Abstract
Mucosal immunity provides efficient protection against upper-airway infections, limiting viral shedding and transmission. However, currently, no nasal spray COVID-19 vaccines are approved by WHO for global use. Here we develop a two-component intranasal vaccine that combines an adenovirus vector expressing the spike protein of the XBB.1.5 variant (Ad5XBB.1.5) with a self-assembled trimeric recombinant protein derived from the receptor binding domain (RBDXBB.1.5-HR). This two-component vaccine elicits superior humoral and cellular immunity against XBB.1.5 variants compared with the individual components. It also provides protective immunity against live XBB.1.16 virus challenges in mice, and prevents XBB.1.5 virus transmission in a hamster model. Notably, the activation of the STING signalling pathway in mucosal dendritic cells is essential for the adjuvant effect of the adenovirus vector. We also incorporate another trimeric protein from the BA.5 variant (RBDBA.5-HR), creating a three-component vaccine (Ad5XBB.1.5 + RBDXBB.1.5-HR + RBDBA.5-HR) that shows enhanced broad-spectrum neutralization. The two-component vaccine demonstrates high tolerability and safety in humans, inducing enhanced mucosal immunity and high levels of neutralizing antibodies in all participants. Our findings underscore this strategy for clinical COVID-19 intranasal vaccine development.
Similar content being viewed by others
Main
The Omicron variant (B.1.1.529) of SARS-CoV-2 continues to circulate globally and has diversified into various subvariants. Among these, the XBB lineages, known for enhanced immune evasion, have produced subvariants such as XBB.1.5, XBB.1.16 (ref. 1), EG.5.1 (ref. 2) and HV.1 (ref. 3). Recently, subvariants from BA.2.86, including JN.1 (ref. 4), KP.2 and KP.3, have also arisen and are competing globally. Given their resistance to neutralizing antibodies from previous vaccinations and infections, and the potential effects of immune imprinting, the US Food and Drug Administration (US FDA) has recommended using antigens from the XBB descendant lineage in the next generation of vaccines5.
To enhance the effectiveness of respiratory virus vaccines, it is crucial to optimize vaccine delivery for a robust mucosal immune response. Currently, most COVID-19 vaccines approved for emergency use are administered intramuscularly, providing protection against symptomatic infections and severe disease6,7,8,9,10. However, theses vaccines may not completely prevent SARS-CoV-2 shedding and transmission, as it does not effectively induce mucosal immunity in the upper airways, leading to asymptomatic or milder infections that can still spread the virus11,12. Leveraging the advantages of activating mucosal immunity, the US government has launched Project NextGen, a US$5 billion initiative to fast-track coronavirus vaccines, including the development of a nasal vaccine. This strategy uses intranasal delivery to stimulate local mucosal immunity at the entry point of SARS-CoV-2, focusing on the production of secretory IgA (sIgA) antibodies and tissue-resident memory T (TRM) cells13,14,15,16.
Great efforts have been made to develop the next-generation intranasal COVID-19 vaccines. Various platforms are being explored, including viral vectors (such as adenovirus14,16,17, live-attenuated influenza18, parainfluenza19, respiratory syncytial virus20 and Newcastle disease virus)21, adjuvanted-protein approaches (using polymersomes22, membrane vesicles23, nanoparticles24,25 and Toll-like receptor agonists26,27), as well as nucleic acid-based vaccines28,29 (both DNA and RNA). These diverse strategies are currently in preclinical study to advance intranasal vaccine development. Currently, more than ten intranasal vaccine candidates are under clinical investigation, yet none have received World Health Organization approval for global use. An effective intranasal SARS-CoV-2 vaccine should ideally stimulate both mucosal and systemic immune responses30,31,32,33, leading to the production of neutralizing antibodies that could curb transmission and protect against symptomatic and severe disease. However, recent clinical trials have shown disappointing results, particularly with viral vector vaccines. For instance, a Phase I trial of the adenovirus-vectored ChAdOx1 nCoV-19 vaccine revealed inadequate levels of plasma and nasal mucosal antibodies, with mucosal responses seen only in a minority of participants34. These findings underscore the need for safe and effective platforms for intranasal vaccination. In addition, many of these intranasal vaccines are based on pre-Omicron variants, lacking neutralizing efficacy against newly emerged variants35,36.
The subunit protein-based vaccine platform has been widely explored for intranasal delivery. Research indicates that intranasal delivery of recombinant protein antigens alone often fails to induce protective immunity due to their low immunogenicity, necessitating the use of adjuvants to improve the magnitude and durability of the mucosal immunity induced by protein antigens24,26,37,38,39. Various adjuvants, including toxoids (CTA1DD and LThaK), polymers (chitosan and PEI), pattern recognition receptor agonists (Poly I:C, monophosphoryl lipid A (MPL), c-di-GMP) and cytokines (IL-12, IFN-I, IL-1 family and TNF family), have shown promise in boosting the immunogenicity of recombinant proteins24,30. Nevertheless, concerns over safety have limited their approval for clinical use, leaving no mucosal adjuvanted protein-based COVID-19 vaccines currently authorized for emergency use by WHO.
The adenovirus vector serves as a noteworthy platform for COVID-19 vaccines, having been utilized in the development of intranasal SARS-CoV-2 vaccines in both preclinical and clinical studies30,37, demonstrating protective efficacy in animals14,16,40. Remarkably, adenovirus vectors can activate the host’s defence mechanisms through intricate pathways, including Toll-like receptors (TLR-2, TLR-9), DNA sensors (cGAS along with the adapter STING) and inflammasome signalling (AIM2, NLRP3), thereby amplifying immune responses41,42,43. This underscores their dual role—not only as effective delivery carriers of viral antigens but also as potential adjuvants for subunit protein-based vaccines. By combining adenovirus-vectored and protein-based approaches, we may unveil a platform for intranasal COVID-19 vaccines, offering enhanced protection in local mucosa.
In addition to the idea that adenoviruses may serve as adjuvants for protein-based intranasal vaccines, adenovirus vectors and subunit protein vaccines activate immune responses through distinct mechanisms. Subunit protein antigens are primarily processed via the lysosomal pathway in antigen-presenting cells (APCs), activating CD4+ T cells through the MHC class II pathway to stimulate humoral immunity44. However, their ability to activate CD8+ T cells is limited, as most internalized antigens are not processed in the cytosol. In contrast, adenovirus vector-based antigens are mainly processed by the intracellular proteasome and presented via the MHC class I pathway, effectively eliciting a cellular immune response that involves CD8+ T cells45,46,47. Given these differences, we hypothesize that a two-component vaccine combining an adenovirus vector carrying one SARS-CoV-2 antigen with a subunit protein-based viral antigen could elicit superior immune responses compared with the individual components alone.
Preclinical and clinical studies have already shown that updated vaccines targeting XBB.1.5 can neutralize multiple Omicron subvariants, including JN.1 (refs. 48,49,50,51). In this study, we developed an adenovirus vector carrying the spike protein of XBB.1.5 (Ad5XBB.1.5), and produced self-assembled trimeric proteins derived from XBB.1.5 and BA.5 (RBDXBB.1.5-HR and RBDBA.5-HR) using the spike heptad-repeat (HR) sequence52,53. We then mixed the adenovirus and protein antigen to formulate the two-component (Ad5XBB.1.5 + RBDXBB.1.5-HR) and three-component (Ad5XBB.1.5 + RBDXBB.1.5-HR + RBDBA.5-HR) vaccines. Compared with the adenovirus vector alone, these multicomponent vaccines induced superior cellular and humoral immune responses, effectively neutralizing XBB-lineage variants. The three-component vaccine further enhanced cross-neutralizing responses against a broader range of Omicron subvariants, both as a standalone vaccine and as a sequential booster, underscoring its potential as a multivalent vaccine platform. Mechanistically, activation of the STING signalling pathway, particularly in mucosal dendritic cells, is crucial for the adenovirus vector-induced adjuvant effect. Intranasal administration of the two-component vaccines provided effective protection against live XBB-lineage virus challenges and transmission in animal models. In human participants, the two-component vaccine demonstrated good tolerability and safety, along with improved systemic and mucosal antibody responses.
Results
The two-component intranasal vaccine elicits stronger humoral immunity
To investigate the potential enhanced effects of combining the adenovirus vectors and protein-based vaccines in eliciting superior immune responses, we produced an adenovirus vector carrying the intact full-length spike of XBB.1.5 (Ad5XBB.1.5) (Fig. 1a and Extended Data Fig. 1a) and a trimeric XBB.1.5 RBD-derived protein (RBDXBB.1.5-HR) (Fig. 1b). We formulated the two doses of two-component vaccines by mixing Ad5XBB.1.5 with RBDXBB.1.5-HR in different ratios (Fig. 1c and Extended Data Fig. 1b). First, we added buffer to dilute a certain amount of RBDXBB.1.5-HR protein stock solution, adjusting the pH to 7.6 to ensure consistency with the buffer composition of the Ad5XBB.1.5 adenovirus stock solution. Next, we slowly added the adenovirus stock solution to achieve final concentrations of 5 × 1010 virus particles (VPs) ml−1 and 100 μg ml−1, or 1.0 × 1011 VPs ml−1 and 200 μg ml−1. To prevent adenovirus aggregation during vaccine preparation, the adenovirus should be mixed slowly using magnetic stirring while being added (Extended Data Fig. 1b). We characterized the size distribution and infectivity of the adenovirus in the two-component preparation and found that the mixing process did not affect the particle size or infectivity of the adenovirus component (Extended Data Fig. 1c,d). In addition, stability assay results demonstrated that the two-component vaccine can be stored stably at −20 °C, with unchanged infectivity of the adenovirus component and protein antigenic content (Extended Data Fig. 1e).
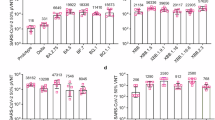
a, Top: schematic representation of the design of full-length XBB.1.5 spike protein-carrying adenovirus. Bottom: western blotting of spike protein expression in A549 cells in the absence or presence of Ad5XBB.1.5. SP, signal peptide; NTD, N-terminal domain; RBD, receptor binding domain; HR1 and HR2, heptad repeats 1 and 2; TM, transmembrane domain; CP, cytoplasmic domain; Mr, relative molecular mass. b, Top: RBDXBB.1.5-HR protein includes an RBD derived from the XBB.1.5 variant, and HR1 and HR2 domains in subunit S2 of spike protein. Bottom: representative elution chromatographs of the recombinant RBDXBB.1.5-HR protein using a calibrated Superdex 200 Increase column. SDS–PAGE analysis of the eluted protein is shown. M, marker; 1, the eluted sample of the ascending part of the protein peak; 2, the eluted sample of the descending part of the peak. Panels a and b are representative of two independent experiments with similar results. mAU, milli absorbance unit. c, Schematic representation of the preparation of the two-component vaccine, immunization and sample collection protocol. BALB/c mice were intranasally immunized with a low dose (2.5 × 109 VPs adenovirus + 5 μg protein) or high dose (5 × 109 VPs adenovirus + 10 μg protein) of the two-component vaccine 3 times at 0, 4 and 8 weeks. Serum and BALF samples in d–i were collected at 11 weeks after the first immunization. d,e, Endpoint titres of anti-RBD IgG in mouse serum (d) and BALF (e) samples (n = 6 mice per group). f, Endpoint titres of anti-RBD IgA in BALF (left) and nasal lavage fluid (NLF) (right) samples (n = 6 mice per group). g,h, Neutralizing antibody titres in serum (g) and BALF (h) samples against Prototype, Delta, BA.2.75, BA.4/5, BF.7, BQ.1, BQ.1.1, XBB, XBB.1.5 and XBB.1.16 pseudoviruses (n = 6 mice per group). pVNT, pseudovirus neutralization titre. i, Sera neutralizing antibody titres against authentic SARS-CoV-2, including Delta, BA.2, BA.5.2.48 and XBB.1.16 (n = 5 mice per group). The numbers above the data points in g–i indicate the GMTs of 50% neutralization for each group, as well as the fold comparisons of GMTs between groups. Data are presented as geometric mean ± s.d. P values in d–f were determined using two-tailed unpaired Student’s t-tests. Schematic illustrations in a–c created with BioRender.com.
