study by Li et al. (2017) reported that the reduction of hydrogen in 1,1,1-TCA molecule using ZVI-containing biochar was the main mechanism of its removal139.
The degradation of organic contaminants represents the ultimate step in their removal from the environment. Various ROS are produced from the oxidants such as PMS, PDS, O2, and H2O2, by the Fe-based biochar activation, where the activation process involves the PFRs and OFGs interaction on the Fe-based biochar and inside the defects of Fe-based biochar followed by the electron transfer between the targeted contaminant and oxidant124. These ROS are responsible for the electron transfer during degradation, mainly involving photocatalytic and Fenton-like reactions61,140. The H2O2 activated by magnetic biochar composites prepared at temperatures 300, 400, and 500 °C produces •OH and O2•−, where the C–OH functional group on the biochar’s surface played an important role in H2O2 activation for MNZ and SMX degradation51,141. Besides C–OH, the Fe3O4, Fe2O3, and FeOOH, were also found on the biochar’s surface142. The Fe3O4 was useful for activating H2O2, generating ROS for aniline degradation143. Meanwhile, the combination of Fe2O3 and TiO2 in the biochar increased the removal efficiency of dye contaminants144. This H2O2 activation by Fe2O3/TiO2–biochar involves the initial adsorption due to the sufficient pores and channels and catalytic reaction attributed to the π–π interaction, ionic bonding, and H-bonding between Fe2O3/TiO2–biochar and dye contaminant144. Besides, the H2O2 can be activated through the decomposition reaction by PFRs, enhancing the one-electron transfer to produce •OH145.
In the case of TCE contaminant removal using nZVI-biochar in the form of Fe-CB600, the adsorption mechanism was involved89. The adsorption isotherms showed type IV isotherms with an H4 hysteresis loop, indicating the presence of biochar mesopores and micropores. For degradation, the SO4•−, •OH, O2•−, and 1O2 were produced from the PMS activation. The well-dispersed Fe0 in the form of nZVI on the biochar’s porous was crucial in increasing the number of active sites, facilitating the activation. In this study, O2•− and 1O2 mediated by the C=O functional group and Fe0 played important roles for TCE de-chlorination. During the PMS activation, the Fe0 was oxidized to Fe3+, where the O–O in the PMS was broken down generating the oxygen-containing groups on the surface of Fe-CB600. After that, the C=O groups were reduced to C–O on the Fe-CB600’s surface, creating O2•− and 1O2. Another nZVI study performed by Hao et al. (2024) reported that the nanoscale zero-valent iron composite (nZVI@NBC) was successful in activating PDS for 2,4-DCP removal146. The pyridine nitrogen and graphitic nitrogen played crucial roles in producing 1O2, leading to the nonradical pathway as major mechanism of 2,4-DCP degradation.
The UBC-x studied by Wang et al. (2020c) was successful in BPA removal by activating the PMS86. The PMS was best activated in the alkali condition for BPA mineralization compared to the neutral and acidic conditions. The 1O2 dominated the degradation reaction attributed to more N–G role than the N–C–Fe during the PMS activation. In comparison to the UBC-x, the BPA removal using PMS activated by Fe–BC-700 consisted of two main mechanisms; 53% of degradation and 47% of adsorption, where the degradation mechanism was contributed by 30% of nonradical pathway and 23% of SO4•− pathway147. Similarly, the Fe/N-PABC, one of the Fe,N-codoped biochar, facilitated the PDS activation for creating the 1O2 nonradical pathway in degrading TCH87. In this study, the Fe/N-PABC combined with PDS contributed as an intermediate oxide for the electron transfer pathway. The electrons were transferred from the C to the N-containing functional group, followed by the transfer to the Fe3C group, where this reaction is irreversible. Another Fe, N-co-doped biochar study by Dai et al. (2020) also reported the success of PDS activation for tetracycline hydrochloride (TCH) removal70. The doping product, known as Fe/N-PABC, consisted of graphite N, pyridine N, Fe0, and Fe2+.
Besides oxidant precursor, the Fe-based biochar can be combined with the photocatalytic reaction. The g-C3N4/FeVO4/Fe@NH2–biochar and g-C3N4/FeVO4 introduced by Kumar et al. (2017) successfully removed methylparaben (MeP) and 2-cholrophenol (2-CP) through the combination of adsorption and photocatalytic mechanisms90. The amino group in the g-C3N4/FeVO4/Fe@NH2–biochar played a role in increasing the magnetic separation and adsorption capacity. In the case of photocatalytic, photo-degradation and photo-ozonation dominated the degradation. In particular, the Fe@NH2–biochar enhanced the MeP removal due to its adsorption capacity and characteristics as electron acceptors. Particularly, the semiconductor characteristic acted as electron–hole pairs. The conduction band and valence band of FeVO4 received electrons from g-C3N4, leading to a charge separation. By using solar illumination, the electron–hole pair was generated leading to the formation of ROS, where in this study, the •OH and O2•− were the dominant radical species in the photocatalysis reaction. The electron–hole can also be facilitated by the heterojunction formation when the band gap is narrow. Firstly, O2 captured the electrons in the FeVO4, forming O2•− and •OH. Secondly, the O2•− reacted with H+ to form H2O2 radical (•HO2), leading to the production of H2O2. The H2O2 was better than the O2 as an electron acceptor. In the presence of ozone (O3) and solar spectrum, the ozone was decomposed into H2O2 and •OH. However, the alkali condition enhanced the formation of −HO2 which interfered with the formation of •OH. In conclusion, the O3 molecules, initial adsorption, and interaction of the organic contaminant are crucial for contaminant removal.
Noorisepehr et al. (2019) invented mesoporous carbon (MNP@C) combined with UV irradiation to activate PMS for acetaminophen (ACT) degradation45. The removal mechanism of ACT involved adsorption and oxidation, where the total removal and TOC removal efficiencies were 97.4% and 63.5%, respectively. This result indicated that 63.5% of ACT was mineralized and the remained ACT was degraded by the ROS or sorbed onto the MNP@C. Firstly, the ACT was attacked by SO4•− through electron transfer to form ACT•+, followed by the ACT•+ reaction with a water molecule, producing (OH)ACT•. On the other hand, the ACT reaction with •OH created (OH)ACT•. Lastly, (OH)ACT• was degraded to byproducts, CO2, and H2O.
Fe-BC’s efficiency can be related to the nature of the specific pollutant. For instance, the antibiotics removal is influenced by pH, functional groups, and redox activity. For example, over 85% removal of tetracycline148, sulfamethoxazole149 and ciprofloxacin150 were performed using different Fe-BC types due to enhanced π–π interactions and electrostatic attraction, hydrogen bonding, surface complexation and oxidative degradation. In contrast, Iron biochar is highly effective for removing heavy metals and phenolic compounds due to strong complexation and redox activity and some adsorbents achieved 99% removal. Antibiotics and pesticides show high but compound-specific efficiency, requiring pH control and optimization. Furthermore, PFAS (an industrial chemical, organic) removal is still a challenge, requiring further modifications (e.g., functionalization with amines or activated carbon integration).
Reactive oxygen species (ROS): radical (e.g., hydroxyl radicals, sulfate superoxide) and non-radical (singlet oxygen)
The degradation of pollutants depends on the existence of active species. In AOPs, reactive oxygen species (ROSs) including sulfate radicals (SO4•−), hydroxyl radicals (•OH), superoxide (O2•−), and singlet oxygen (1O2) have been extensively shown for efficiently degrade organic contaminants in both wastewater and groundwater because of their strong oxidation capabilities and high efficacy118,151. Moreover, Fe-based BC hasthe potential to generate holes, and facilitate electron transfer, which may also contribute to the degradation of organic contaminants118.
The ROS (O2•−, •OH) could be generated by the electron–hole pair (e–h) interaction. For example, Fe0 converted to Fe2+ by interaction with water and oxygen113,117. The conversion of Fe0 to Fe2+ by interaction with H2O and O2 may occur in following manners113,117:
So far, numerous articles have shown that Fe–BC may activate H2O2 and degrade organic contaminants by generating \({{\rm{O}}}_{2}^{{\rm{\bullet }}-}\) and •OH113,117,151. Moreover, peroxymonosulfate (PMS) or persulfate (PDS) based Fe–BC catalytic system could produce (SO4•−) and hydroxyl radicals (•OH), superoxide \(\left({{\rm{O}}}_{2}^{{\rm{\bullet }}-}\right)\) and singlet oxygen (1O2) for degradation of organics substance under the bicarbonate buffer solutions113,151,152.
In photocatalytic systems, Fe species, specially Fe3O4 incorporated BC could also enhance the separation efficiency by generating e− h+ pairs that influence the formation of \({{\rm{O}}}_{2}^{{\rm{\bullet }}-},\) •OH, and also participated in pollutant degradation along with \({{\rm{O}}}_{2}^{{\rm{\bullet }}-},\) •OH118.
Mechanistic insights into electron transfer
There are two main types of Fe-based catalysts used for PDS activation: homogeneous and heterogeneous151. Among the many benefits of heterogeneous Fe-based catalysts are their high recyclability, lack of Fe-sludge formation, ease of solid–liquid separation, and broad pH response boundaries151,153,154,155,156. Extensive research has recently focused on nano- and micro-zero-valent Fe (n(m)ZVI) and its ability to heterogeneously activate PDS157,158 in the degradation of organic pollutants including pesticides159, polycyclic aromatic hydrocarbons160, antibiotics161,162. However, (n(m)ZVI) have also several disadvantages like low electron consumption, delayed electron transfer cycle, strong aggregation propensity, and poor air stability. Therefore, it is necessary to make attempt to improve the limitations of this catalyst151,153,158,163.