We intranasally vaccinated mice with a low dose or high dose of the two-component vaccine containing 2.5 × 109 or 5 × 109 VPs of Ad5XBB.1.5 with 5 μg or 10 μg of RBDXBB.1.5-HR, respectively. Control groups received either naked RBDXBB.1.5-HR or Ad5XBB.1.5 alone. All animals were vaccinated following a prime–boost regimen with a 28-day interval (Fig. 1c). We observed that intranasal delivery of RBDXBB.1.5-HR protein alone hardly induced RBD-specific IgG antibodies in serum samples, while immunization with Ad5XBB.1.5 alone induced a sustained binding antibody response (Fig. 1d). Of note, the combination of Ad5XBB.1.5 and RBDXBB.1.5-HR resulted in a significant increase in the titres of antigen-specific IgG antibodies compared with Ad5XBB.1.5 alone (Fig. 1d), irrespective of the dose. Mucosal antibody response, particularly secretory IgA, plays an important role in mucosal immunity conferred by intranasal COVID-19 vaccines. Recent studies demonstrated that secretory IgA exhibits significantly higher neutralizing potency against Omicron than serum IgG and IgA54,55. Therefore, we further investigated the production of antigen-specific IgG and IgA in the bronchoalveolar lavage fluid (BALF) and nasal lavage fluid from vaccinated mice. Consistent with the results of binding antibodies in sera, the combination of Ad5XBB.1.5 and RBDXBB.1.5-HR induced higher titres of IgG and IgA in the respiratory tract compared with immunization with adenovirus-vectored vaccine alone (Fig. 1e,f). It is worth noting that the endpoint titres of antigen-specific IgG and IgA in the serum and BALF and NLF samples were orders of magnitude higher when RBDXBB.1.5-HR protein was administered with an empty adenovirus vector (Ad5Empty+RBDXBB.1.5-HR), suggesting that the adenovirus vector can improve the immunogenicity of subunit protein and may serve as a favourable adjuvant candidate for protein-based intranasal vaccines (Fig. 1d–f).
We then carried out pseudovirus neutralization assays to evaluate the neutralizing capacities induced by combined spike-carrying adenovirus and RBD-HR proteins. As expected, RBDXBB.1.5-HR protein alone induced only a negligible neutralization response, while the adenovirus-vector vaccine alone induced a relatively strong neutralizing response (Fig. 1g). Notably, the combination of Ad5XBB.1.5 and RBDXBB.1.5-HR remarkably enhanced the cross-neutralization capacities against all types of pseudoviruses. For instance, the geometric mean titres (GMTs) of 50% neutralization in the high-dose Ad5XBB.1.5 group against prototype, Delta and Omicron subvariants BA.2.75, BA.4/5, BF.7, BQ.1, BQ.1.1, XBB, XBB.1.5 and XBB.1.16 were determined to be 387, 34, 2,694, 5,218, 6,791, 4,635, 3,820, 11,803, 26,965 and 8,419, respectively, whereas the GMTs in the combination group of high-dose Ad5XBB.1.5 and RBDXBB.1.5-HR protein were improved by 5.1-, 23.4-, 2.0-, 1.6-, 1.7-, 2.4-, 2.8-, 2.5-, 2.5- and 2.9-fold, respectively. In addition, compared with immunization with low-dose Ad5XBB.1.5 alone, the neutralizing antibody titres in the group receiving the low-dose two-component vaccine increased by 2.8-, 15.5-, 2.6-, 1.4-, 1.4-, 2.0-, 1.3-, 3.0-, 1.6- and 3.7-fold, respectively. Even when comparing the high-dose adenoviral group with the low-dose two-component vaccine group, the latter still exhibited enhanced neutralizing activity to some extent. Similar enhanced effects in neutralization were also observed in the BALF samples (Fig. 1h). It is worth noting that the addition of Ad5Empty also improved the neutralizing antibody titres in sera and respiratory tracts induced by RBDXBB.1.5-HR (Fig. 1g,h). To further confirm the neutralizing potency of antibodies induced by the combination of Ad5XBB.1.5 and RBDXBB.1.5-HR, authentic virus neutralizing assays were performed (Fig. 1i). Combining Ad5XBB.1.5 and RBDXBB.1.5-HR protein exhibited superior neutralization with cross-neutralizing activities against live BA.2 and BA.5.2.48 and XBB.1.16 viruses. Specifically, compared with Ad5XBB.1.5 alone, the GMTs for 50% neutralization against XBB.1.16 in the low-dose group of Ad5XBB.1.5 + RBDXBB.1.5-HR were improved by 3.5-fold, and by 5.3-fold in the high-dose group, and reached 1,176 and 1,783, respectively. The lower doses of adenovirus (5 × 107 VPs and 5 × 108 VPs) combined with RBD-HR protein induce similar superior humoral immune response compared with individual components (Extended Data Fig. 2a–d). These results indicated that the combination of adenovirus and subunit protein antigens can elicit superior humoral immune responses compared with the individual vaccine components.
The two-component vaccine induces superior airway cellular immunity
In addition to IgA antibodies, TRM cells are another dominant component of mucosal immunity, rapidly responding to prevent virus infection. In the next set of experiments, we detected the frequencies of TRM cells in BALF samples after vaccination. Consistent with our expectations, a significant increase in the frequency of antigen-experienced CD8+ TRM (CD44+CD69+CD103+), but not CD4+ TRM cells, was observed when combining the adenovirus and RBD-HR protein (Fig. 2a,b). Enzyme-linked immunospot assay (ELISpot) was employed to determine IFNγ-spot-forming cells (SFCs) in BALF samples after stimulation with full-length spike peptide pools, and the results showed greatest abundance of IFNγ-secreting cells in the group of the two-component vaccine (Fig. 2c). To further ascertain the antigen-specific T cells in the mucosal tissue, we isolated lung tissues processed into a single-cell suspension and subsequently stimulated with spike peptide pools to detect the expression of intracellular IFNγ and TNF cytokines via intracellular cytokine staining (ICS). The combination of Ad5XBB.1.5 and RBDXBB.1.5-HR resulted in increased numbers of spike-specific IFNγ- and TNF-secreting memory T cells, indicating that the two-component vaccine induced more robust T cell responses in the local mucosal environment compared with the adenovirus-vectored vaccine alone (Fig. 2d,e). Further analysis revealed that these increased mucosal TRM cells are spike protein antigen-responsive, as evidenced by their cytokine IFNγ production (antiviral effect) after stimulation with antigens (Extended Data Fig. 3).
a, Left: representative flow cytometry graphs showing antigen-experienced CD8+ TRM cells induced by Ad5XBB.1.5 or the two-component vaccine. Right: the absolute number of CD8+ TRM cells in BALF analysed by flow cytometry. Antigen-experienced TRM cells were gated on CD44+CD69+CD103+. b, Absolute numbers of antigen-experienced CD4+ TRM cells in BALF. c, ELISpot analysis of IFNγ-SFCs in BALF samples after stimulation with peptide pools of SARS-CoV-2 XBB.1.5 spike. d,e, Representative graphs and quantitative percentages of XBB.1.5 spike-specific IFNγ (d) and TNF (e)-producing memory T cells in lung tissue were analysed after stimulation with XBB.1.5 spike peptide pools. f,g, Frequency of T follicular helper cells (CD4+CXCR5+PD-1+) (f) and RBDXBB.1.5-specific germinal centre B cells (CD19+GL7+CD95+) (g) in mediastinal lymph nodes (mLN). h, IFNγ-SFCs among splenocytes after stimulation with XBB.1.5 spike peptide pools. All tissue samples in Fig. 2, including BALF, lung, mLN and spleen samples, were collected at week 11 after the first immunization. In a–h, n = 6 mice per group; data are presented as mean ± s.e.m. P values between the RBDXBB.1.5-HR and Ad5Empty + RBDXBB.1.5-HR groups, as well as between Ad5XBB.1.5 and Ad5XBB.1.5 + RBDXBB.1.5-HR were calculated using two-tailed unpaired Student’s t-tests. NS, not significant.
Germinal centre B cell (GC B) and T follicular helper (Tfh) cell responses are critical for long-term protective immunity and the formation of memory B cells56,57. Thus, we evaluated the Tfh (CD4+CXCR5+PD-1+) and RBD-specific GC B (CD19+GL7+CD95+) cell responses in mediastinal lymph nodes. As expected, combining adenovirus and protein induced the highest frequencies of Tfh and the largest total number of antigen-specific GC B cells, compared with immunization with Ad5XBB.1.5 alone (Fig. 2f,g). In addition to the stronger mucosal cellular immunity, we also noticed the improved antigen-specific splenocytes in the group receiving the two-component vaccine vs the adenovirus vector alone, suggesting a superior systemic cellular immune response (Fig. 2h). Similar enhancements in cellular immune response can be observed in the lower dose of the adenovirus plus protein antigens (Extended Data Fig. 2e,f). Thus, these results demonstrate that the two-component vaccines, which combine Ad5XBB.1.5 and RBDXBB.1.5-HR proteins, can provide superior mucosal and systemic cellular response against SARS-CoV-2 infection.
The STING pathway is critical for the adenovirus-vector adjuvant effect
It is remarkable that the combination of adenovirus and subunit protein can elicit superior immunity compared with individual components. Even the empty adenovirus vector can serve as an adjuvant to improve the immunogenicity of naked RBD-HR protein owing to its immunomodulatory property. To discover the potential signalling pathways involved in the adjuvant effects of the adenovirus vector, we intranasally administered the two-component vaccine to multiple transgenic mice deficient in signalling pathways associated with host immune response to adenovirus41,43, including Tlr2−/−, Tlr9−/−, Myd88−/−, Nlrp3−/−, Casp1−/−, Il1b−/−, Sting−/− and Cd8a−/− mice, and evaluated the improvements in antibodies induced by the two-component vaccine. Since the XBB lineages were not yet prevalent worldwide during our investigation of the signalling pathways, we utilized a two-component vaccine that combined BA.5 spike-carrying adenovirus (Ad5BA.5) and a BA.5-derived RBD-HR (RBDBA.5-HR) in this experiment (Extended Data Fig. 4a–c). The improvements in RBD-specific antibody titres were still observed in wildtype (WT) and most transgenic mice. However, there was no significant difference between the endpoint titres of sera IgG and BALF IgA antibodies induced by adenovirus alone compared with the two-component vaccines in Sting−/− mice (Fig. 3a,b). To further investigate the mechanism of adjuvant effect exerted by the adenovirus, we immunized the mice with the adenovirus vector without spike antigen (Ad5Empty) along with the RBDBA.5-HR protein. Consistent with the result above, the adjuvant effect of the adenovirus vector on systemic and mucosal humoral immune responses was remarkably impaired in the Sting−/− mice (Fig. 3c,d). Since MYD88 is the most important adaptor protein for TLR-2 and TLR-9 signalling pathways, the Myd88−/− mice were also utilized as controls for the subsequent evaluation of the adjuvant effect of the adenovirus vector on cellular immunity.
a, Endpoint titres of anti-RBD IgG in serum samples from WT or transgenic (Tlr9−/−, Tlr2−/−, Myd88−/−, Nlrp3−/−, Casp1−/−, Il1b−/−, Sting−/−, Cd8a−/−) mice immunized with Ad5BA.5 alone or Ad5BA.5 plus RBDBA.5-HR (n = 6 mice per group). b, Endpoint titres of anti-RBD IgA in BALF samples from WT or Sting−/−mice immunized with Ad5BA.5 alone or Ad5BA.5 plus RBDBA.5-HR (n = 6 mice per group). c, Endpoint titres of sera anti-RBD IgG from WT or transgenic mice immunized with Ad5Empty plus RBDBA.5-HR. WT mice intranasally vaccinated with naked RBDBA.5-HR were used as control (n = 6 mice per group). d, Endpoint titres of BALF IgA from WT or Sting−/−mice immunized with Ad5Empty plus RBDBA.5-HR (n = 6 mice per group). e, Absolute number of antigen-experienced CD8+ TRM cells in BALF from vaccinated WT, Myd88−/− or Sting−/− mice (n = 6 mice per group). f, t-SNE maps were generated by concentrating CD3+ T cells in the BALF from WT or Sting−/− mice vaccinated with Ad5BA.5 plus RBDBA.5-HR, and heat map projections of CD69 and CD103 expression on t-SNE maps are shown (n = 3 mice each group). Analysis was carried out using FlowJo v.10. g,h, Percentage of spike-specific CD8+ (g) and CD4+ (h) memory T cells in lung tissue of vaccinated WT, Myd88−/− or Sting−/− mice (n = 6 mice per group). i, Percentage of antigen-specific IFNγ-producing CD8+ T cells in lung from mice vaccinated with Ad5Empty + RBDBA.5-HR (n = 6 mice per group). Data are presented as geometric mean ± s.d. (a–d), and as mean with s.e.m. (e,g–i). P values were determined using two-tailed unpaired Student’s t-tests (a,b), one-way ANOVA followed by Dunnett’s multiple comparisons test (c) or Tukey’s multiple comparisons test (d), two-way ANOVA followed by Sidak’s multiple comparisons test (e–h), and one-way ANOVA followed by Tukey’s multiple comparisons test (i).
Next, we evaluated the role of STING signalling in the induction of mucosal cellular immunity. As expected, the increase in the number of CD8+ TRM induced by the combination of Ad5BA.5 and RBDBA.5-HR was abrogated in Sting−/− mice (Fig. 3e). We then established t-distributed stochastic neighbour embedding (t-SNE) maps based on pooled CD3+ T cells in BAL fluid from wildtype and Sting−/− mice. Heat maps were generated to visualize the expression intensities of CD69 and CD103, two classic surface markers for TRM cells (Fig. 3f). In wildtype mice, the expressions of CD69 and CD103 in CD3+ T cells were significantly increased after immunization with the two-component vaccine. In contrast, the expression of these two markers was remarkably decreased in Sting−/− mice, suggesting that STING signalling is critical for the induction of mucosal TRM cells. In addition, the enhancements of antigen-specific CD8+ and CD4+ T memory cells were significantly impaired in Sting knockout mice (Fig. 3g,h), and the adjuvant effect of the Ad5Empty on cellular immune response was completely abolished in the Sting−/− mice (Fig. 3i). These results indicate that the STING signalling pathway is crucial for the establishment of adenovirus-adjuvanted cellular immune response.
Single-cell RNA sequencing (scRNA-seq) was conducted to investigate the STING signalling pathway, which influences the immunization efficacy of the two-component vaccine. The cell populations were visualized using a t-SNE map (Extended Data Fig. 5a,b), revealing a significant increase in B memory cells in the lung tissues of wildtype mice following immunization, compared with Sting−/− mice. Gene expression analysis indicated that transcript levels of Tmem173, the gene encoding STING, were predominantly enriched in dendritic cell (DC) populations, including CCR7+ DCs, DC1 and plasmacytoid DCs (pDCs) (Extended Data Fig. 5c,d). Notably, these levels were significantly upregulated after administration of the two-component vaccine (Extended Data Fig. 5e). Next, we focused on the activation of the STING pathway by selecting key genes involved in the signalling cascade, including Adar, Ccl5, Ddx41, Ifi203, Ifit1, Irf7, Irf9, Isg15, Oas1a and Trim21. Our findings showed that this pathway was significantly activated in wildtype mice, whereas it was diminished in STING knockout mice immunized with the two-component vaccine (Extended Data Fig. 5f). This suggests that STING pathway activation in DC populations is crucial for the protective efficacy of the vaccine. Indeed, the expression levels of genes involved in antigen processing and presentation were markedly upregulated in DC populations from wildtype mice after immunization, while these levels were significantly inhibited in Sting−/− mice (Extended Data Fig. 5g). In addition, adenoviral vectors, including Ad5Empty and Ad5XBB.1.5, facilitated protein antigen capture in DCs in vitro, as evidenced by enhanced phagocytosis of fluorescence-conjugated RBDXBB.1.5-HR proteins in DCs in the presence of adenoviral vectors (Extended Data Fig. 5h). Furthermore, assessments of plasma cell formation and B cell activation revealed impaired humoral immunity in Sting−/− mice following immunization, consistent with reduced antibody production (Extended Data Fig. 5i). Therefore, these results demonstrate that the activation of the STING signalling pathway, particularly in antigen-presenting DCs, is imperative for the induction of protective immunity by the combination of adenovirus and protein antigens.
The three-component vaccine enhances cross-neutralizing activity
We have demonstrated that the combination of adenovirus-vectored and protein vaccines (Ad5XBB.1.5 + RBDXBB.1.5-HR, Ad5BA.5 + RBDBA.5-HR) can induce superior protective immunity. To investigate the potential of combining adenovirus and subunit protein antigens as a platform for developing a universal COVID-19 vaccine with broader-spectrum neutralizing activities, we generated a multivalent vaccine by incorporating additional antigens and adjusting component ratios. The goal was to target the transmission of multiple circulating Omicron subvariants. We developed an intranasal three-component vaccine consisting of Ad5XBB.1.5 (5 × 109 VPs), RBDXBB.1.5-HR (7.5 μg) and RBDBA.5-HR (2.5 μg) (Ad5XBB.1.5 + RBDXBB.1.5-HR + RBDBA.5-HR) (Fig. 4a). Similar to the two-component vaccines, mice immunized with the three-component vaccine exhibited superior humoral and cellular immune responses compared with those immunized with Ad5XBB.1.5 vaccine alone.
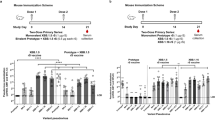
a, Schematic representations of the preparation of a three-component vaccine, consisting of Ad5XBB.1.5 (5 × 109 VPs), RBDXBB.1.5-HR (7.5 μg) and RBDBA.5-HR (2.5 μg). b,c, Endpoint titres of sera anti-RBD IgG (b), and BALF anti-RBD IgG and IgA (c) (n = 6 mice per group). d, Neutralizing antibody titres in sera samples (n = 6 mice per group). e, Absolute number of antigen-experienced CD8+ TRM cells in BALF (left), and the percentage of spike-specific IFNγ-secreting CD8+ T cells in lung tissue (right) from vaccinated mice (n = 6 mice per group). f, Frequency of Tfh cells (left) and the absolute number of RBD-specific GC B cells (right) in mediastinal lymph nodes (n = 6 mice per group). g–m, BALB/c mice were intramuscularly (i.m.) immunized with 3 doses of 5 μg mRNA/50 μl of encapsulated liposome (LPX)/Spike-mRNA, followed by 1 dose of homologous injection of mRNA vaccine (4× mRNA), or twice intranasal (i.n.) delivery of three-component vaccine (3× mRNA + 2× three-component vaccine) (g); n = 6 mice per group. The anti-RBD IgG in sera (h), anti-RBD IgG, IgA antibody (i) and TRM cells in BALF (j), spike-specific T cells in lung tissue (k), and neutralization antibody titres of sera (l) and BALF (m) samples were detected. The numbers above the data points in d, l and m indicate the GMTs of 50% neutralization for each group, as well as the fold comparisons of GMTs between groups in d. Data are presented as geometric mean ± s.d. (b–d,h,i,l,m), and as mean ± s.e.m. (e,f,j,k). P values between the RBDXBB.1.5-HR + RBDBA.5-HR and Ad5Empty + RBDXBB.1.5-HR + RBDBA.5-HR groups, as well as between Ad5XBB.1.5 and Ad5XBB.1.5 + RBDXBB.1.5-HR + RBDBA.5-HR were determined using two-tailed unpaired Student’s t-tests (b,c,e,f). P values between the homologous and heterologous immunization groups were determined using two-tailed unpaired Student’s t-tests (h,l,m); two-way ANOVA followed by Sidak’s multiple comparisons test was performed for i–k. Schematic in a and schematic illustration in g created with BioRender.com.
The three-component vaccine induced higher levels of binding antibodies in sera and the local respiratory tract (Fig. 4b,c). Pseudovirus neutralization assays further confirmed the improvements in neutralizing capacities (Fig. 4d). It is worth noting that the three-component vaccine further increased the neutralizing activities against other Omicron variants without compromising the neutralization of XBB lineages (Extended Data Fig. 6a). Specifically, the GMTs of 50% neutralization in the high dose of the two-component vaccine group against BA.2.75, BA.5, BF.7, BQ.1 and BQ.1.1 were improved by 2.0-, 1.6-, 1.7-, 2.4- and 2.8-fold, respectively, compared with Ad5XBB.1.5 (Fig. 1g), while the GMTs were improved by 3.6-, 3.3-, 3.3-, 3.2- and 2.5-fold after immunization of the three-component vaccine (Fig. 4d and Extended Data Fig. 6a). In addition, the GMTs in the group that received the three-component vaccine against XBB, XBB.1.5 and XBB.1.16 were also increased by 3.0-, 1.6- and 2.1-fold, respectively, compared with Ad5XBB.1.5 alone.
In addition to the humoral immune response, the three-component vaccine also resulted in increased frequencies of antigen-experienced TRM in BALF samples and IFNγ-secreting memory CD8+ T cells in lung tissues (Fig. 4e). Furthermore, Tfh and RBD-specific GC B cells in the mediastinal lymph node were significantly increased in the three-component vaccine group (Fig. 4f). Two additional three-component vaccines were prepared and evaluated, featuring adjusted RBDXBB.1.5-HR and RBDBA.5-HR antigen ratios of 1:1 and 1:3 (Extended Data Fig. 6b). Both vaccines elicited comparable cross-neutralization responses, with the neutralizing activity against pre-XBB subvariants progressively enhanced by increasing the proportion of RBDBA.5-HR protein antigen in the intranasal formulation. Notably, the intranasal vaccine with a 3:1 antigen ratio of RBDXBB.1.5-HR to RBDBA.5-HR demonstrated the strongest neutralization against XBB lineages, prompting its selection for further studies. These results strongly demonstrate that combining adenovirus-vectored and subunit protein antigens can be used as an intranasal vaccine platform. Moreover, multivalent vaccines can be developed by adjusting the proportions of different components in the vaccine, allowing for the elicitation of broader-spectrum neutralizing capacities against multiple circulating Omicron subvariants.
The three-component vaccine as a heterologous booster to improve immunity
The messenger RNA-based COVID-19 vaccines have been widely used around the world6. Therefore, we investigated whether the three-component vaccine could serve as a heterologous booster in sequential immunization. Animals received three intramuscular injections of an mRNA vaccine on days 0, 21 and 42 (ref. 52), followed by a two-dose intranasal immunization with the three-component vaccine (protein components usually work better after two immunizations) (Fig. 4g). As expected, heterologous immunization with the three-component vaccine significantly improved the titres of RBD-specific IgG (Fig. 4h) and neutralizing antibodies (Fig. 4l) in the sera compared with homologous vaccination with mRNA vaccines. Consistent with previous studies, the circulating Omicron subvariants, particularly the XBB lineages, exhibited extensive resistance to neutralization by mRNA vaccines. However, intranasal delivery of the three-component vaccine rescued the compromised neutralizing activities against Omicron subvariants that included the XBB lineages (Fig. 4l). Remarkably, the GMTs of 50% sera neutralization in the heterologous vaccination group against BA.5, BF.7, BQ.1, BQ.1.1, XBB and XBB.1.5 subvariants were improved by 17.6-, 14.3-, 11.8-, 17.8-, 20.5- and 20.5- fold, respectively, compared with the homologous vaccination group.
In addition to enhancing systemic immune response, heterologous intranasal immunization with the three-component vaccine conferred additional respiratory mucosal immunity, characterized by higher levels of antigen-specific IgA and IgG in BALF (Fig. 4i), increased frequencies of TRM cells in BALF and lung tissue (Fig. 4j and Extended Data Fig. 6c), elevated Tfh and GC B cells in mediastinal lymph nodes (Extended Data Fig. 6d,e), and abundant spike-specific T memory cells in lung tissues (Fig. 4k). Unlike homologous immunization with mRNA vaccine, the heterologous immunization regimen resulted in high levels of neutralizing antibodies in the respiratory tract against all variants in the panel (Fig. 4m). The GMT of 50% neutralization against XBB and XBB.1.5 variants in BALF reached 621 and 795, respectively, indicating potent neutralizing activity against the circulating XBB lineages. Remarkably, the antibody responses in the blood and mucosal immunity were apparently elevated in a quicker way, even with only one dose of the three-component vaccine following three injections of the mRNA vaccine (Extended Data Fig. 6f–k). These findings indicate that the combination of adenovirus and protein antigens can be a promising candidate for heterologous immunization after mRNA vaccine administration.