In Fe–BC electrons may transfer from non-zero valent Fe to PDS molecules and break off O–O bonds in PDS to produce \({{\rm{O}}}_{2}^{{\rm{\bullet }}-}\)151. However, various quenching agents, including MeOH, TBA, p-BQ, FFA, and L-histidine (Fig. 2a), were utilized to ascertain the role of •OH, \({\mathrm{SO}}_{4}^{\bullet -}\), \({{\rm{O}}}_{2}^{{\rm{\bullet }}-},\) and 1O2 in various catalysis process (Fig. 2b and c). The electron could also be directly transferred to the pollutant, leading to the degradation of the organic pollutant (Fig. 2d–f and g). For example, the breakdown of tetracycline hydrochloride (TH) has been demonstrated to occur directly with Fe species when a Fe-BC catalyst is used.
a Scavenger test, electron spin resonance (ESR) analysis for b radical, c non-radical. e and f Direct electron transfer and g proposed reaction mechanism of ciprofloxacin (CIP) degradation in the Fe-KBC800/PMS system11.
Moreover, a hydroxylated product was formed from the TH cation radical through hydroxylation. A theoretical study was conducted to investigate the position at which •OH attacked TH. Due to the electrophilic nature of •OH, it selectively targets atoms or groups with a high electron density. The site with the highest electron density in a molecule is most susceptible to assault by electrophilic or oxidizing chemicals. The frontier molecular orbital analyses of TH indicate that the phenol and enol regions are electron-rich and susceptible to •OH attack, as shown in Fig. 3a and b. However, the Gibbs free energies of various reactive sites of TH were also shown to display a range of intermediate and transition states, together with the corresponding reaction energy barriers (Fig. 3c–e)117.
a and b HOMO and HOMO-1 orbitals of TH. c Schematic energy diagrams of oxidative degradation of TH with initial •OH attacked onto TH. d and e Spin electron distribution of IN1o and IN1c, respectively, based on relative Gibbs free energy, and the enthalpy. Adapted from ref. 117.
Moreover, to quantify quantum efficiency, charge separation, charge flow, and formation of ROS (\({{\rm{O}}}_{2}^{{\rm{\bullet }}-},\) •OH), electrochemical impedance spectroscopy (EIS) and photocurrent response analyses could be utilized118,164.
Regeneration efficacy of iron–biochar composites for sustained pollutant removal
The potential for repeated use of iron–biochar composites (Fe–BC) in pollutant removal is critical for their economic and environmental sustainability. Recent studies have demonstrated that Fe–BC composites can be effectively regenerated and reused in various water treatment applications, maintaining a high capacity for the adsorption of pollutants over multiple cycles11. One key aspect of Fe–BC composites is their robust structure, which supports the adsorption and catalytic breakdown of complex organic compounds. Zhang et al. (2020) reported that Fe–BC composites could be regenerated using a simple thermal process, which restored adsorption efficiency up to 90% after three cycles of use for dye removal from wastewater165. This finding suggests that the thermal stability of iron within the biochar matrix is crucial for maintaining the composite’s functionality after regeneration. Furthermore, the presence of iron enhances the generation of reactive species during the treatment processes, aiding in the breakdown of pollutants. Park et al. (2021) observed that the oxidative regeneration of Fe–BC using hydrogen peroxide not only recovered adsorption capacity but also activated new reactive sites on the biochar surface166. This dual functionality underscores the catalytic role of iron, facilitating both regeneration and enhanced pollutant removal. However, the long-term effectiveness and the mechanisms underlying the regeneration of Fe–BC remain areas needing further investigation. Masud et al. (2023) highlighted the challenge of iron leaching during successive regeneration cycles, which could potentially reduce the composite’s effectiveness and pose secondary contamination risks11. Addressing these challenges is crucial for developing sustainable practices for the use of Fe–BC in environmental applications.
Advantages, limitations, and future directions
Comparative life-cycle assessment
A crucial aspect of the sustainability of Fe–BC is its energy requirement during production. Typically, the synthesis of Fe–BC involves pyrolysis, which can be energy-intensive. However, when compared to other advanced materials like activated carbon or synthetic resins, which require higher temperatures and longer processing times, Fe–BC might exhibit lower overall energy consumption. A comparative analysis, as suggested, would involve quantifying the total energy input from raw material extraction through to production, providing insights into the efficiency of Fe–BC relative to these alternatives. Another critical factor is the amount of waste generated during the production of Fe–BC. The use of biomass as a precursor for biochar can significantly reduce waste, as it utilizes agricultural by-products that might otherwise contribute to waste streams. In contrast, the production of synthetic adsorbents typically involves chemicals and processes that generate hazardous waste. A detailed assessment of waste types, quantities, and disposal methods used in the production of Fe–BC compared to other materials would elucidate its environmental footprint. The sustainability of raw materials used in Fe–BC production also plays a fundamental role. The renewable nature of biochar, derived from plant-based or organic waste, contrasts with the finite mineral resources required for some other adsorbents or catalysts. Discussing the sourcing, renewability, and processing of raw materials for Fe–BC versus non-renewable resource-dependent materials would provide a comprehensive sustainability perspective.
Technical challenges in the production and application of Fe–BC
Biochar production for the removal of organic contaminants depends on various properties and conditions. The physical and chemical properties of the biochar surface also affect the removal methods167,168. The factors that affect the production of biochar include pyrolysis temperature, biomass feedstock, heating rate, temperature, residence time, etc.3. The properties of biochar vary depending on the source materials and pyrolysis conditions used.
Pyrolysis temperature is critical in the synthesis of biochar. It is an important characteristic that affects the yield and quality of biochar169. According to Pariyar’s research, pyrolysis has an impact on the other properties of biochar, including pH, surface area, and functional group170. Because high temperatures increase the volume of micropores, biochar produced at these temperatures has a high carbon content and a large surface area. This may be because volatile organic compounds are removed during the production process, which is favorable to the migration of organic pollutants to micropores171. The production of biochar at high temperatures enhances the different characteristics of biochar, such as pore structure and surface area. Also, many research experiments show that biochar produced at high temperatures has a higher adsorption capacity than biochar produced at low temperatures172. Preparation of biochar at an exceeding temperature of 500 °C enhances the porous structure with a high surface area and a high pH value. Many studies have shown that biochar produced at high temperatures reduces the adsorption of the contaminant173. When the biochar is prepared at a higher temperature, it leads to chemical rearrangement of biochar; the change in the structure blocks the pores and reduces the adsorption capacity of biochar169. On the other side, biochar prepared at low temperatures is more efficient in the removal of contaminants172,174,175. There is much research that has been carried out at high and low temperatures that shows different outcomes. It has been investigated to determine the removal rate of Congo red by using magnetic biochar synthesized at various temperatures176. The result showed that the removal rate was higher at a high temperature of 1073 K104. In the other research carried out by one of the researchers, Auricularia auricula dregs waste biochar was prepared at different pyrolysis temperatures, and the experiment showed that biochar prepared at higher temperatures had higher adsorption of tetracycline as compared to the biochar prepared at the lowest temperature177.
Biomass feedstock has a key role in contaminant adsorption. The elemental content of varied biomass affects its adsorption capacity171,174. The feedstock composition is the most important variable influencing biochar adsorption. The type of feedstock material used in the manufacturing of biochar determines the final product. The type of raw material used to make the adsorbent dictates which one should be used to remove developing contaminants, such as organic, inorganic, heavy metals, and biological pollutants178. The biomass for biochar has a wide range of resources, such as agricultural lignocellulose residue, sugarcane bagasse, banana stalks, corn straw, rice biomass, organic sludge, and so on179,180. Several studies have demonstrated that biomass with high lignin yields high biochar. Qiu et al. (2022b) prepared biochar from various biomasses at the same pyrolysis temperature181. The biomass included wheat straw, rice husk, sunflower stem, corn cob, walnut shell, poplar sawdust, and conifer sawdust. The varied material of the biomass experiment demonstrated that residues are mostly related to the lignin concentration of the source material. The heating rate of the pyrolysis temperature during biochar production indirectly affects the output result (biofuel, bio-oil, and syngas). The heating rate also affects the surface area and porosity of biochar. It has been observed that when the heating rate reaches a certain value, then the surface area and porosity also increase; it shows that at a certain value, surface area and porosity are at their maximum. The heating rate also influences the transport of mass and heat within the particles. Several studies have shown that heat and mass transfer are slow at lower heating rates, but at higher heating rates, they also increase. Also, the results have shown that a quick heating rate allows the biochar particles to melt and flatten the surfaces169,178. Residence time also plays a role in the production of biochar, which influences the characteristics and preparation cost of biochar181. During the production of biochar, when sufficient residence time is given, it leads to the release of a volatile substance that is accelerated and promotes the formation of the basic pore structure182.
Fe–BC has been widely studied for its catalytic properties and contaminant removal efficiency; several limitations hinder its practical applications in aquatic systems. Fe–BC exhibits high reactivity toward certain organic pollutants, such as dyes and phenolic compounds, but its effectiveness in degrading highly stable and recalcitrant contaminants (e.g., perfluoroalkyl substances, chlorinated hydrocarbons) remains limited. The catalytic efficiency of Fe–BC in advanced oxidation processes (AOPs) depends on the generation of reactive oxygen species (ROS), which may not be sufficient to break down highly persistent organic pollutants. Fe–BC can suffer from surface passivation, where iron species become oxidized over time, reducing their reactivity in redox reactions. The formation of iron oxides (e.g., Fe₃O₄, Fe₂O₃) can decrease the availability of Fe²⁺, which is crucial for Fenton-like reactions. Leaching of iron ions into aquatic systems has been reported in several studies183,184, leading to secondary contamination concerns and reduced long-term efficiency of Fe–BC. Strategies such as incorporating stabilizing agents (e.g., carbon nanotubes, clay minerals) or designing composite materials with other metals have been proposed to mitigate this issue.