The two-component vaccine protects against viral infection and transmission
Due to the existence of imprinted immunity in humans with a hybrid immune background, the US FDA has recommended the use of the component derived from the XBB descendant lineage as the vaccine antigen. Therefore, we next use the two-component (Ad5XBB.1.5 + RBDXBB.1.5-HR) rather than the three-component (Ad5XBB.1.5 + RBDXBB.1.5-HR + RBDBA.5-HR) intranasal vaccine for virus challenge assay and clinical trial.
To evaluate the efficacy of the Ad5XBB.1.5 + RBDXBB.1.5-HR vaccine against the XBB.1.16 variant in vivo, we conducted a live virus challenge assay. Mice were intranasally immunized with three doses of Ad5Empty + RBDXBB.1.5-HR, Ad5XBB.1.5 or Ad5XBB.1.5 + RBDXBB.1.5-HR, and challenged with 1 × 106 plaque-forming units (p.f.u.s) of XBB.1.16 Omicron via the intranasal route (Fig. 5a). Mice administered with PBS and naked RBDXBB.1.5-HR served as controls. Changes in viral loads of throat swab samples were monitored daily using quantitative PCR with reverse transcription (RT–qPCR). Throughout the experiment, a large amount of viral load was detected in throat swab samples from mice administered with PBS and RBDXBB.1.5-HR, while immunization with Ad5Empty + RBDXBB.1.5-HR, Ad5XBB.1.5 and Ad5XBB.1.5 + RBDXBB.1.5-HR markedly reduced the viral loads in throat swabs (Fig. 5b). Of note, on day 4 post infection, the group receiving Ad5XBB.1.5 + RBDXBB.1.5-HR vaccine had the lowest viral loads with almost undetectable genomic RNA.
a, BALB/c mice were intranasally immunized three times with Ad5XBB.1.5 alone and two-component (Ad5XBB.1.5 + RBDXBB.1.5-HR) vaccines. Mice treated with PBS, naked RBDXBB.1.5-HR and Ad5Empty plus RBDXBB.1.5-HR were used as controls. Immunized BALB/c mice were challenged with 1 × 106 p.f.u.s of live SARS-CoV-2 XBB.1.16 Omicron viruses. Wks, weeks. b, Changes in viral loads of throat swabs post SARS-CoV-2 infection were determined. c,d, Nasal turbinates, trachea and lung tissue were collected on day 4 post infection to determine the levels of gRNA (c) and sgRNA (d). e,f, Representative histopathological images (e) and corresponding pathological scores (f) of lung tissues from mice challenged with Omicron. n = 6 mice per group (b–d,f). g, Schematic illustration of the contact/airborne transmission protection experiment. Syrian golden hamsters were first intranasally inoculated with 1 × 104 p.f.u.s of XBB.1.5 variants and placed in a cage with four recipient groups that had previously received PBS, Ad5Empty + RBDXBB.1.5-HR, Ad5XBB.1.5 or Ad5XBB.1.5 + RBDXBB.1.5-HR vaccines. After 24 h of co-caging, they were separated into individual cages. On day 4 post exposure, all hamsters were killed for tissue collection. h–j, The gRNA levels in throat swabs (h) and respiratory tissues (i), and sgRNA levels in tissues (j) were determined. k,l, Representative histopathological images (k) and corresponding pathological scores (l) of lung tissues from hamsters post infection. n = 6 hamsters per group (h–j,l). Scale bars, 100 μm (e,k). Data are presented as mean ± s.e.m. P values were determined using two-way ANOVA followed by Tukey’s multiple comparisons test after logarithmic transformation (b–d,h–j), and one-way ANOVA followed by Dunnett’s multiple comparisons test (f,l). The coloured P values in b and h correspond to the comparison between each group and the two-component intranasal vaccine group. Schematic illustrations in a and g created with BioRender.com.
The viral loads in multiple tissues of the respiratory system were detected on day 4 post infection. As expected, the Ad5XBB.1.5 and Ad5XBB.1.5 + RBDXBB.1.5-HR vaccines provided significant reductions in gRNA levels in the upper and lower respiratory tracts, including the nasal turbinates, trachea and lung tissues (Fig. 5c). Even the RBDXBB.1.5-HR protein mixed with empty adenovirus vector (Ad5Empty+RBDXBB.1.5-HR) notably reduced the viral burden in respiratory tracts. To further evaluate the protective effects conferred by our two-component vaccine, we tested the levels of viral subgenomic RNA (E gene) in tissues, which serves as an indicator of active viral replication. High levels of sgRNA were detected in the collected samples in PBS (geometric mean copy numbers of E gene per mg tissue: turbinate, 2.52 × 105; trachea, 10.3; lung, 9.64 × 103) and RBDXBB.1.5-HR (turbinate, 3.45 × 103; trachea, 4.60; lung, 2.51 × 102). Consistent with the gRNA results, the levels of sgRNA were significantly decreased in the Ad5Empty + RBDXBB.1.5-HR and Ad5XBB.1.5 groups, with undetectable sgRNA in the majority of samples. However, a small number of tissues still exhibited active viral replication in these two groups (one nasal turbinate sample in the Ad5XBB.1.5 group, two nasal turbinate samples, one trachea and two lung tissue samples in the Ad5Empty + RBDXBB.1.5-HR group). In contrast to the other groups, intranasal delivery of Ad5XBB.1.5 + RBDXBB.1.5-HR vaccine was more effective in preventing virus replication in the upper and lower respiratory tracts, as evidenced by undetectable sgRNA in all samples from mice immunized with Ad5XBB.1.5 + RBD XBB.1.5-HR (Fig. 5d).
Moreover, the effective protection conferred by the Ad5XBB.1.5 + RBDXBB.1.5-HR vaccine correlated with a remarkable reduction in lung pathology. Histopathological evaluation revealed mild pathologic changes in lung tissue from mice in the PBS and RBDXBB.1.5-HR protein groups, characterized by multifocal areas of consolidation, mild alveolar septa thickening, alveolar congestion and small patches of inflammation (Fig. 5e,f). AdEmpty + RBDXBB.1.5-HR partially alleviated the pathological changes. Notably, no evident pathological changes were observed in the lung tissues collected from mice immunized with Ad5XBB.1.5 and Ad5XBB.1.5 + RBDXBB.1.5-HR vaccines, with all pathological scores reduced.
Besides using direct intranasal instillation of live viruses for the viral challenge, the ‘contact/airborne’ infection model was employed to evaluate the efficacy of the Ad5XBB.1.5 + RBDXBB.1.5-HR vaccine in preventing viral host-to-host transmission (Fig. 5g). In this model, a group of Syrian golden hamsters was first intranasally inoculated with 1 × 104 p.f.u.s of live XBB.1.5 variants and designated as ‘donor animals’. At 1 day post infection, the donor animals were placed in a cage with four recipient groups that had previously received PBS, Ad5Empty + RBDXBB.1.5-HR, Ad5XBB.1.5 or Ad5XBB.1.5 + RBDXBB.1.5-HR vaccines, sharing diet, bedding and air. After 24 h of co-caging, the donor and recipient animals were separated into individual cages, and viral loads from throat swab samples were monitored daily throughout the study. On day 4 post exposure to the donor, all hamsters were killed, and various respiratory tissues were collected for viral load detection and histopathological examination. Consistent with findings in mice, all animals that had received intranasal immunization demonstrated both viral transmission and reduced lung pathological changes (Fig. 5h–l). Notably, the Ad5XBB.1.5 + RBDXBB.1.5-HR vaccine resulted in the lowest viral burden in throat swab samples (Fig. 5h) and respiratory tissues, particularly in the nasal turbinates (Fig. 5i). In the group receiving Ad5Empty + RBDXBB.1.5-HR, five nasal turbinate samples and one trachea sample showed active viral replication with high levels of sgRNA, and one nasal turbinate and one trachea sample exhibited detectable sgRNA in the group receiving Ad5XBB.1.5, while all samples from the Ad5XBB.1.5 + RBDXBB.1.5-HR group showed undetectable sgRNA (Fig. 5j). These results strongly indicate that the Ad5XBB.1.5 + RBDXBB.1.5-HR vaccine is an effective intranasal vaccine candidate for protecting against viral challenges and transmission of Omicron sublineages in vivo.
The two-component vaccine improves systemic and mucosal immunity in humans
To further investigate the tolerability and immunogenicity of this intranasal two-component vaccine in the clinic, we conducted a clinical investigator-initiated trial (IIT), and 70 human participants were recruited (Registration number ChiCTR2300069022). All participants in this study provided written informed consent, and the trial was performed according to the principles of the Biomedical Ethics Review Committee of West China Hospital. Adults 18 years and older with a documented history of 2 or 3 doses of the COVID-19 vaccine and with more than 3 months after the last vaccination were eligible for enrolment. In addition, the participants were advised to undergo detection of SARS-CoV-2 antigen within the study to rule out elevated antibody response caused by infection. Most participants received 2 or 3 doses of an inactivated virus vaccine and experienced a BA.5 infection wave from December 2022 to January 2023 in mainland China58. Since then, they have not been infected, and had a 6-month interval between the infection and the vaccination. Therefore, it is likely that most participants were not infected with XBB subvariants. Detailed information about individual participants, including their vaccination and infection histories, can be found in Supplementary Table 1.
The first objective of this investigation was to evaluate the tolerability and safety of the intranasal two-component vaccine. Seventy human participants were assigned to two groups, receiving 2 doses of the two-component vaccine. The first group received 1 × 1010 VPs of Ad5XBB.1.5 plus 20 μg of RBDXBB.1.5-HR protein (comprising 20 participants), while the second group received 2 × 1010 VPs of Ad5XBB.1.5 plus 40 μg of RBDXBB.1.5-HR (comprising 50 participants). The immunization schedule follows a prime–boost regimen spaced 14 days apart (Fig. 6a). In general, there were no significant differences in adverse reactions between these two dosage groups. The most common adverse reactions were predominantly mild (grades 1 and 2) and were transient and self-limiting, and total solicited adverse reactions were reported by 7.14% of all 70 human participants, with local symptoms including nasal obstruction (2.86%), sore throat (1.43%), nasal discharge (1.43%) and systemic symptoms of headache (4.29%) (Fig. 6b). The frequency and severity of solicited adverse events were not related to the dose level. In addition, total unsolicited adverse events within 30 days of vaccination were reported by 1.43% of all participants. No adverse reaction of grade Ⅲ and serious adverse events occurred in the trial.
a, Schematic representation of the investigator-initiated trial to evaluate the safety and immunogenicity of the two-component vaccine in humans. Participants were intranasally administered with Ad5XBB.1.5 + RBDXBB.1.5-HR (n = 20 participants received 1 × 1010 VPs of adenovirus plus 20 μg protein and n = 50 participants received 2 × 1010 VPs of adenovirus plus 40 μg protein) on days 0 and 14, and blood and nasal wash samples were collected to detect the levels of binding and neutralization antibodies. b, Common adverse reactions within 14 days of each vaccination in all human participants (n = 70 human participants). c, Endpoint titres of anti-RBDXBB.1.5 IgA in nasal swab samples from participants in the group receiving 2 × 1010 VPs of Ad5XBB.1.5 plus 40 μg of RBDXBB.1.5-HR (n = 50 participants). d, Neutralizing antibodies against prototype, XBB.1.5 and XBB.1.16 pseudoviruses in nasal swab samples in the group receiving 2 × 1010 VPs of Ad5XBB.1.5 plus 40 μg of RBDXBB.1.5-HR (n = 50 participants). e, Endpoint titres of sera IgG and IgA in human participants on day 28 (n = 50). f, Sera neutralizing antibodies against pseudoviruses (prototype, BA.5, XBB.1.5, XBB.1.6, XBB.1.9.1, XBB.1.16 and XBB.2.3) were determined (n = 50 participants). g, Neutralizing antibodies against authentic ancestral, XBB.1.5 and XBB.1.16 viruses in sera collected on day 28 (n = 50 participants). The numbers above the data points in c–g indicate the GMTs of 50% neutralization measured for pre- and post-immunization samples, as well as the fold changes between them. Data are presented as geometric mean ± s.d. Schematic in a created with BioRender.com.
Another objective of this investigation was to assess the mucosal and systemic immune response induced by the intranasal two-component vaccine. Blood and nasal swab wash samples were collected before the first vaccination (used as pre-immunization control) and on days 14 and 28 for immunological assays. We first measured the mucosal binding antibodies in nasal wash samples from 50 participants in the group receiving 2 × 1010 VPs of Ad5XBB.1.5 plus 40 μg of RBDXBB.1.5-HR, and found significant enhancement of antigen (RBDXBB.1.5)-specific IgA levels after intranasal vaccination (Fig. 6c). Compared with pre-immunization levels, the titres of IgA antibody were 107-fold higher after the first intranasal delivery, and further increased by 203-fold after the second vaccination. In addition to the improvement in mucosal IgA, neutralizing activities were also significantly improved by the two-component vaccines (Fig. 6d). The GMTs of neutralizing antibody against prototype, XBB.1.5 and XBB.1.16 pseudoviruses were improved by 5.83-, 20.05- and 9.00-fold, respectively, after the first dose of immunization, and improved by 13.50-, 49.50- and 18.75-fold, respectively, after the second dose.
In the next set of experiments, sera binding and neutralizing antibody responses were examined, and the GMTs for sera binding IgG and IgA reached 445,127 and 4,718, respectively (Fig. 6e). The results of pseudovirus neutralization showed that most participants have detectable sera neutralizing antibodies induced by previous vaccination and infection, while the neutralizing capacities were extensively compromised by XBB lineages. Intranasal delivery of the two-component vaccine rescued the neutralizing activities against all circulating XBB subvariants (Fig. 6f). The plasma neutralizing antibodies were significantly increased at 14 days after the first vaccination, and the GMTs of 50% neutralization against Prototype, BA.5, XBB.1.5, XBB.1.6, XBB.1.16, XBB.1.9.1 and XBB.2.3 were improved by 2.37-, 2.28-, 7.74-, 5.13-, 5.68-, 7.39- and 6.54-fold, respectively. At 28 days after the first vaccination, the GMTs of 50% neutralization against XBB.1.5, XBB.1.6, XBB.1.16, XBB.1.9.1 and XBB.2.3 variants reached 801, 515, 659, 794 and 581, respectively, highlighting strong neutralizing potency against all XBB lineages. Furthermore, serum samples were subjected to authentic virus neutralization assays. The results exhibited similar significant improvements in serum neutralizing activities against pseudoviruses after the second intranasal vaccination, with GMTs against the ancestral, XBB.1.5 and XBB.1.16 viruses reaching 352, 248 and 193, respectively (Fig. 6g). Similar improvements in neutralization were also observed in the group receiving a two-component intranasal vaccine containing 1 × 1010 VPs of Ad5XBB.1.5 plus 20 μg of RBDXBB.1.5-HR protein (Extended Data Fig. 7). These results suggest a good immunogenicity of the nasal two-component vaccine. Therefore, this clinical trial demonstrates the safety and strong immunogenicity of the intranasal two-component vaccine in humans, inducing robust mucosal and systemic immune responses.
Discussion
Considering the remarkable increase in the ability of new Omicron subvariants to evade immune responses, coupled with the limitations of currently administered vaccines in providing mucosal immunity to prevent SARS-CoV-2 transmission, there is an urgent need for the development of COVID-19 vaccines via intranasal delivery. Various vaccine platforms, including viral vectors14,16,17,18,19,21, subunit proteins22,23,24,25,26,27 and nucleic acids28,29, have been explored in preclinical studies for this purpose. The WHO COVID-19 vaccine tracker indicates that over a dozen intranasal candidates have entered clinical trials, but clinical data remain limited and have unsatisfactory results, and none have received WHO approval. For instance, the adenovirus ChAdOx1 nCoV-19 intranasal vaccine and the live-attenuated influenza virus vectored vaccine CA4-dNS1-nCoV-RBD demonstrated suboptimal systemic and mucosal antibody responses in only a minority of volunteers34,59, highlighting the necessity to improve existing mucosal vaccine technologies or to develop new intranasal vaccine platforms.
In the current study, we developed a two-component intranasal vaccine by combining an adenovirus vector Ad5XBB.1.5 with the trimeric RBDXBB.1.5-HR protein antigen. This combination synergistically enhanced both humoral and mucosal immune responses, yielding higher levels of antigen-specific IgG and IgA, increased neutralization capacity and greater frequencies of TRM, lung spike-specific T cells, as well as germinal centre B cells compared with individual components. Even with a 10-fold difference in adenovirus doses, the two-component vaccine with a lower-dose adenovirus vector (5 × 107 VPs of Ad5XBB.1.5 + 5 μg of RBDXBB.1.5-HR) can still generate an immune response comparable to that of a single high-dose adenovirus component (5 × 108 VPs of Ad5XBB.1.5) (Extended Data Fig. 2). The two-component intranasal vaccines effectively protected mice against the highly contagious XBB.1.16 virus challenge, and completely blocked XBB.1.5 virus transmission in a ‘contact/airborne’ hamster model. Although there was no statistically significant difference in respiratory viral load reduction in mice between the two-component intranasal vaccine and the single adenovirus component, we observed that hamsters in the transmission model showed the lowest viral loads in their throat swabs and respiratory tissues after receiving the two-component intranasal vaccine (Fig. 5h,i). Furthermore, all samples from the two-component vaccine group showed undetectable viral replication indicator sgRNA (Fig. 5d,j), compared with the adenovirus immunization group, further indicating that the two-component vaccine can provide a more effective local mucosal antiviral protective immune response. Notably, the two-component intranasal vaccine fulfills the criteria for inducing both mucosal and systemic immune responses, eliciting strong immunity in all participants, as evidenced by elevated nasal IgA and high neutralization levels against various variants in both nasal and serum samples. The vaccine also demonstrated good tolerability and safety in groups receiving two doses, with only mild and transient adverse reactions. These results suggest that this two-component vaccine represents a safe and advanced technology for intranasal vaccine development, warranting further evaluation in large-scale clinical trials.
One of the novelties in the design of our two-component intranasal vaccine is the employment of adenovirus as a mucosal adjuvant for subunit protein-based antigens, utilizing the immune-stimulating properties of adenovirus as a viral vector. With advantages such as safety, ease of mass production and convenient transportation, subunit proteins represent a promising platform for the development of intranasal vaccines. Multiple mucosal adjuvants22,23,25,26,27,39, including polyethyleneimine (PEI)38 and cationic crosslinked carbon dots (CCD)24 developed by our team, have been designed to overcome the poor immunogenicity of pure subunits in local mucosa; however, safety concerns have restricted their use in human applications. Therefore, selecting a material that has been clinically evaluated as an adjuvant for intranasal protein antigens could significantly simplify the clinical translation process. We thus turned our attention to another safe intranasal vaccine platform, adenoviral vaccines, hypothesizing that the adenovirus vector could serve both as an antigen delivery system and as an adjuvant for intranasal subunit proteins. Indeed, even empty adenoviral vectors significantly enhanced the immunogenicity of the protein antigen, highlighting the adjuvant effect of the adenovirus vector.
Similar to other intracellular pathogens, adenoviruses can be recognized by the host’s innate defence system, triggering an innate immune response through multiple receptors and signalling pathways41,42,43; thus, the activation of this response induced by the adenovirus vector may enhance the antigenicity and immunogenicity of the carried antigen, thereby accelerating the immune response42. In this study, we found that the enhancement of humoral and cellular immune responses conferred by the multicomponent vaccine was abolished in Sting−/− mice, but not in Tlr2−/−, Tlr9−/− and Myd88−/− mice. This suggests the involvement of the STING signalling pathway in the adenovirus vector-induced adjuvant effect, which appears to be largely independent of TLR/MYD88. Importantly, scRNA-seq data further elucidate that the activation of the STING signalling pathway in mucosal CCR7+ DCs, DC1 and pDCs contributes to antigen processing and presentation in the mucosa with the two-component vaccine.
mRNA-based COVID-19 vaccines have proven highly effective against symptomatic and severe disease60. Moderna and Pfizer/BioNTech have swiftly updated their vaccine sequences to include the XBB.1.5 spike protein, demonstrating strong neutralization against subvariants XBB and JN.1 (ref. 48). Considering the widespread global administration of mRNA-based COVID-19 vaccines, there is increasing interest in heterologous vaccination strategies. Using alternative vaccine platforms as boosters may produce a stronger immune response than repeated mRNA doses. In addition, the intramuscular delivery of mRNA vaccines limits mucosal immunity, essential for upper respiratory tract protection, as seen with the BNT162b2 booster, which fails to effectively activate such immunity61. However, recent trials of the intranasal ChAdOx1 nCoV-19 vaccine in human participants previously receiving an injection of ChAdOx1 or BNT162b2 did not show a clear boosting effect or additional benefits34. Here we demonstrated that a heterologous boost with the three-component vaccine following three mRNA injections in mice not only enhanced serum neutralization but also strengthened local mucosal immunity. The highest neutralizing antibody levels against the BA.5 and BF.7 variants were achieved with two doses of Ad5XBB.1.5 + RBDXBB.1.5-HR + RBDBA.5-HR, rather than against XBB lineages. The weaker neutralization of new Omicron subvariants may be affected by previous antigenic exposure, suggesting immune imprinting62. Due to the unfavourable effect of imprinted immunity, especially among those who repeatedly received the original COVID-19 vaccines and suffered infections with pre-XBB-lineage variants, the FDA has recommended using a monovalent antigen derived from the XBB lineage for the next-generation vaccine. Following this guidance, we evaluated the efficacy of a two-component vaccine for live virus challenge assays and clinical trials, despite the three-component intranasal vaccine offering broader neutralizing activity. Nevertheless, this highlights the potential of a multivalent vaccine platform to address the simultaneous circulation of multiple variants in the future.
Despite most participants with a history of two or three doses of COVID-19 vaccines contracting BA.5 between December 2022 and January 2023, neutralization against XBB lineages remained inadequate 6 months post infection. Preliminary results showed that human participants exhibited elevated antibody responses in both serum and the respiratory tract after just one dose of intranasal vaccination with the two-component vaccine. Concerns about preexisting immunity to adenoviruses in the population may affect the use of adenoviral vectors, particularly since our immunization requires two doses. However, our findings indicate that the two-component vaccine demonstrates good immunogenicity in individuals with a hybrid immune background. A recent study also suggested that a bolus of Ad5 vaccine might overcome anti-Ad5 immunity in the nasal passage63, warranting further investigation in future studies. In summary, the combination of an antigen-carrying adenovirus vector and subunit protein antigens can serve as a promising platform for developing intranasal vaccines against not only COVID-19 but also other respiratory diseases.
Methods
Animals
Specific pathogen-free (SPF) female BALB/c mice (6–8 weeks), C57BL/6 mice (6–8 weeks) and Syrian Golden hamsters (6–10 weeks) were purchased from Beijing Vital River. The Sting−/−, Myd88−/−, Tlr9−/−, Tlr2−/−, Cd8a−/− and Casp1−/− mice were obtained from The Jackson Laboratory. Nlrp3−/− mice were obtained from Genentech, and Il1b−/− mice were provided by the Tokyo University of Science. All genetically modified mice used in this study were 6–12 weeks old. All animals were maintained in an SPF animal facility (temperature: 21–25 °C; humidity: 30–70%; dark/light cycle: 12 h/12 h) in the animal centre of the State Key Laboratory of Biotherapy. All animal experiments were approved by the Institutional Animal Care and Use Committee of Sichuan University.