Environmental impact
During the evolution of biochar, its quality alters, and it is exposed to environmental processes that pose a negative impact on the ecosystem. This not only affects the medium but also its interface. Biochar is a relatively low-cost carbonaceous material. It is distinguished by a wide range of abundant feedstocks, a high surface area, micro-porosity, and ion exchange capacity171. With the increase in pollutants in the environment, concern over climate change has increased. Soil is an important carbon sink in the global carbon cycle that affects climate change. For reducing carbon emissions from the environment, carbon sequestration plays a vital role, and biochar has high resistance to biodegradation due to its highly condensed aromatic structure169,176,185. Kim et al. (2019) prepared iron-modified biochar for arsenite removal using Miscanthus, and the results showed that it was successful and environmentally friendly because the adsorption capacity of iron-modified biochar was high, resulting in a reduction in arsenic toxicity to a level safe for aquatic life186. Samaraweera et al. (2023) developed Douglas Fir biochar (FDBC), which successfully eliminates dyes from wastewater, hence reducing water pollution44. The investigation demonstrated that the modified biochar not only effectively removes dyes but also allows for regeneration, increasing its sustainability in wastewater treatment applications. Soares produces biochar from sugarcane straws to remove arsenic and lead from wastewater. The author synthesized the biochar at various temperatures and then modified it using ferric chloride. The experiment demonstrated that chemical change and temperature enhanced the sorption capacity. The removal of arsenic and lead from wastewater improves the quality of the wastewater and reduces the environmental risk of saturated biochar.
The leaching of iron is important during the adsorption and oxidation processes. Many studies have discovered that biochar may easily improve adsorption in the soil, which leads to reducing the danger of leaching into the environment4. Wu et al. (2020) prepared rice straw-derived biochar modified with ferrous chloride and ferric chloride for the removal of the phosphate187. The result showed that in the column experiment, all treatments were found to increase the phosphorus content significantly. The result showed that Fe (II) biochar decreased the leaching by 86.4% as BC improves water retention, enhancing P immobilization187. Cheng et al. (2022) synthesized iron-modified biochar for simazine adsorption in soil188. The experiment showed that 63.36% of simazine was leached. Liu et al. (2022) used a single-step pyrolysis process to create iron-modified biochar from oily sludge; the experiment demonstrated increased removal capacity with decreased iron leaching189. Similarly, another experiment was carried out using rice husks. Islam et al. (2021) prepared rice husk magnetic biochar through liquification190. In this experiment, it was noticed that, due to the acidic reaction, iron leached from the surface of the adsorbent, resulting in a loss of adsorption capacity. This increased leaching can affect soil and water quality over time, particularly in acidic environments, potentially altering microbial communities and nutrient dynamics. Kim et al. (2019) developed Miscanthus charcoal and treated it with iron oxide for the elimination of arsenite186. The experiment indicated the leaching of arsenite concentrations from the adsorbent. The results showed that using adsorbent in powder form was suitable for pollutant removal. While biochar enhances pollutant removal and carbon sequestration, its long-term stability and potential for secondary contamination remain uncertain191. Over extended periods, biochar may degrade, releasing adsorbed heavy metals and persistent pollutants into the environment, potentially leading to bioaccumulation in aquatic and terrestrial ecosystems192. Additionally, the long-term impact of biochar amendments on soil fertility, organic matter decomposition, and carbon storage needs further study to ensure its sustainability193. A comprehensive risk assessment addressing biochar’s degradation pathways, accumulation of heavy metals, and ecological consequences is crucial for evaluating its long-term environmental safety.
Economic consideration
The availability, collection, and transportation of raw biomass, as well as scale and production technique, handling, and supply, all influence biochar costs. The most important aspect is gasification and transportation for biochar fabrication. Many researchers have studied the economic potential of producing two types of biochar in three states and discovered that the net current cost of biochar rises with the reduction in movement of the mobile pyrolysis unit194. The cost of biochar labor fabrication varies globally. Many researchers have carried out experiments on biochar for the removal of contaminants from the environment. In different countries, the cost differs, as Gupta et al. showed that the production of biochar in Selangor was at $532/yr and the total income after the sale was $8012/yr. This shows the positive aspect of the production of biochar195. The cost-effectiveness of producing biochar is determined by the price at which it can be sold. Yard waste, for example, horse and cattle dung were discovered to be an excellent feedstock for biochar production, with a net profit margin (e.g., ratio of net income by revenue) of $16 and $69 for the low and high-income scenarios of carbon dioxide equivalents (method to determine how much a greenhouse gas contributes to global warming compared to carbon dioxide). The break-even point (e.g., a point at which total cost equals total revenue) for pyrolysis at 450 °C was around $280/t, while at 300 °C it was roughly $220/t196,197. This shows that the net profit margin of biochar production can be improved with low-cost feedstock and an optimized processing strategy.
Comparison with existing water treatment technologies
When compared to conventional water treatment technologies, biochar offers a cost-effective and sustainable alternative. Traditional adsorption techniques, such as activated carbon, are widely used for contaminant removal but come with high production and regeneration costs. The cost of activated carbon can range between $1500 and $3500 per ton, depending on raw material and processing198. In contrast, biochar can be produced from agricultural and forestry waste at a significantly lower cost—ranging between $300 and $800 per ton199. Additionally, biochar can be regenerated, further enhancing its economic viability. Other treatment methods, including chemical precipitation, ion exchange, and membrane filtration, require high operational and maintenance costs. For instance, membrane filtration systems (e.g., reverse osmosis) typically cost between $0.50 and $3.00 per cubic meter of treated water, depending on scale and membrane type200. In contrast, biochar-based filtration can be implemented at a fraction of this cost, particularly in decentralized and low-resource settings. Furthermore, biochar production can be integrated with bioenergy generation, where syngas and bio-oil co-products help offset the costs, making the process more economically feasible. Overall, biochar’s cost-effectiveness depends on optimizing feedstock selection, pyrolysis conditions, and application strategies to maximize adsorption efficiency and long-term sustainability. A more detailed techno-economic assessment would provide further insights into its economic feasibility compared to traditional water treatment technologies.
Challenges and future research
Iron-modified biochar has gained recognition for its ability to remove and degrade contaminants in wastewater. The addition of iron enhances the biochar adsorption capacity, catalytic properties, and reactivity, making it effective for treating pollutants such as heavy metals, PFAS, and organic compounds. The increased surface area, magnetic behavior, and enhanced porous structure also enhance removal capabilities (Fig. 4). As soil and water pollution continue to increase, biochar treatment offers a long-term solution that has the advantage of better adsorption capacities and catalytic potential. This strategy is promising; however, more research is needed to obtain an improved iron-modified biochar interaction with higher adsorption capacities. This review article summarized biochar modification with iron for organic contaminant adsorption, highlighting cutting-edge research on the topic. The article covers Fe–BC synthesis and organic contaminant adsorption, and adsorption mechanisms. Limited knowledge exists regarding the remediation process involving iron-modified biochar.
The diagram displays how various biomass sources, including sugarcane waste and rice straw, are transformed into iron-modified biochar, enhancing properties like porosity for contaminant removal through different methods. It also outlines key benefits and challenges, illustrating the comprehensive potential of this technology in environmental remediation.
Considering major challenges and future research, emphasis should be placed on the characteristics of biochar that may be influenced by biomass material, pyrolysis conditions, reaction time, and temperature. Moreover, several factors need to be considered during the iron modification of biochar and the adsorption of contaminants, as detailed below.
-
More research is required to fill knowledge gaps and enhance this sustainable development strategy in light of the possible advantages and disadvantages of using iron-modified biochar.
-
The mechanism of iron-modified biochar should be studied, as should the environmental regulation of essential active components.
-
Relatively little information is available regarding the environmental effects of iron-modified biochar. Therefore, further investigation is warranted to facilitate its broader application in real-world environments.
-
The pollutants emitted during the adsorption process are adsorbed onto the biochar. Thus, a thorough investigation and a well-defined monitoring system should be built to prevent secondary pollution throughout the process.
-
So far, most research has been conducted in laboratory settings, but to achieve better scalability and results, actual wastewater treatment processes should be explored. Advanced techniques should be explored to elucidate the remediation mechanism of organic contaminants through iron-modified biochar.
-
The leaching behavior of iron has not been thoroughly investigated to date, highlighting the need for focused research on the leaching characteristics of iron biochar.
-
Recent studies have demonstrated that bimetallic biochar composites can significantly enhance catalytic activity in aquatic systems due to synergistic effects between different metals. While iron-based biochar has been extensively studied for its electron transfer capabilities, the addition of other transition metals, such as copper (Cu), manganese (Mn), cobalt (Co), and nickel (Ni), has been shown to improve redox reactions, reactive oxygen species (ROS) generation, and overall pollutant degradation efficiency184. Moreover, the presence of secondary metals can enhance the structural stability of iron in biochar composites, reducing iron leaching and prolonging catalytic activity Fe–Ni systems have been found to promote electron transfer processes and enhance the degradation of organic pollutants through combined redox and adsorption mechanisms183.
In conclusion, this study has elucidated how iron-modified biochar (Fe–BC) facilitates the remediation of organic contaminants in aquatic environments. Through comprehensive characterization and batch degradation experiments, it has been demonstrated that Fe–BC significantly enhances organic pollutant degradation compared to unmodified biochar. The incorporation of iron into the biochar matrix promotes reactive oxygen species (ROS) formation and electron transfer dynamics, which are crucial for degradation processes. Additionally, Fe–BC has exhibited stability and reusability across multiple treatment cycles, reinforcing its economic feasibility and operational reliability for continuous water purification applications.