Preparation of recombinant trimeric RBD-HR protein
The trimeric RBD-HR proteins were generated by tandemly linking the RBD (residues 320–545) of the SARS-CoV-2 BA.5 or XBB.1.5 variant with the HR1 (residues 916–966) and HR2 (residues 1,157–1,203) regions of the S2 subunit, and expressing the resulting fusion proteins using the Bac-to-Bac baculovirus expression system (Invitrogen) in insect cells52. The synthetic gene was amplified and incorporated into the pFastBac1 vector via BamHI and HindIII restriction sites, then the plasmid was transformed into Escherichia coli DH10b cells for cloning. The bacmids were then transfected into Sf9 insect cells (Thermo Fisher, 11496015) for protein expression. The proteins were primarily purified using a prepacked Ni-TED HisTrap column (Nuptec Biosciences, NRPB58S). Next, the proteins were cleaved using EK protease and further purified using the Superdex 200 Increase 10/300 GL column (GE Healthcare, 28990944). Finally, the purified recombinant RBD-HR proteins were determined by SDS‒PAGE and Coomassie blue staining.
Construction of adenovirus vectors expressing the spike protein
Full spike proteins with proline stabilizing mutations64 of BA.4/5 and XBB.1.5 SARS-CoV-2 variants (https://covariants.org/variants) were codon optimized for expression in human cell lines and synthesized by Genewiz. Then, these adenoviral vectors expressing spike proteins were packaged and generated in HEK-293 cells (ATCC, CRL-1573) through an AdMax adenovirus system (Microbix). Briefly, these spike genes were cloned into the adenovirus shuttle plasmid pDC316 to generate the recombinant pDC316-S by Gibson assembly. HEK-293 cells were co-transfected with the recombinant shuttle plasmid pDC316-S and an E1/E3-deficient Adenovirus-5 genomic backbone plasmid pBHGloxΔE1,3Cre. These rescued replication-incompetent adenoviral vector vaccines were amplified in HEK-293 cells and purified by caesium chloride density-gradient ultracentrifugation. The number of viral particles in each adenoviral vector was detected using ultraviolet spectrophotometry at 260 nm. Spike protein expression was verified by western blot using Spike antibody (1:2,000, Sino Biological, 40591-MM42) after infection of A549 cells (ATCC, CCL-185) by Ad5XBB.1.5 and Ad5BA.5 viruses.
Vaccinations of mice
For the preparation of two-component vaccines, either a low dose (2.5 × 109 VPs) or a high dose (5 × 109 VPs) of Ad5XBB.1.5 was mixed with 5 μg or 10 μg RBDXBB.1.5-HR, respectively, in a total volume of 50 μl. BALB/c mice were immunized with three doses of (1) the low dose or (2) high dose of Ad5XBB.1.5 alone, (3) the low dose or (4) the high dose of two-component vaccine (Ad5XBB.1.5 + RBDXBB.1.5-HR), (5) 10 μg of RBDXBB.1.5-HR protein alone, or (6) 5 × 109 VPs of Ad5Empty mixed with RBDXBB.1.5-HR protein. The immunization programmes were performed with a 28-day interval between doses. The three-component vaccine, comprising 5 × 109 VPs of Ad5XBB.1.5, 7.5 μg of RBDXBB.1.5-HR and 2.5 μg of RBDBA.5-HR, as well as lower doses of adenovirus (5 × 107 or 5 × 108 VPs of Ad5XBB.1.5) and a two-component vaccine (5 × 107 or 5 × 108 VPs of Ad5XBB.1.5 plus 5 μg of RBDXBB.1.5-HR), were administered intranasally according to the same immunization regimen. Serum samples were collected from vaccinated animals at 11 weeks after immunization to assess binding and neutralizing antibody responses. To evaluate the mucosal immunity induced by vaccines, mice were euthanized on day 21 after the third booster vaccination to collect BALF and lung tissues.
To investigate the potential signalling pathways activated by the adenovirus vector vaccine, 5 × 109 VPs of Ad5BA.5 or Ad5Empty mixed with 10 μg RBDBA.5-HR were intranasally immunized into wildtype, Tlr2−/−, Tlr9−/−, Myd88−/−, Nlrp3−/−, Il1b−/−, Casp1−/−, Sting−/− and Cd8a−/− mice, following the same immunization programme.
For the heterologous booster vaccination assay, we developed an mRNA vaccine based on the full-length spike protein sequence of the SARS-CoV-2 Delta variant, incorporating several proline mutations to enhance stability52,65. BALB/c mice were intramuscularly immunized with four doses of 5 μg mRNA per 50 μl of encapsulated liposome (LPX)/SpikeDelta-mRNA vaccine (4× mRNA). In the heterologous vaccination group, mice were immunized with three doses of SpikeDelta-mRNA vaccine on day 0, 21 and 42, followed by one or two intranasal immunizations with the three-component vaccine (Ad5XBB.1.5 + RBDXBB.1.5-HR + RBDBA.5-HR).
ELISA for antibody measurement
To detect anti-SARS-CoV-2 RBD-specific IgG and IgA, 96-well plates (Corning, 42592) were coated with 1 μg ml−1 recombinant RBD proteins in carbonate–bicarbonate buffer and incubated at 4 °C overnight. The plates were then washed three times with 1× PBS containing 0.1% Tween-20 (PBST) and blocked with PBST containing 1% BSA for 1 h at room temperature. Serially diluted sera, BALF, NLF or nasal swab washes in dilution buffer were added to the wells (100 μl per well). After incubation for 1 h at 37 °C, the plates were washed three times. Then, diluted horseradish peroxidase (HRP)-conjugated antibodies, including goat anti-mouse IgG (1:10,000, Thermo Fisher, 31430), goat anti-mouse IgA (1:5,000, Abcam, ab97235), goat anti-human IgG (1:4,000, Invitrogen, 62-8420) or goat anti-human IgA (1:4,000, Southern Biotech, 2050-05) were added and incubated at 37 °C for 1 h. The plates were washed three times and developed with 3,3′,5,5′-tetramethyl biphenyl diamine (TMB, NeoBioscience, TMS.1000) for 10 min at room temperature. The reaction was stopped with 1 M H2SO4, and the absorbance was measured at 450 nm using a SpectraMax ABS microplate reader with SoftMax Pro 7.1 (Molecular Devices). The endpoint titre was defined as the highest reciprocal dilution of serum with an absorbance ≥2.1 times the negative control serum value.
SARS-CoV-2 pseudovirus neutralization assay
To determine the titres of neutralizing antibodies in sera, BALF and nasal swab wash samples, a pseudovirus neutralization assay was performed. Pseudoviruses expressing luciferase, including Prototype (GM-0220PV07), Delta (GM-0220PV45) and various Omicron sublineages: BA.2.75 (GM-0220PV99), BA.5 (GM-0220PV90), BF.7 (GM-0220PV100), BQ.1 (GM-0220PV103), BQ.1.1 (GM-0220PV105), XBB (GM-0220PV102), XBB.1.5 (GM-0220PV102), XBB.1.16 (GM-0220PV120), XBB.1.6 (GM-0220PV116), XBB.1.9.1 (GM-0220PV113) and XBB.2.3 (GM-0220PV115), were obtained from Genomeditech.
In brief, the inactivated sera, BALF, NLF and nasal swab wash samples (56 °C for 30 min) were subjected to a 3-fold serial dilution, ranging from 30 to 65,610-fold, and then incubated with equal volumes of different diluted pseudoviruses at 37 °C for 1 h. Subsequently, 1.2 × 104 HEK293T cells (ATCC, CRL-11268) expressing human ACE2 receptor (293T/ACE2) were added to each well and incubated at 37 °C for 48 h to allow for luciferase expression. Finally, the supernatants were removed and a lysis reagent with luciferase substrate (Beyotime, RG005) was added. The luminescence emitted by the 293T/ACE2 cells was measured using a PerkinElmer EnSight multimode plate reader with Kaleido 3.0 software (PerkinElmer). The 50% neutralization of pseudovirus was determined and calculated using GraphPad Prism 8.0.2. The assay included a positive control group with cells and viruses, a negative control group with only cells, and a sample group with cells, samples and viruses. The percentage of neutralization was calculated using equation (1):
Live SARS-CoV-2 virus neutralization assay
Neutralizing antibodies against live ancestral and mutated SARS-CoV-2 viruses in samples from vaccinated mice and human participants were evaluated using the authentic virus neutralization assay. Diluted samples were incubated with live SARS-CoV-2 viruses at 50% tissue-culture infectious doses (TCID50). The mixture was then added to 96-well microplates containing Vero cells (ATCC, CCL-81) (5 × 104 cells per well) and incubated at 37 °C for 72 h. The cytopathogenic effects (CPE) were observed and measured using a microscope. The titres of neutralizing antibodies that resulted in 50% neutralization (EC50) in samples were calculated.
ELISpot
To assess the presence of IFNγ-secreting cells in the samples of BALF and spleen, 96-well ELISpot plates (MABTECH, 3321-4AST) were initially washed four times with PBS. They were then filled with complete RPMI 1640 medium (10% FBS, 100 µl per well) and incubated at 37 °C for 1 h. After carefully removing the supernatant, isolated cells in BALF and splenocytes (1 × 105 cells per well) were introduced into each well. The cells were stimulated overnight with pools of XBB.1.5 spike protein peptides (GenScript, Customer designed) (1 μg ml−1) in an incubator set to 37 °C with 5% CO2. Following this incubation, the cells were washed away with PBS, and the plates were treated with detection antibodies (R4-6A2, biotin, 1 μg ml−1) at room temperature for 2 h. After five additional washes with PBS, Streptavidin-ALP (1:1,000) was applied and incubated for 1 h at room temperature. The plates were washed five more times with PBS, and substrate solution (BCIP/NBT-plus substrate, 100 μl per well) was then added to facilitate spot formation. After incubation at room temperature for 10 min, the plates were rinsed with water and then dried. Finally, the spots were read using an IRIS FluoroSpot/ELISpot reader with Mabtech Apex v.1.1.45.114 (MABTECH).
Flow cytometry
Affinity between the expressed spike protein and the human ACE2 receptor was confirmed using flow cytometry. A549 cells infected with Ad5XBB.1.5 (multiplicity of infection (MOI) = 30) were incubated with His-tagged recombinant human ACE2 protein (ACROBiosystems, AC2-H52H4) (1 μg ml−1) for 30 min. Following washing steps, the cells were incubated with an APC anti-His Tag antibody (BioLegend, 362605) to assess the percentage of cells binding ACE2.
BALF fluid was used to collect cells for the analysis of TRM cells in the respiratory tract. CD4+ and CD8+ TRM cells were stained using the following antibodies: PerCP/Cyanine5.5 anti-mouse CD3 (BioLegend, 100218), Brilliant Violet 421 anti-mouse CD4 (BioLegend, 100438), Brilliant Violet 510 anti-mouse CD8a (BioLegend, 100751), PE anti-mouse CD44 (BioLegend, 103008), FITC anti-mouse CD69 (BioLegend, 104506) and APC anti-mouse CD103 antibodies (BioLegend, 121414). For Tfh cells in mediastinal lymph nodes, the cells were stained with PerCP/Cyanine5.5 anti-mouse CD3, Brilliant Violet 421 anti-mouse CD4, PE/Cyanine7 anti-mouse B220 (BioLegend, 103222), FITC anti-mouse CD279 (PD-1; BioLegend, 135214) and APC anti-mouse CD185 (CXCR5; BioLegend, 145506). To analyse the frequency of RBDXBB.1.5-specific GC B cells, cells were first incubated with biotin-tagged RBDXBB.1.5 (ACROBiosystems, SPD-C82Q3), followed by staining with Brilliant Violet 421 anti-mouse CD19 (BioLegend, 115549), PerCP/Cyanine5.5 anti-mouse CD95 (BioLegend, 152610), APC anti-mouse GL-7 (BioLegend, 144618) and PE-conjugated anti-biotin antibodies (BioLegend, 409004). The absolute number of cells was counted with Precision Count beads (BioLegend, 424902) according to manufacturer instructions.
For ICS staining, lung tissues were minced into small pieces (<1 mm3) and digested in 6 ml digestion buffer consisting of collagenase I (Gibco,17100-017; 1 mg ml−1), collagenase IV (Gibco, 17104-019; 0.5 mg ml−1) and DNase I (KeyGen biotech, KGA1506-1; 40 U ml−1) in DMEM medium (Gibco, C11995500). After incubation, the single-cell suspensions were obtained by passing through a 70-µm nylon mesh filter (Falcon, 352350), followed by red blood cell lysis. Then, the lung mononuclear cells were cultured with 1640 medium (Gibco, C11875500) with 10% FBS (Gibco, 10099-141 C), 100 μg ml−1 streptomycin, 100 U ml−1 penicillin (Beyotime, ST488S), 1 mM pyruvate (Sigma-Aldrich, S8636), 50 μM β-mercaptoethanol (Sigma-Aldrich, M6250) and 20 U ml−1 IL-2 (Sigma-Aldrich, I0523), and stimulated with 1 μg ml−1 of overlapping 15-amino-acid peptides covering BA.4/5 (GenScript, RP30223CN) or XBB.1.5 spike protein for 12 h. Brefeldin A (BFA, eBioscience, 00-4506-51) was used to block intracellular cytokine secretion before sample collection. The cells were then incubated with the following antibodies: PerCP/Cyanine5.5 anti-mouse CD3, APC anti-mouse CD4 (BioLegend, 100412), FITC anti-mouse CD8a (BioLegend, 100706) and PE anti-mouse CD44. Cells were fixed and permeabilized using the Fixation/Permeabilization kit with BD GolgiStop (BD, 554715), and then stained with PE/Cyanine7 anti-mouse IFNγ (BioLegend, 505826) and Brilliant Violet 510 anti-mouse TNF (BioLegend, 506339) antibodies for 1 h at room temperature.
To evaluate phagocytosis of protein antigens by DCs, monocyte-derived DCs were generated using GM-CSF (Novoprotein, CJ46; 20 ng ml−1) and IL-4 (Novoprotein, CK74; 10 ng ml−1) in complete 1640 medium. Six days after initiating the culture, the DCs, pre-treated with either Ad5Empty or Ad5XBB.1.5, were incubated with FITC-labelled RBDXBB.1.5-HR (labelling performed using a FluoReporter FITC Protein Labelling kit from Invitrogen, F6434) for 30 min. Excess unphagocytosed proteins were then washed away with PBS, and the cells were stained with PE/Cyanine7 anti-mouse CD11c antibody (BioLegend, 117318). All primary antibodies were used at a 1:100 dilution for flow cytometry. All cells were detected and analysed using a NovoCyte flow cytometer with NovoExpress 1.4.1 software (ACEA Biosciences). Gating strategies for all flow cytometry analyses are provided in Supplementary Fig. 1.
Single-cell data generation and processing
scRNA-seq was performed on female C57BL/6 mice treated with PBS, as well as on groups receiving the Ad5XBB.1.5 + RBDXBB.1.5-HR vaccine, including Sting−/− mice that also received Ad5XBB.1.5 + RBDXBB.1.5-HR immunization, as described above. At 48 h after the final vaccination, the mice were euthanized and lung tissues were collected. The lungs were minced with scissors and treated with a tissue dissociation solution to generate a single-cell suspension. After treatment with a red blood cell lysis reagent, the suspension was filtered through 40-µm strainers and resuspended in PBS. Single-cell suspensions from three mice per group were pooled and loaded onto a 10x Genomics Chromium Single Cell and VDJ Library Construction system. The concentration of single-cell suspensions was adjusted to 500–1,200 cells per µl. Between 10,000 and 18,000 cells were loaded and subsequent steps were performed following standard protocols provided by the manufacturer. Purified libraries were analysed by LC-Bio using an Illumina NovaSeq 6000 sequencing system with 150-bp paired-end reads.
Live SARS-CoV-2 virus challenge
BALB/c mice received intranasal immunization with Ad5XBB.1.5 (5 × 109 VPs) alone, Ad5Empty (5 × 109 VPs) + RBDXBB.1.5-HR (10 μg), or the two-component vaccine, Ad5XBB.1.5 (5 × 109 VPs) + RBDXBB.1.5-HR (10 μg) on days 0, 28 and 56. Mice immunized with PBS or naked RBDXBB.1.5-HR (10 μg) served as controls. On day 21 after the last vaccination, all mice were intranasally challenged with live SARS-CoV-2 XBB.1.16 Omicron variant (1 × 106 p.f.u.s per mouse). At 4 days post infection, the mice were euthanized, and their tissues were collected for viral detection and histopathological evaluation.
To evaluate the vaccine’s ability to block viral transmission, we adapted the ‘contact/airborne’ animal model. A total of 24 hamsters were randomly divided into four groups receiving three intranasal doses of either PBS, Ad5Empty (1 × 010 VPs) + RBDXBB.1.5-HR (20 μg), Ad5XBB.1.5 (1 × 1010 VPs) or Ad5XBB.1.5 (1 × 1010 VPs) + RBDXBB.1.5-HR (20 μg) at weeks 0, 4 and 8. At 40 days after the final immunization, several naïve hamsters were inoculated intranasally with 1 × 104 p.f.u.s of the XBB.1.5 Omicron variant as donor animals. At 24 h post infection, these donor animals were co-housed with the vaccinated recipients, sharing diet, bedding and air for 24 h. On the second day, the donor and recipient animals were separated and individually housed for an additional 4 days, after which they were euthanized for tissue collection.
Haematoxylin and eosin staining was performed to observe pathological changes in lung tissues. Pathologic slides were digitized using a Pannoramic MIDI scanner with CaseViewer 2.1 (3DHISTECH) and a NanoZoomer S360 scanner with NDP.view 2.9.22 RUO software (HAMAMATSU PHOTONICS). Viral loads in nasal turbinate, trachea and lung tissue samples were quantified by RT–qPCR targeting viral loads using a Bio-Rad CFX384 Touch Real-Time PCR Detection System and CFX Manager 3.1 software (Bio-Rad). The primer sequences used were 5′-GACCCCAAAATCAGCGAAAT-3′ (forward) and 5′-TCTGGTTACTGCCAGTTGAATCTG-3′ (reverse), and the probe sequence was 5′-FAM-ACNGCCGCATTACGTTTGGTGGACC-BHQ1-3′. Subgenomic RNA gene was detected using the following primer and probe sequences: 5′-CGATCTCTTGTAGATCTGTTCTC-3′ (forward); 5′-ATATTGCAGCAGTACGCACACA-3′ (reverse); 5′-FAM-CGAAGCGCAGTAA GGATGGCTAGTGT-BHQ1-3′ (probe). The primers and probes were custom synthesized by Sangon Biotech.
All procedures related to the challenge of mice with the SARS-CoV-2 Omicron variant were approved by the Institutional Animal Care and Use Committee of the Institute of Medical Biology, Chinese Academy of Medical Sciences, and performed in the ABSL-4 facility of Kunming National High-level Biosafety Primate Research Center.
Intranasal delivery of Ad5XBB.1.5 plus RBDXBB.1.5-HR vaccine in human participants
The clinical IIT was conducted to evaluate the safety and immunogenicity of the two-component (Ad5XBB.1.5 plus RBDXBB.1.5-HR) vaccine in human participants (Registration number: ChiCTR2300069022). The research involving humans was reviewed and approved by the Biomedical Ethics Review Committee of West China Hospital of Sichuan University. We obtained written informed consent from all volunteers in the study. All participants were advised to undergo detection of SARS-CoV-2 antigen within the study to rule out elevated antibody response caused by infection. Participants testing positive for SARS-CoV-2 antigen were excluded.
In this clinical IIT trial, we recruited 70 adult participants who were 18 years and older with a history of 2 or 3 doses of COVID-19 vaccines with an interval of >3 months after the last vaccination. The participants were assigned to two groups and received two doses of two-component vaccines (1 × 1010 VPs of Ad5XBB.1.5 plus 20 μg of RBDXBB.1.5-HR, or 2 × 1010 VPs of Ad5XBB.1.5 plus 40 μg of RBDXBB.1.5-HR). Two-component vaccines formulated with 2 × 1010 VPs of Ad5XBB.1.5 and 40 μg RBDXBB.1.5-HR in a total volume of 0.2 ml were prepared and provided by WestVac Biopharma. Human participants were intranasally administered with two-component vaccines twice using a disposable intranasal atomization device (Xinfuda Medical, XFD-07-K-01) following a prime–boost regimen with a 14-day interval. All participants were requested to complete an online diary to record any adverse reactions within 14 days of each vaccination. The blood and nasal swab wash samples from human participants were collected on days 0, 14 and 28, and stored at −80 °C for subsequent binding and neutralization antibody assays.
Statistics and reproducibility
All experiments, except for the SARS-CoV-2 challenge experiment in animals, and in the clinical trial, were repeated independently at least twice with similar results. Statistical analysis was performed using GraphPad Prism 8.0.2. Data are presented as geometric mean ± s.d, or mean ± s.e.m., as indicated in figure captions. Significance values were calculated using two-tailed unpaired Student’s t-tests for comparisons between two groups; one-way analysis of variance (ANOVA) followed by Tukey’s or Dunnett’s multiple comparisons test; or two-way ANOVA followed by Sidak’s or Tukey’s multiple comparisons post hoc test for comparisons involving multiple groups with multiple timepoints. P < 0.05 was considered significant.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data that support the findings of this study are available in the paper and its Supplementary Information. The scRNA-seq data are available for reproducing the results. The FASTQ files generated from the current scRNA-seq study have been deposited in the NCBI BioProject repository (accession number GSE279004; W.H., X.P., X.W.). The files for the two-component intranasal vaccine against SARS-CoV-2 Omicron variant have been deposited in NCBI GEO (GSE279004) at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE279004 (ref. 66). Source data are provided with this paper.
Code availability
All scripts used to process the data are available in GitHub at https://github.com/pangxueyu233/Vaccine-against-SARS-CoV-2 (ref. 67).
References
Uriu, K. et al. Enhanced transmissibility, infectivity, and immune resistance of the SARS-CoV-2 omicron XBB.1.5 variant. Lancet Infect. Dis. 23, 280–281 (2023).
Faraone, J. N. et al. Immune evasion and membrane fusion of SARS-CoV-2 XBB subvariants EG.5.1 and XBB.2.3. Emerg. Microbes Infect. 12, 2270069 (2023).
Ciccozzi, A. et al. The mutation point of view of the SARS-CoV-2 HV.1 lineage. J. Med. Virol. 96, e29359 (2024).
Yang, S. et al. Fast evolution of SARS-CoV-2 BA.2.86 to JN.1 under heavy immune pressure. Lancet Infect. Dis. 24, e70–e72 (2024).
Huang, C. Q., Vishwanath, S., Carnell, G. W., Chan, A. C. Y. & Heeney, J. L. Immune imprinting and next-generation coronavirus vaccines. Nat. Microbiol. 8, 1971–1985 (2023).
Baden, L. R. et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 384, 403–416 (2021).
Polack, F. P. et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 383, 2603–2615 (2020).
Voysey, M. et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 397, 99–111 (2021).
Logunov, D. Y. et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 397, 671–681 (2021).
Sadoff, J. et al. Interim results of a phase 1–2a trial of Ad26.COV2.S Covid-19 vaccine. N. Engl. J. Med. 384, 1824–1835 (2021).
Bleier, B. S., Ramanathan, M. Jr. & Lane, A. P. COVID-19 vaccines may not prevent nasal SARS-CoV-2 infection and asymptomatic transmission. Otolaryngol. Head Neck Surg. 164, 305–307 (2021).
V’Kovski, P., Kratzel, A., Steiner, S., Stalder, H. & Thiel, V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat. Rev. Microbiol. 19, 155–170 (2021).
Wang, Z. et al. Enhanced SARS-CoV-2 neutralization by dimeric IgA. Sci. Transl. Med. 13, eabf1555 (2021).
Afkhami, S. et al. Respiratory mucosal delivery of next-generation COVID-19 vaccine provides robust protection against both ancestral and variant strains of SARS-CoV-2. Cell 185, 896–915.e19 (2022).
Lapuente, D. et al. Protective mucosal immunity against SARS-CoV-2 after heterologous systemic prime-mucosal boost immunization. Nat. Commun. 12, 6871 (2021).
Hassan, A. O. et al. A single-dose intranasal ChAd vaccine protects upper and lower respiratory tracts against SARS-CoV-2. Cell 183, 169–184.e13 (2020).
van Doremalen, N. et al. Intranasal ChAdOx1 nCoV-19/AZD1222 vaccination reduces viral shedding after SARS-CoV-2 D614G challenge in preclinical models. Sci. Transl. Med. 13, eabh0755 (2021).