The integration of Fe–BC into existing wastewater treatment infrastructure presents a scalable, cost-effective, and environmentally sustainable approach to addressing organic contamination. Beyond localized applications, Fe–BC holds promise for large-scale environmental remediation efforts, particularly in the context of sustainable water management, climate change mitigation, and circular economy principles. Its ability to immobilize pollutants, regenerate through low-energy processes, and function under varying environmental conditions aligns with global efforts to reduce water contamination and promote resource recovery.
Moving forward, further research should focus on optimizing Fe–BC synthesis for large-scale deployment, ensuring its effectiveness in complex environmental matrices, and evaluating long-term environmental trade-offs, such as carbon footprint and secondary pollution risks. Additionally, techno-economic assessments and life-cycle analyses will be essential to determine Fe–BC’s feasibility in industrial wastewater treatment plants, agricultural runoff management, and decentralized water purification systems. By aligning Fe–BC applications with broader environmental sustainability goals, this material has the potential to become a key component in next-generation green remediation technologies.
Methodology
The bibliometric analysis was used to visualize the perspective of Fe-based biochar studies quantitatively. In this study, the data is extracted from a database and subsequently used to create maps of trends and networks for Fe-based biochar research. The networks include authors’ collaboration networks, country networks, keyword networks, other related topics, and article citation networks. The visualization figures are generated using Bibliophagy and VOSviewer (version 1.6.20) for science mapping. The criteria for choosing the data were based on co-authorship, keyword co-occurrence, citation, bibliographic coupling, and co-citation map. The current article trends in the Fe-based biochar are chosen from 1 January 2017 to 15 February 2025. The chosen keywords and Boolean operators (AND, OR, AND NOT) are important in gaining reliable and representative bibliometric data using the Scopus, Web of Science, Dimensions, Lens, and PubMed databases. The details of data mining are shown in Table S1, where 163 research articles were collected as main data sources. The 20 most crucial articles are shown in Table S2.
The potential countries that lead to the Fe-based biochar research and development are shown in Fig. 1a. The global distribution of Fe-based biochar research was generated using VOSviewer with a minimum threshold of 1 document and 1 citation for each country. Out of 29 countries, 26 countries met the set threshold. China was the leading contributor to the Fe-based biochar research, with 110 documents and 4514 citations, as shown in Fig. S2 by the blue circle. The other leading countries with the most Fe-based biochar publications are Hong Kong (11), South Korea (10), the United States (12), the Czech Republic (8), the United Kingdom (5), and Canada (4). However, there are several countries having significant contributions to Fe-based biochar studies even though they do not reach the top 20 leading countries, such as Finland, France, Germany, Italy, Oman, Brazil, Malaysia, Morocco, Serbia, and Turkey, with a total of 224 citations. The thematic map of the Fe-based biochar is illustrated in Fig. S3. Among four quadrants, such as niche themes, motor themes, emerging or declining themes, and basic themes, the quadrant for emerging or declining themes determines the importance of the Fe-based biochar. In this quadrant, the three topics are iron compounds, zero-valent iron, and redox reactions, as marked with the red dash boxes. To assist us in determining the emerging topic, Fig. S4 generated using VOSviewer can be used. In this figure, green to yellow indicates a novel publication year, whereas green to blue shows an old publication year. The green to yellow node colors signify the emerging topic, while the blue colors are the declining topic. The zero-valent iron biochar has a yellow color, indicating it is an emerging topic. Iron compound/iron biochar and redox reaction have a blue color, indicating they are declining topics. Fig. S5 generated by Bibliophagy depicts the organic- and inorganic-contaminated wastewater remediation using Fe-based biochar over a period of time. The size of the blue node illustrates the number of documents published in a certain year. Chlorobenzene and chlorpyrifos were the pioneers of the organic contaminants treated by the Fe-based biochar in 2017. The study of Fe-based biochar reached its peak in 2022, where charcoal, biochar, and iron as the most frequent keywords that appeared in the publications with an average citation of around 120. Recently, the study of tetracycline removal, denitrification, and thermodynamics investigation using Fe-based biochar started to gain attention in 2024.
Data availability
No datasets were generated or analysed during the current study.
References
Sarker, A. et al. Biological and green remediation of heavy metal contaminated water and soils: a state-of-the-art review. Chemosphere 332, 138861 (2023).
Septian, A., Masud, M. A. A. & Shin, W. S. Potassium nickel hexacyanoferrate–polyacrylonitrile composite for removing cobalt, strontium, and cesium from radioactive laundry wastewater. Clean (Weinheim) 1–12 https://doi.org/10.1002/clen.202300057 (2023).
Masud, M. A. A. et al. A critical review of sustainable application of biochar for green remediation: research uncertainty and future directions. Sci. Total Environ. 904, 166813 (2023).
Sarker, A. et al. A critical review of sustainable pesticide remediation in contaminated sites: research challenges and mechanistic insights. Environ. Pollut. 341, 122940 (2024).
He, Y. et al. Fe–Mn oxycarbide anchored on N-doped carbon for enhanced Fenton-like catalysis: importance of high-valent metal-oxo species and singlet oxygen. Appl. Catal. B 340, 123204 (2024).
Huang, Q. et al. Enhanced PMS activation through CuMo2S3 chalcogenide: Crucial role of MoXSX in restoring the Cu active sites of catalyst for pollutants removal. J. Environ. Chem. Eng. 13, 115324 (2025).
Pei, X. Y. et al. Electron transfer mechanism of peroxydisulfate activation by sewage sludge-derived biochar for enhanced degradation of sulfamethoxazole. Environ. Sci.7, 1563–1575 (2021).
Ali, J. et al. MoS4-LDH: a dual centre Fe-based layered double hydroxide catalyst for efficient atrazine removal and peroxymonsulfate activation. Chem. Eng. J. 456, 141161 (2023).
Mao, J. et al. Ultrafast oxidation of refractory organics via PMS activation by Si–O doped biomimetic montmorillonite: Simultaneous enhanced radical/electron transfer pathways and efficient catalytic membrane system. Appl. Catal. B 342, 123428 (2024).
Wu, S. et al. Rapid activation of peroxymonosulfate with iron(III) complex for organic pollutants degradation via a non-radical pathway. Water Res. 233, 119725 (2023).
Masud, M. A. A., Shin, W. S. & Kim, D. G. Fe-doped kelp biochar-assisted peroxymonosulfate activation for ciprofloxacin degradation: Multiple active site-triggered radical and non-radical mechanisms. Chem. Eng. J. 471, 144519 (2023).
Ali, J. et al. Metal sulfides as emerging materials for advanced oxidation of wastewater: recent developments, challenges, and prospects. Coord. Chem. Rev. 509, 215765 (2024).
Duan, P. et al. Activation of peroxymonosulfate via mediated electron transfer mechanism on single-atom Fe catalyst for effective organic pollutants removal. Appl. Catal. B 299, 120714 (2021).
Zhang, S. et al. Atomic Fe sites embedded within carbon nanotubes for the efficient photodegradation of multiple tetracyclines. Sep. Purif. Technol. 287, 120530 (2022).
Wang, Z. et al. Single-atom iron catalyst activating peroxydisulfate for efficient organic contaminant degradation relying on electron transfer. Chem. Eng. J. 458, 141513 (2023).
Shang, Y., Xu, X., Gao, B., Wang, S. & Duan, X. Single-atom catalysis in advanced oxidation processes for environmental remediation. Chem. Soc. Rev. 50, 5281–5322 (2021).
Masud, M. A. A., Kim, D. G. & Shin, W. S. Highly efficient degradation of phenolic compounds by Fe(II)-activated dual oxidant (persulfate/calcium peroxide) system. Chemosphere 299, 134392 (2022).
Masud, M. A. A., Kim, D. G. & Shin, W. S. Degradation of phenol using Fe(II)-activated CaO2: effect of ball-milled activated carbon (ACBM) addition. Environ. Res. 214, 113882 (2022).
Xu, L. et al. Degradation of organic pollutants by Fe/N co-doped biochar via peroxymonosulfate activation: synthesis, performance, mechanism and its potential for practical application. Chem. Eng. J. 400, 125870 (2020).
Wang, H. et al. Biochar-induced Fe(III) reduction for persulfate activation in sulfamethoxazole degradation: insight into the electron transfer, radical oxidation and degradation pathways. Chem. Eng. J. 362, 561–569 (2019).
Zhao, D. et al. Effect and mechanism of persulfate activated by different methods for PAHs removal in soil. J. Hazard. Mater. 254–255, 228–235 (2013).
Zhao, L. et al. Generating high-valent iron-oxo ≡FeIV=O complexes by calcium sulfite activation in neutral microenvironments for enhanced degradation of CIP. Environ. Pollut. 336, 122449 (2023).
Feng, M., Jinadatha, C., McDonald, T. J. & Sharma, V. K. Accelerated oxidation of organic contaminants by ferrate(VI): the overlooked role of reducing additives. Environ. Sci. Technol. 52, 11319–11327 (2018).
Jian, H. et al. Efficient removal of pyrene by biochar supported iron oxide in heterogeneous Fenton-like reaction via radicals and high-valent iron-oxo species. Sep. Purif. Technol. 265, 118518 (2021).
Mei, Y. et al. Effect of Fe–N modification on the properties of biochars and their adsorption behavior on tetracycline removal from aqueous solution. Bioresour. Technol. 325, 124732 (2021).
Luo, H., Zeng, Y., He, D. & Pan, X. Application of iron-based materials in heterogeneous advanced oxidation processes for wastewater treatment: a review. Chem. Eng. J. 407, 127191 (2021).
Liu, M. et al. Application of functional modification of iron-based materials in advanced oxidation processes (AOPs). Water (Basel) 14, 1498 (2022).
Masud, M. A. A. & Shin, W. S. Recycling of spent superabsorbent polymer into nitrogen-doped carbon catalyst for enhanced antibiotic degradation in groundwater. J. Water Process Eng. 64, 105567 (2024).
Septian, A., Masud, M. A. A., Nugroho, R. & Shin, W. S. Evaluating the potential of wax impregnated reactive components for long-term acenaphthene removal. J. Water Process Eng. 56, 104466 (2023).
Haddaoui, I. & Mateo-Sagasta, J. A review on occurrence of emerging pollutants in waters of the MENA region. Environ. Sci. Pollut. Res. 28, 68090–68110 (2021).
Syafrudin, M. et al. Pesticides in drinking water—a review. Int. J. Environ. Res. Public Health 18, 468 (2021).
Singh, S. et al. Global distribution of pesticides in freshwater resources and their remediation approaches. Environ. Res. 225, 115605 (2023).
Bae, S., Masud, M. A. A., Annamalai, S. & Shin, W. S. The inherent nature of N/P heteroatoms in Sargassum fusiforme seaweed biochar enhanced the nonradical activation of peroxymonosulfate for acetaminophen degradation in aquatic environments. Chemosphere 356, 141877 (2024).
Masud, M. A. A. et al. Exploring the environmental pathways and challenges of fluoroquinolone antibiotics: a state-of-the-art review. Sci. Total Environ. 926, 171944 (2024).
Masud, M. A. A., Annamalai, S. & Shin, W. S. Remediation of ciprofloxacin in soil using peroxymonosulfate activated by ball-milled seaweed kelp biochar: performance, mechanism, and phytotoxicity. Chem. Eng. J. 465, 142908 (2023).
Wang, Z., Lin, X., Yang, K. & Lin, D. Differential photodegradation processes of adsorbed polychlorinated biphenyls on biochar colloids with various pyrolysis temperatures. Water Res. 251, 121174 (2024).
Masud, M. A. A., Narendra Kumar, A. V. & Shin, W. S. Fe(II) activated calcium peroxide/peroxymonosulfate: a practical system for phenanthrene degradation and upholding ecological pH. Sep. Purif. Technol. 317, 123902 (2023).
Zeghioud, H., Fryda, L., Djelal, H., Assadi, A. & Kane, A. A comprehensive review of biochar in removal of organic pollutants from wastewater: characterization, toxicity, activation/functionalization and influencing treatment factors. J. Water Process Eng. 47, 102801 (2022).
Jiang, T. et al. Degradation of organic pollutants from water by biochar-assisted advanced oxidation processes: mechanisms and applications. J. Hazard. Mater. 442, 130075 (2023).
Schuijt, L. M., Peng, F.-J., van den Berg, S. J. P., Dingemans, M. M. L. & van den Brink, P. J. (Eco)toxicological tests for assessing impacts of chemical stress to aquatic ecosystems: facts, challenges, and future. Sci. Total Environ. 795, 148776 (2021).
Kløve, B. et al. Overview of groundwater sources and water-supply systems, and associated microbial pollution, in Finland, Norway and Iceland. Hydrogeol. J. 25, 1033–1044 (2017).
Akhtar, N., Syakir Ishak, M. I., Bhawani, S. A. & Umar, K. Various natural and anthropogenic factors responsible for water quality degradation: a review. Water (Basel) 13, 2660 (2021).
Aziz, F. F. A. et al. A review on synergistic coexisting pollutants for efficient photocatalytic reaction in wastewater remediation. Environ. Res. 209, 112748 (2022).
Samaraweera, H. et al. Green iron oxide-modified biochar for methylene blue removal from aqueous solutions. Groundw. Sustain Dev. 21, 100945 (2023).
Noorisepehr, M., Ghadirinejad, K., Kakavandi, B., Ramazanpour Esfahani, A. & Asadi, A. Photo-assisted catalytic degradation of acetaminophen using peroxymonosulfate decomposed by magnetic carbon heterojunction catalyst. Chemosphere 232, 140–151 (2019).
Masud, M. A. A., Shin, W. S. & Kim, D. G. Degradation of phenol by ball-milled activated carbon (ACBM) activated dual oxidant (persulfate/calcium peroxide) system: effect of preadsorption and sequential injection. Chemosphere 312, 137120 (2023).
Bolobajev, J. et al. Reuse of ferric sludge as an iron source for the Fenton-based process in wastewater treatment. Chem. Eng. J. 255, 8–13 (2014).
Priyadarshini, M., Das, I., Ghangrekar, M. M. & Blaney, L. Advanced oxidation processes: performance, advantages, and scale-up of emerging technologies. J. Environ. Manag. 316, 115295 (2022).
Li, X. et al. Preparation and application of Fe/biochar (Fe-BC) catalysts in wastewater treatment: a review. Chemosphere 274, 129766 (2021).
Hoch, L. B. et al. Carbothermal synthesis of carbon-supported nanoscale zero-valent iron particles for the remediation of hexavalent chromium. Environ. Sci. Technol. 42, 2600–2605 (2008).
Zhang, X., Sun, P., Wei, K., Huang, X. & Zhang, X. Enhanced H2O2 activation and sulfamethoxazole degradation by Fe-impregnated biochar. Chem. Eng. J. 385, 123921 (2020).
Yang, X. et al. Mechanistic insights into removal of norfloxacin from water using different natural iron ore–biochar composites: more rich free radicals derived from natural pyrite-biochar composites than hematite-biochar composites. Appl. Catal. B 255, 117752 (2019).
Han, Z. et al. Magnetite impregnation effects on the sorbent properties of activated carbons and biochars. Water Res. 70, 394–403 (2015).
Ding, Y., Tang, H., Zhang, S., Wang, S. & Tang, H. Efficient degradation of carbamazepine by easily recyclable microscaled CuFeO2 mediated heterogeneous activation of peroxymonosulfate. J. Hazard. Mater. 317, 686–694 (2016).
Li, S., Tang, J., Liu, Q., Liu, X. & Gao, B. A novel stabilized carbon-coated nZVI as heterogeneous persulfate catalyst for enhanced degradation of 4-chlorophenol. Environ. Int. 138, 105639 (2020).
Zhang, S. et al. A novel biochar supported CMC stabilized nano zero-valent iron composite for hexavalent chromium removal from water. Chemosphere 217, 686–694 (2019).
Luo, H. et al. New insights into the formation and transformation of active species in nZVI/BC activated persulfate in alkaline solutions. Chem. Eng. J. 359, 1215–1223 (2019).
Ma, H. et al. Novel synthesis of a versatile magnetic adsorbent derived from corncob for dye removal. Bioresour. Technol. 190, 13–20 (2015).
Huang, J., Zimmerman, A. R., Chen, H. & Gao, B. Ball milled biochar effectively removes sulfamethoxazole and sulfapyridine antibiotics from water and wastewater. Environ. Pollut. 258, 113809 (2020).
Wang, K., Sun, Y., Tang, J., He, J. & Sun, H. Aqueous Cr(VI) removal by a novel ball milled FeO–biochar composite: role of biochar electron transfer capacity under high pyrolysis temperature. Chemosphere 241, 125044 (2020).
Li, X., Jia, Y., Zhou, M., Su, X. & Sun, J. High-efficiency degradation of organic pollutants with Fe, N co-doped biochar catalysts via persulfate activation. J. Hazard. Mater. 397, 122764 (2020).
Ma, Q. et al. Iron nanoparticles in situ encapsulated in lignin-derived hydrochar as an effective catalyst for phenol removal. Environ. Sci. Pollut. Res. 25, 20833–20840 (2018).
Wang, X. et al. Metal-rich hyperaccumulator-derived biochar as an efficient persulfate activator: role of intrinsic metals (Fe, Mn and Zn) in regulating characteristics, performance and reaction mechanisms. J. Hazard. Mater. 424, 127225 (2022).
Tong, J. et al. Catalytic microenvironment regulation by introducing biochar into MIL-101Fe derivative for enhanced persulfate activation: efficient antibiotic removal and RSM optimization. Sep. Purif. Technol. 331, 125719 (2024).
Yang, B. et al. Fe3O4/biochar modified with molecularly imprinted polymer as efficient persulfate activator for salicylic acid removal from wastewater: performance and specific recognition mechanism. Chemosphere 355, 141680 (2024).
Ding, S., Wan, J., Wang, Y., Yan, Z. & Ma, Y. Activation of persulfate by molecularly imprinted Fe-MOF-74@SiO2 for the targeted degradation of dimethyl phthalate: effects of operating parameters and chlorine. Chem. Eng. J. 422, 130406 (2021).
Zeng, C. et al. Targeted degradation of TBBPA using novel molecularly imprinted polymer encapsulated C–Fe–Nx nanocomposite driven from MOFs. J. Hazard. Mater. 424, 127499 (2022).
Jing, X.-R., Wang, Y.-Y., Liu, W.-J., Wang, Y.-K. & Jiang, H. Enhanced adsorption performance of tetracycline in aqueous solutions by methanol-modified biochar. Chem. Eng. J. 248, 168–174 (2014).
Meischl, F. et al. Synthesis and evaluation of a novel molecularly imprinted polymer for the selective isolation of acetylsalicylic acid from aqueous solutions. J. Environ. Chem. Eng. 4, 4083–4090 (2016).
Dai, J., Meng, X., Zhang, Y. & Huang, Y. Effects of modification and magnetization of rice straw derived biochar on adsorption of tetracycline from water. Bioresour. Technol. 311, 123455 (2020).
Wang, Y. et al. High-efficiency recognition and detection of sulindac in sewage using hydrophilic imprinted resorcinol–formaldehyde resin magnetic nano-spheres as SPE adsorbents combined with HPLC. Chem. Eng. J. 392, 123716 (2020).
Steigerwald, J. M., Peng, S. & Ray, J. R. Novel Perfluorooctanesulfonate-imprinted polymer immobilized on spent coffee grounds biochar for selective removal of perfluoroalkyl acids in synthetic wastewater. ACS EST Eng. 3, 520–532 (2023).
Wang, Y., Ma, X., Peng, Y., Liu, Y. & Zhang, H. Selective and fast removal and determination of β-lactam antibiotics in aqueous solution using multiple templates imprinted polymers based on magnetic hybrid carbon material. J. Hazard. Mater. 416, 126098 (2021).
Zhao, T., Chen, R. & Wang, J. A mild method for preparation of highly selective magnetic biochar microspheres. Int J. Mol. Sci. 21, 3752 (2020).
Kong, X. et al. Molecularly imprinted polymer functionalized magnetic Fe3O4 for the highly selective extraction of triclosan. J. Sep Sci. 43, 808–817 (2020).
Wang, S. & Wang, J. Activation of peroxymonosulfate by sludge-derived biochar for the degradation of triclosan in water and wastewater. Chem. Eng. J. 356, 350–358 (2019).
Xie, X. et al. Novel magnetic multi-templates molecularly imprinted polymer for selective and rapid removal and detection of alkylphenols in water. Chem. Eng. J. 357, 56–65 (2019).
Guo, H. et al. Surface molecular imprinting on carbon microspheres for fast and selective adsorption of perfluorooctane sulfonate. J. Hazard. Mater. 348, 29–38 (2018).
Zhang, P. et al. Catalytic degradation of estrogen by persulfate activated with iron-doped graphitic biochar: process variables effects and matrix effects. Chem. Eng. J. 378, 122141 (2019).
Rong, X. et al. The magnetic biochar derived from banana peels as a persulfate activator for organic contaminants degradation. Chem. Eng. J. 372, 294–303 (2019).
Liu, T. et al. Applications and influencing factors of the biochar-persulfate based advanced oxidation processes for the remediation of groundwater and soil contaminated with organic compounds. Sci. Total Environ. 836, 155421 (2022).
Liu, J. et al. Activation of persulfate with biochar for degradation of bisphenol A in soil. Chem. Eng. J. 381, 122637 (2020).
Wang, J. et al. One-step preparation and application of magnetic sludge-derived biochar on acid orange 7 removal via both adsorption and persulfate based oxidation. RSC Adv. 7, 18696–18706 (2017).
Takdastan, A., Kakavandi, B., Azizi, M. & Golshan, M. Efficient activation of peroxymonosulfate by using ferroferric oxide supported on carbon/UV/US system: a new approach into catalytic degradation of bisphenol A. Chem. Eng. J. 331, 729–743 (2018).
Li, H. et al. Magnetic nitrogen-doped nanocarbons for enhanced metal-free catalytic oxidation: integrated experimental and theoretical investigations for mechanism and application. Chem. Eng. J. 354, 507–516 (2018).
Wang, J., Kou, L., Zhao, L. & Duan, W. One-pot fabrication of sludge-derived magnetic Fe,N-codoped carbon catalysts for peroxymonosulfate-induced elimination of phenolic contaminants. Chemosphere 248, 126076 (2020).
Deng, Y. et al. Phytic acid pre-modulated and Fe/N co-doped biochar derived from ramie fiber to active persulfate for efficient degradation of tetracycline via radical and non-radical pathways. Sep. Purif. Technol. 342, 126976 (2024).
Yu, J. et al. Non-radical oxidation by N,S,P co-doped biochar for persulfate activation: different roles of exogenous P/S doping, and electron transfer path. J. Clean. Prod. 374, 133995 (2022).
Li, Z. et al. Biochar-supported nanoscale zero-valent iron as an efficient catalyst for organic degradation in groundwater. J. Hazard. Mater. 383, 121240 (2020).
Kumar, A. et al. Sustainable nano-hybrids of magnetic biochar supported g-C3N4/FeVO 4 for solar powered degradation of noxious pollutants—synergism of adsorption, photocatalysis & photo-ozonation. J. Clean. Prod. 165, 431–451 (2017).
Wang, S., Gao, B., Li, Y., Wan, Y. & Creamer, A. E. Sorption of arsenate onto magnetic iron–manganese (Fe–Mn) biochar composites. RSC Adv. 5, 67971–67978 (2015).
Baig, S. A., Zhu, J., Muhammad, N., Sheng, T. & Xu, X. Effect of synthesis methods on magnetic Kans grass biochar for enhanced As(III, V) adsorption from aqueous solutions. Biomass Bioenergy 71, 299–310 (2014).
Huff, M. D., Kumar, S. & Lee, J. W. Comparative analysis of pinewood, peanut shell, and bamboo biomass derived biochars produced via hydrothermal conversion and pyrolysis. J. Environ. Manag. 146, 303–308 (2014).
Cai, W. et al. Preparation of amino-functionalized magnetic biochar with excellent adsorption performance for Cr(VI) by a mild one-step hydrothermal method from peanut hull. Colloids Surf. A Physicochem. Eng. Asp. 563, 102–111 (2019).
Zhu, S., Huang, X., Wang, D., Wang, L. & Ma, F. Enhanced hexavalent chromium removal performance and stabilization by magnetic iron nanoparticles assisted biochar in aqueous solution: mechanisms and application potential. Chemosphere 207, 50–59 (2018).
Shang, J. et al. Chromium removal using magnetic biochar derived from herb-residue. J. Taiwan Inst. Chem. Eng. 68, 289–294 (2016).
Yi, Y., Tu, G., Zhao, D., Tsang, P. E. & Fang, Z. Biomass waste components significantly influence the removal of Cr(VI) using magnetic biochar derived from four types of feedstocks and steel pickling waste liquor. Chem. Eng. J. 360, 212–220 (2019).
Xu, Z. et al. Unraveling iron speciation on Fe-biochar with distinct arsenic removal mechanisms and depth distributions of As and Fe. Chem. Eng. J. 425, 131489 (2021).
Shi, C. et al. Fe-biochar as a safe and efficient catalyst to activate peracetic acid for the removal of the acid orange dye from water. Chemosphere 307, 135686 (2022).
Liao, C. Z., Zeng, L. & Shih, K. Quantitative X-ray diffraction (QXRD) analysis for revealing thermal transformations of red mud. Chemosphere 131, 171–177 (2015).
Hussain, I. et al. Insights into the mechanism of persulfate activation with nZVI/BC nanocomposite for the degradation of nonylphenol. Chem. Eng. J. 311, 163–172 (2017).
Deng, J. et al. Nanoscale zero-valent iron/biochar composite as an activator for Fenton-like removal of sulfamethazine. Sep. Purif. Technol. 202, 130–137 (2018).
Ren, J. et al. Granulation and ferric oxides loading enable biochar derived from cotton stalk to remove phosphate from water. Bioresour. Technol. 178, 119–125 (2015).
Thines, K. R., Abdullah, E. C. & Mubarak, N. M. Effect of process parameters for production of microporous magnetic biochar derived from agriculture waste biomass. Microporous Mesoporous Mater. 253, 29–39 (2017).
Chon, K., Mo Kim, Y. & Bae, S. Advances in Fe-modified lignocellulosic biochar: impact of iron species and characteristics on wastewater treatment. Bioresour. Technol. 395, 130332 (2024).
Liu, Y. et al. Adsorption and reductive degradation of Cr(VI) and TCE by a simply synthesized zero valent iron magnetic biochar. J. Environ. Manag. 235, 276–281 (2019).
Fu, H., Ma, S., Zhao, P., Xu, S. & Zhan, S. Activation of peroxymonosulfate by graphitized hierarchical porous biochar and MnFe2O4 magnetic nanoarchitecture for organic pollutants degradation: structure dependence and mechanism. Chem. Eng. J. 360, 157–170 (2019).
Liu, T. et al. FeS/MgO–biochar composites for boosting the adsorption–photocatalytic degradation of RhB. Mater. Sci. Semicond. Process. 174, 108178 (2024).
Yang, F., Jiang, Y., Dai, M., Hou, X. & Peng, C. Active biochar-supported iron oxides for Cr(VI) removal from groundwater: kinetics, stability and the key role of FeO in electron-transfer mechanism. J. Hazard. Mater. 424, 127542 (2022).
Xin, S. et al. High efficiency heterogeneous Fenton-like catalyst biochar modified CuFeO2 for the degradation of tetracycline: economical synthesis, catalytic performance and mechanism. Appl. Catal. B 280, 119386 (2021).
Lam, S. S., Liew, R. K., Lim, X. Y., Ani, F. N. & Jusoh, A. Fruit waste as feedstock for recovery by pyrolysis technique. Int. Biodeterior. Biodegrad. 113, 325–333 (2016).
Bao, D. et al. Metal-modified sludge-based biochar enhance catalytic capacity: characteristics and mechanism. J. Environ. Manag. 284, 112113 (2021).
Cao, B. et al. One-step self-assembly of Fe–biochar composite for enhanced persulfate activation to phenol degradation: different active sites-induced radical/non-radical mechanism. Chemosphere 322, 138168 (2023).
Qian, L. et al. Nanoscale zero-valent iron supported by biochars produced at different temperatures: synthesis mechanism and effect on Cr(VI) removal. Environ. Pollut. 223, 153–160 (2017).
Feng, Z. et al. Influence mechanisms of different stalks on iron species type of magnetic biochar prepared from Fe2O3. Sci. Total Environ. 903, 166790 (2023).
Yan, N. et al. Twice-milled magnetic biochar: a recyclable material for efficient removal of methylene blue from wastewater. Bioresour. Technol. 372, 128663 (2023).
Liu, K. et al. High Fe utilization efficiency and low toxicity of Fe3C@FeO loaded biochar for removing of tetracycline hydrochloride in wastewater. J. Clean. Prod. 353, 131630 (2022).
Wang, J., Yuan, S., Dai, X. & Dong, B. Application, mechanism and prospects of Fe-based/Fe–biochar catalysts in heterogenous ozonation process: a review. Chemosphere 319, 138018 (2023).
Pi, Z. et al. Persulfate activation by oxidation biochar supported magnetite particles for tetracycline removal: performance and degradation pathway. J. Clean. Prod. 235, 1103–1115 (2019).
Li, X. et al. Removal of bisphenol A in a heterogeneous Fenton system via biochar synthesized using different Fe precursors: properties, effects, and mechanisms. Sci. Total Environ. 912, 168855 (2024).
Liang, C. et al. Pyrolysis temperature-switchable Fe–N sites in pharmaceutical sludge biochar toward peroxymonosulfate activation for efficient pollutants degradation. Water Res. 228, 119328 (2023).
Zhang, D. et al. Enhanced nitrobenzene reduction by modified biochar supported sulfidated nano zerovalent iron: comparison of surface modification methods. Sci. Total Environ. 694, 133701 (2019).
Du, S. et al. Environmental fate and impacts of microplastics in aquatic ecosystems: a review. RSC Adv. 11, 15762–15784 (2021).
Wang, J. et al. Treatment of refractory contaminants by sludge-derived biochar/persulfate system via both adsorption and advanced oxidation process. Chemosphere 185, 754–763 (2017).
Wang, Y. et al. Mechanochemical synthesis of biochar encapsulated FeMn nanoparticles with strong metal–carbon interactions for efficient degradation of tetracycline via activating peroxymonosulfate. Chem. Eng. J. 479, 147525 (2024).
Kumar, R. et al. Adsorptive behavior of micro(nano)plastics through biochar: co-existence, consequences, and challenges in contaminated ecosystems. Sci. Total Environ. 856, 159097 (2023).
Wu, H. et al. Modified biochar supported Ag/Fe nanoparticles used for removal of cephalexin in solution: characterization, kinetics and mechanisms. Colloids Surf. A Physicochem. Eng. Asp. 517, 63–71 (2017).
Diao, Z.-H. et al. Synergistic oxidation of Bisphenol A in a heterogeneous ultrasound-enhanced sludge biochar catalyst/persulfate process: reactivity and mechanism. J. Hazard Mater. 384, 121385 (2020).
Mei, S. C., Li, L., Huang, G. X., Pan, X. Q. & Yu, H. Q. Heterogeneous Fenton water purification catalyzed by iron phosphide (FeP). Water Res. 241, 120151 (2023).
Xu, Y. et al. Defects and oxygen functional groups on sludge biochar synergistic activation of PMS for degradation of sulfamethoxazole and practical application. J. Environ. Chem. Eng. 12, 114958 (2024).
Zheng, J. et al. Peroxymonosulfate activation by Mg-introduced Fe-N carbon nanotubes to accelerate sulfamethoxazole degradation: singlet oxygen-dominated nonradical pathway. Chem. Eng. J. 452, 139233 (2023).
Ruan, X. et al. Formation, characteristics, and applications of environmentally persistent free radicals in biochars: a review. Bioresour. Technol. 281, 457–468 (2019).
Cheng, X. et al. Metal-free carbocatalysis for persulfate activation toward nonradical oxidation: enhanced singlet oxygen generation based on active sites and electronic property. Chem. Eng. J. 396, 125107 (2020).
Tang, Y., Dou, J., Lu, Z., Xu, J. & He, Y. Accelerating Fe2+/Fe3+ cycle via biochar to improve catalytic degradation efficiency of the Fe3+/persulfate oxidation. Environ. Pollut. 316, 120669 (2023).
Zhang, C., Lu, J. & Wu, J. One-step green preparation of magnetic seaweed biochar/sulfidated Fe0 composite with strengthen adsorptive removal of tetrabromobisphenol A through in situ reduction. Bioresour. Technol. 307, 123170 (2020).
Oh, S.-Y., Seo, Y.-D. & Ryu, K.-S. Reductive removal of 2,4-dinitrotoluene and 2,4-dichlorophenol with zero-valent iron-included biochar. Bioresour. Technol. 216, 1014–1021 (2016).
Zhang, Y. et al. Novel carbothermal synthesis of Fe, N co-doped oak wood biochar (Fe/N-OB) for fast and effective Cr(VI) removal. Colloids Surf. A Physicochem. Eng. Asp. 600, 124926 (2020).
Zhong, D. et al. Mechanistic insights into adsorption and reduction of hexavalent chromium from water using magnetic biochar composite: key roles of Fe3O4 and persistent free radicals. Environ. Pollut. 243, 1302–1309 (2018).
Li, H. et al. Biochar supported Ni/Fe bimetallic nanoparticles to remove 1,1,1-trichloroethane under various reaction conditions. Chemosphere 169, 534–541 (2017).
Tao, S. et al. Enhanced sludge dewatering via homogeneous and heterogeneous Fenton reactions initiated by Fe-rich biochar derived from sludge. Chem. Eng. J. 372, 966–977 (2019).
Yi, Y., Tu, G., Eric Tsang, P. & Fang, Z. Insight into the influence of pyrolysis temperature on Fenton-like catalytic performance of magnetic biochar. Chem. Eng. J. 380, 122518 (2020).
Rubeena, K. K., Hari Prasad Reddy, P., Laiju, A. R. & Nidheesh, P. V. Iron impregnated biochars as heterogeneous Fenton catalyst for the degradation of acid red 1 dye. J. Environ. Manag. 226, 320–328 (2018).
Zhang, H., Xue, G., Chen, H. & Li, X. Hydrothermal synthesizing sludge-based magnetite catalyst from ferric sludge and biosolids: formation mechanism and catalytic performance. Sci. Total Environ. 697, 133986 (2019).
Chen, X.-L., Li, F., Chen, H., Wang, H. & Li, G. Fe2O3/TiO2 functionalized biochar as a heterogeneous catalyst for dyes degradation in water under Fenton processes. J. Environ. Chem. Eng. 8, 103905 (2020).
Xue, G. et al. Simultaneous removal of aniline, antimony and chromium by ZVI coupled with H2O2: implication for textile wastewater treatment. J. Hazard. Mater. 368, 840–848 (2019).
Hao, M. et al. Efficient degradation of 2,4-dichlorophphenol in groundwater using persulfate activated by nitrogen-doped biochar-supported nano zero-valent iron. J. Clean. Prod. 458, 142415 (2024).
Jiang, S.-F. et al. High efficient removal of bisphenol A in a peroxymonosulfate/iron functionalized biochar system: mechanistic elucidation and quantification of the contributors. Chem. Eng. J. 359, 572–583 (2019).
Alidadi, H. et al. Enhanced removal of tetracycline using modified sawdust: optimization, isotherm, kinetics, and regeneration studies. Process Saf. Environ. Prot. 117, 51–60 (2018).
Jin, R. et al. Competitive adsorption of sulfamethoxazole and bisphenol A on magnetic biochar: mechanism and site energy distribution. Environ. Pollut. 329, 121662 (2023).
Alawa, B. et al. Development of novel biochar adsorbent using agricultural waste biomass for enhanced removal of ciprofloxacin from water: insights into the isotherm, kinetics, and thermodynamic analysis. Chemosphere 375, 144252 (2025).
Huang, P., Zhang, P., Wang, C., Tang, J. & Sun, H. Enhancement of persulfate activation by Fe-biochar composites: synergism of Fe and N-doped biochar. Appl. Catal. B 303, 120926 (2022).
Fang, G., Liu, C., Gao, J., Dionysiou, D. D. & Zhou, D. Manipulation of persistent free radicals in biochar to activate persulfate for contaminant degradation. Environ. Sci. Technol. 49, 5645–5653 (2015).
Zhao, G. et al. Iron-based catalysts for persulfate-based advanced oxidation process: microstructure, property and tailoring. Chem. Eng. J. 421, 127845 (2021).
Wang, W. et al. Catalyst-free activation of persulfate by visible light for water disinfection: efficiency and mechanisms. Water Res. 157, 106–118 (2019).
Wang, W., Wang, H., Li, G., Wong, P. K. & An, T. Visible light activation of persulfate by magnetic hydrochar for bacterial inactivation: efficiency, recyclability and mechanisms. Water Res. 176, 115746 (2020).
Kim, C., Ahn, J.-Y., Kim, T. Y., Shin, W. S. & Hwang, I. Activation of persulfate by nanosized zero-valent iron (NZVI): mechanisms and transformation products of NZVI. Environ. Sci. Technol. 52, 3625–3633 (2018).
Xiao, S. et al. Iron-mediated activation of persulfate and peroxymonosulfate in both homogeneous and heterogeneous ways: a review. Chem. Eng. J. 384, 123265 (2020).
Rodriguez, S., Santos, A. & Romero, A. Oxidation of priority and emerging pollutants with persulfate activated by iron: effect of iron valence and particle size. Chem. Eng. J. 318, 197–205 (2017).
Wu, S. et al. Insights into atrazine degradation by persulfate activation using composite of nanoscale zero-valent iron and graphene: performances and mechanisms. Chem. Eng. J. 341, 126–136 (2018).
Zhang, B. et al. Combining chemical oxidation and bioremediation for petroleum polluted soil remediation by BC-nZVI activated persulfate. Chem. Eng. J. 382, 123055 (2020).
Jiang, X., Guo, Y., Zhang, L., Jiang, W. & Xie, R. Catalytic degradation of tetracycline hydrochloride by persulfate activated with nano FeO immobilized mesoporous carbon. Chem. Eng. J. 341, 392–401 (2018).
Yu, J. et al. Efficient degradation of chloramphenicol by zero-valent iron microspheres and new insights in mechanisms. Appl. Catal. B 256, 117876 (2019).
Ansaf, K. V. K., Ambika, S. & Nambi, I. M. Performance enhancement of zero valent iron based systems using depassivators: optimization and kinetic mechanisms. Water Res. 102, 436–444 (2016).
Xu, Z. et al. Electroactive Fe–biochar for redox-related remediation of arsenic and chromium: distinct redox nature with varying iron/carbon speciation. J. Hazard. Mater. 430, 128479 (2022).
Zhang, P. et al. A green biochar/iron oxide composite for methylene blue removal. J. Hazard. Mater. 384, 121286 (2020).
Park, J. H., Wang, J. J. & Seo, D. C. Comparison of catalytic activity for treating recalcitrant organic pollutant in heterogeneous fenton oxidation with iron-impregnated biochar and activated carbon. J. Water Process Eng. 42, 102141 (2021).
Liu, B.-L. et al. Adsorption of microcystin contaminants by biochars derived from contrasting pyrolytic conditions: characteristics, affecting factors, and mechanisms. Sci. Total Environ. 763, 143028 (2021).
Rosales, E., Meijide, J., Pazos, M. & Sanromán, M. A. Challenges and recent advances in biochar as low-cost biosorbent: from batch assays to continuous-flow systems. Bioresour. Technol. 246, 176–192 (2017).
Qiu, B., Shao, Q., Shi, J., Yang, C. & Chu, H. Application of biochar for the adsorption of organic pollutants from wastewater: modification strategies, mechanisms and challenges. Sep. Purif. Technol. 300, 121925 (2022).
Pariyar, P., Kumari, K., Jain, M. K. & Jadhao, P. S. Evaluation of change in biochar properties derived from different feedstock and pyrolysis temperature for environmental and agricultural application. Sci. Total Environ. 713, 136433 (2020).
Wang, J. & Wang, S. Preparation, modification and environmental application of biochar: a review. J. Clean. Prod. 227, 1002–1022 (2019).
Li, N. et al. Municipal solid waste derived biochars for wastewater treatment: production, properties and applications. Resour. Conserv Recycl 177, 106003 (2022).
Raj, A. et al. Preparation, characterization and agri applications of biochar produced by pyrolysis of sewage sludge at different temperatures. Sci. Total Environ. 795, 148722 (2021).
Cai, M. et al. An efficient, economical, and easy mass production biochar supported zero˗valent iron composite derived from direct˗reduction natural goethite for Cu(II) and Cr(VI) remove. Chemosphere 285, 131539 (2021).
Sakhiya, A. K., Anand, A. & Kaushal, P. Production, activation, and applications of biochar in recent times. Biochar 2, 253–285 (2020).
Wang, J., Sun, C., Huang, Q.-X., Chi, Y. & Yan, J.-H. Adsorption and thermal degradation of microplastics from aqueous solutions by Mg/Zn modified magnetic biochars. J. Hazard. Mater. 419, 126486 (2021).
Dai, Y., Li, J. & Shan, D. Adsorption of tetracycline in aqueous solution by biochar derived from waste Auricularia auricula dregs. Chemosphere 238, 124432 (2020).
Jagadeesh, N. & Sundaram, B. Adsorption of pollutants from wastewater by biochar: a review. J. Hazard. Mater. Adv. 9, 100226 (2023).
Bai, L., Su, X., Feng, J. & Ma, S. Preparation of sugarcane bagasse biochar/nano-iron oxide composite and mechanism of its Cr (VI) adsorption in water. J. Clean. Prod. 320, 128723 (2021).
Das, S. K., Ghosh, G. K., Avasthe, R. K. & Sinha, K. Compositional heterogeneity of different biochar: effect of pyrolysis temperature and feedstocks. J. Environ. Manag. 278, 111501 (2021).
Qiu, B. et al. Research progress in the preparation of high-quality liquid fuels and chemicals by catalytic pyrolysis of biomass: a review. Energy Convers. Manag. 261, 115647 (2022).
Leng, L. et al. An overview on engineering the surface area and porosity of biochar. Sci. Total Environ. 763, 144204 (2021).
Hao, J. et al. A stable Fe/Co bimetallic modified biochar for ofloxacin removal from water: adsorption behavior and mechanisms. RSC Adv. 12, 31650–31662 (2022).
Liu, Y. & Wang, J. Multivalent metal catalysts in Fenton/Fenton-like oxidation system: a critical review. Chem. Eng. J. 466, 143147 (2023).
Medha, I. et al. (3-Aminopropyl)triethoxysilane and iron rice straw biochar composites for the sorption of Cr (VI) and Zn (II) using the extract of heavy metals contaminated soil. Sci. Total Environ. 771, 144764 (2021).
Kim, J., Song, J., Lee, S.-M. & Jung, J. Application of iron-modified biochar for arsenite removal and toxicity reduction. J. Ind. Eng. Chem. 80, 17–22 (2019).
Wu, L., Zhang, S., Wang, J. & Ding, X. Phosphorus retention using iron (II/III) modified biochar in saline-alkaline soils: adsorption, column and field tests. Environ. Pollut. 261, 114223 (2020).
Cheng, H. et al. Iron-Modified biochar strengthens simazine adsorption and decreases simazine decomposition in the soil. Front. Microbiol. 13, 1–10 (2022).
Liu, Y. et al. Iron-biochar production from oily sludge pyrolysis and its application for organic dyes removal. Chemosphere 301, 134803 (2022).
Islam, T. et al. Synthesis of rice husk-derived magnetic biochar through liquefaction to adsorb anionic and cationic dyes from aqueous solutions. Arab. J. Sci. Eng. 46, 233–246 (2021).
Ahmad, M. et al. Biochar as a sorbent for contaminant management in soil and water: a review. Chemosphere 99, 19–33 (2014).
Nasrollahpour, S., Pulicharla, R. & Brar, S. K. Functionalized biochar for the removal of poly- and perfluoroalkyl substances in aqueous media. iScience 28, 112113 (2025).
Wang, J. et al. Analysis of the long-term effectiveness of biochar immobilization remediation on heavy metal contaminated soil and the potential environmental factors weakening the remediation effect: a review. Ecotoxicol. Environ. Saf. 207, 111261 (2021).
Pan, J., Deng, H., Du, Z., Tian, K. & Zhang, J. Design of nitrogen–phosphorus-doped biochar and its lead adsorption performance. Environ. Sci. Pollut. Res. 29, 28984–28994 (2022).
Gupta, R. et al. Potential and future prospects of biochar-based materials and their applications in removal of organic contaminants from industrial wastewater. J. Mater. Cycles Waste Manag. 24, 852–876 (2022).
Panwar, N. L. & Pawar, A. Influence of activation conditions on the physicochemical properties of activated biochar: a review. Biomass Convers. Biorefin. 12, 925–947 (2022).
Shang, H. et al. Preparation of nitrogen doped magnesium oxide modified biochar and its sorption efficiency of lead ions in aqueous solution. Bioresour. Technol. 314, 123708 (2020).
Samaraweera, H. et al. Pyrolyzed Ca-impregnated lignite for aqueous phosphate removal: batch and column studies. J. Environ. Chem. Eng. 9, 106077 (2021).
Micah Elias, J. H. J. R. P. S. D. L. S. Biochar Carbon Credit Market Analysis (Blue Forest, 2022).
Reverse Osmosis to Remove Arsenic from Drinking Water.
Shao, F., Wang, Y., Mao, Y., Shao, T. & Shang, J. Degradation of tetracycline in water by biochar supported nanosized iron activated persulfate. Chemosphere 261, 127844 (2020).
Sun, P. et al. Efficient removal of crystal violet using Fe3O4-coated biochar: the role of the Fe3O4 nanoparticles and modeling study their adsorption behavior. Sci. Rep. 5, 12638 (2015).
Shan, D. et al. Preparation of ultrafine magnetic biochar and activated carbon for pharmaceutical adsorption and subsequent degradation by ball milling. J. Hazard. Mater. 305, 156–163 (2016).
Acknowledgements
The authors wish to acknowledge partial funding through the National Science Foundation Established Program to Stimulate Competitive Research projects Grant RII Track 2 (# 2019561) and Grant RII Track 4 (# 2228903). This publication has not been formally reviewed by NSF. The views expressed in this publication are solely those of authors and do not necessarily reflect those of NSF.
Author information
Authors and Affiliations
Contributions
Md Abdullah Al Masud: Conceptualization, investigation, methodology, data curation, software, writing—original draft, and editing. Hasara Samaraweera, Md. Mahmudul Hassan Mondol, Ardie Septian, and Rakesh Kumar: Writing—original draft and editing. Leigh Terry: Funding acquisition, conceptualization, methodology, investigation, writing—reviewing, and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Masud, M.A.A., Samaraweera, H., Mondol, M.M.H. et al. Iron biochar synergy in aquatic systems through surface functionalities electron transfer and reactive species dynamics. npj Clean Water 8, 46 (2025). https://doi.org/10.1038/s41545-025-00471-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41545-025-00471-5