Chen, J. et al. A live attenuated virus-based intranasal COVID-19 vaccine provides rapid, prolonged, and broad protection against SARS-CoV-2. Sci. Bull. 67, 1372–1387 (2022).
An, D. et al. Protection of K18-hACE2 mice and ferrets against SARS-CoV-2 challenge by a single-dose mucosal immunization with a parainfluenza virus 5-based COVID-19 vaccine. Sci. Adv. 7, eabi5246 (2021).
Tioni, M. F. et al. Mucosal administration of a live attenuated recombinant COVID-19 vaccine protects nonhuman primates from SARS-CoV-2. npj Vaccines 7, 85 (2022).
Sun, W. et al. A Newcastle disease virus expressing a stabilized spike protein of SARS-CoV-2 induces protective immune responses. Nat. Commun. 12, 6197 (2021).
Lam, J. H. et al. Artificial cell membrane polymersome-based intranasal beta spike formulation as a second generation Covid-19 vaccine. ACS Nano 16, 16757–16775 (2022).
van der Ley, P. A., Zariri, A., van Riet, E., Oosterhoff, D. & Kruiswijk, C. P. An intranasal OMV-based vaccine induces high mucosal and systemic protecting immunity against a SARS-CoV-2 infection. Front. Immunol. 12, 781280 (2021).
Lei, H. et al. Cationic crosslinked carbon dots-adjuvanted intranasal vaccine induces protective immunity against Omicron-included SARS-CoV-2 variants. Nat. Commun. 14, 2678 (2023).
Jearanaiwitayakul, T. et al. Intranasal administration of RBD nanoparticles confers induction of mucosal and systemic immunity against SARS-CoV-2. Vaccines 9, 768 (2021).
Ashhurst, A. S. et al. Mucosal TLR2-activating protein-based vaccination induces potent pulmonary immunity and protection against SARS-CoV-2 in mice. Nat. Commun. 13, 6972 (2022).
Diallo, B. K. et al. Intranasal COVID-19 vaccine induces respiratory memory T cells and protects K18-hACE mice against SARS-CoV-2 infection. npj Vaccines 8, 68 (2023).
Chandrasekar, S. S. et al. Localized and systemic immune responses against SARS-CoV-2 following mucosal immunization. Vaccines 9, 132 (2021).
Baldeon Vaca, G. et al. Intranasal mRNA-LNP vaccination protects hamsters from SARS-CoV-2 infection. Sci. Adv. 9, eadh1655 (2023).
Alu, A. et al. Intranasal COVID-19 vaccines: from bench to bed. EBioMedicine 76, 103841 (2022).
Knisely, J. M. et al. Mucosal vaccines for SARS-CoV-2: scientific gaps and opportunities–workshop report. npj Vaccines 8, 53 (2023).
Marks, P. W., Gruppuso, P. A. & Adashi, E. Y. Urgent need for next-generation COVID-19 vaccines. JAMA 329, 19–20 (2023).
Nakahashi-Ouchida, R., Fujihashi, K., Kurashima, Y., Yuki, Y. & Kiyono, H. Nasal vaccines: solutions for respiratory infectious diseases. Trends Mol. Med. 29, 124–140 (2023).
Madhavan, M. et al. Tolerability and immunogenicity of an intranasally-administered adenovirus-vectored COVID-19 vaccine: an open-label partially-randomised ascending dose phase I trial. EBioMedicine 85, 104298 (2022).
Singh, C. et al. Phase III pivotal comparative clinical trial of intranasal (iNCOVACC) and intramuscular COVID 19 vaccine (Covaxin®). npj Vaccines 8, 125 (2023).
Ponce-de-León, S. et al. Interim safety and immunogenicity results from an NDV-based COVID-19 vaccine phase I trial in Mexico. npj Vaccines 8, 67 (2023).
Chavda, V. P., Vora, L. K., Pandya, A. K. & Patravale, V. B. Intranasal vaccines for SARS-CoV-2: from challenges to potential in COVID-19 management. Drug Discov. Today 26, 2619–2636 (2021).
Lei, H. et al. Intranasal administration of a recombinant RBD vaccine induces long-term immunity against Omicron-included SARS-CoV-2 variants. Signal Transduct. Target. Ther. 7, 159 (2022).
Stark, F. C. et al. Intranasal immunization with a proteosome-adjuvanted SARS-CoV-2 spike protein-based vaccine is immunogenic and efficacious in mice and hamsters. Sci. Rep. 12, 9772 (2022).
Wu, S. et al. A single dose of an adenovirus-vectored vaccine provides protection against SARS-CoV-2 challenge. Nat. Commun. 11, 4081 (2020).
Hendrickx, R. et al. Innate immunity to adenovirus. Hum. Gene Ther. 25, 265–284 (2014).
Fejer, G., Freudenberg, M., Greber, U. F. & Gyory, I. Adenovirus-triggered innate signalling pathways. Eur. J. Microbiol. Immunol. 1, 279–288 (2011).
Greber, U. F. & Flatt, J. W. Adenovirus entry: from infection to immunity. Annu. Rev. Virol. 6, 177–197 (2019).
Blum, J. S., Wearsch, P. A. & Cresswell, P. Pathways of antigen processing. Annu. Rev. Immunol. 31, 443–473 (2013).
McCann, N., O’Connor, D., Lambe, T. & Pollard, A. J. Viral vector vaccines. Curr. Opin. Immunol. 77, 102210 (2022).
Burgdorf, S., Kautz, A., Böhnert, V., Knolle, P. A. & Kurts, C. Distinct pathways of antigen uptake and intracellular routing in CD4 and CD8 T cell activation. Science 316, 612–616 (2007).
Saeland, E. et al. Combination Ad26.RSV.preF/preF protein vaccine induces superior protective immunity compared with individual vaccine components in preclinical models. npj Vaccines 8, 45 (2023).
Wang, Q. et al. XBB.1.5 monovalent mRNA vaccine booster elicits robust neutralizing antibodies against XBB subvariants and JN.1. Cell Host Microbe 32, 315–321.e3 (2024).
Stankov, M. V. et al. Humoral and cellular immune responses following BNT162b2 XBB.1.5 vaccination. Lancet Infect. Dis. 24, e1–e3 (2024).
Lin, D. Y. et al. Durability of XBB.1.5 vaccines against Omicron subvariants. N. Engl. J. Med. 390, 2124–2127 (2024).
He, C. et al. A recombinant spike-XBB.1.5 protein vaccine induces broad-spectrum immune responses against XBB.1.5-included Omicron variants of SARS-CoV-2. MedComm 4, e263 (2023).
He, C. et al. A self-assembled trimeric protein vaccine induces protective immunity against Omicron variant. Nat. Commun. 13, 5459 (2022).
Yang, J. et al. Trivalent recombinant protein vaccine induces cross-neutralization against XBB lineage and JN.1 subvariants: preclinical and phase 1 clinical trials. Nat. Commun. 15, 10778 (2024).
Chen, S. et al. Intranasal adenovirus-vectored Omicron vaccine induced nasal immunoglobulin A has superior neutralizing potency than serum antibodies. Signal Transduct. Target. Ther. 9, 190 (2024).
Chen, S. et al. Nasal mucosal secretory immunoglobulin A but not serum antibodies is resistant to Omicron evasion. hLife 2, 488–491 (2024).
Laidlaw, B. J. & Ellebedy, A. H. The germinal centre B cell response to SARS-CoV-2. Nat. Rev. Immunol. 22, 7–18 (2022).
Mudd, P. A. et al. SARS-CoV-2 mRNA vaccination elicits a robust and persistent T follicular helper cell response in humans. Cell 185, 603–613.e15 (2022).
Yang, J. et al. Low levels of neutralizing antibodies against XBB Omicron subvariants after BA.5 infection. Signal Transduct. Target. Ther. 8, 252 (2023).
Zhu, F. et al. Safety and immunogenicity of a live-attenuated influenza virus vector-based intranasal SARS-CoV-2 vaccine in adults: randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Respir. Med. 10, 749–760 (2022).
Haas, E. J. et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet 397, 1819–1829 (2021).
Azzi, L. et al. Mucosal immune response after the booster dose of the BNT162b2 COVID-19 vaccine. EBioMedicine 88, 104435 (2023).
Park, Y. J. et al. Imprinted antibody responses against SARS-CoV-2 Omicron sublineages. Science 378, 619–627 (2022).
Sun, B. et al. An intranasally administered adenovirus-vectored SARS-CoV-2 vaccine induces robust mucosal secretory IgA. JCI Insight 9, e180784 (2024).
Wrapp, D. et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 367, 1260–1263 (2020).
Huang, H. et al. The investigation of mRNA vaccines formulated in liposomes administrated in multiple routes against SARS-CoV-2. J. Control. Release 335, 449–456 (2021).
Hong, W., Pan, X. & Wei, X. Two-component intranasal vaccine against SARS-CoV-2 Omicron variant. NCBI GEO https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE279004 (2025).
Pan, X. Vaccine-against-SARS-CoV-2. Source code. GitHub https://github.com/pangxueyu233/Vaccine-against-SARS-CoV-2 (2025).
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2024YFC2310700, X.W.), the Sichuan Science and Technology Program (2023ZYD0169, X.W.) and the National Natural Science Foundation of China Young Student Basic Research Program (323B2050, W.H.). Schematic illustrations in Figs. 1a–c, 4a,g, 5a,g and 6a, and Extended Data Figs. 1b, 2a, 4a–c and 6f were created with BioRender.com.
Author information
Authors and Affiliations
Contributions
X.W. conceived of and supervised the research and designed the experiments. P.C. performed the construction of adenovirus vector vaccines. G.L. and Jiong Li performed gene cloning, expression and protein purification. X.S. prepared the mRNA vaccine. W.H., H.L., D.P., C.H. and Jingyun Yang performed vaccine formulation and vaccinations in animals, and performed binding antibodies assay and the pseudovirus neutralization experiment. W.H., H.L., D.P., C.H., Y. Huang, A.A., F.Q., B.W., J.A., Li Chen, H.Q., Z.Z., J. Liu, Y. Hao, D.A., Y. Zhang, X. Huang, C.Y. and M.F. collected the serum and BALF samples, and performed flow cytometry to assay the frequency of TRM, GC B and Tfh cells, and to evaluate the T cellular immune response in lung tissues. X.P. performed the analysis of scRNA-seq data. Y. Zhou and M.Y. performed live SARS-CoV-2 neutralization assays. Y. Wang, Q.S., S.L., Y.Y., W.Y., C.T., Q.H., B.L., Jingmei Li and J.W. performed SARS-CoV-2 challenge in animals and determined the viral loads. W.L., H.S. and Z.W. performed the evaluation of the efficiency and adverse reaction of the two-component vaccine in human participants. X.W., W.H., Jingyun Yang, H.L., D.P., C.H., W.R., X. He, Z.B., X. Han, M.L., H.H., W.C., H.D., J. Lei, Lu Chen, X.Z., W.W., G.S., Jinliang Yang, Y. Wei and L.Y. analysed and interpreted the data, and assisted with the adjustments of directions and interpretation of the mechanistic aspects of the results. X.W. and W.H. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
This work was supported by WestVac Biopharma Co. Ltd. P.C., Jingyun Yang, Z.W., Jiong Li, W.W., G.S., Jinliang Yang, Y. Wei, G.L., L.Y. and X.W. are employees of WestVac Biopharma Co. Ltd. The other authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Ling Chen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Characterizations of the Ad5XBB.1.5 + RBDXBB.1.5-HR vaccine.
a, Flow cytometric analysis of A549 cells infected with Ad5XBB.1.5 (MOI = 30) demonstrates high-affinity binding to recombinant human ACE2 (hACE2) protein. Control cells included uninfected A549 cells and those infected with Ad5Empty (MOI = 30). b, The diagram illustrates how to prepare a two-component intranasal vaccine by mixing adenoviruses with trimeric protein antigens. First, dilute a specific amount of RBDXBB.1.5-HR protein stock solution using a buffer, adjusting the pH to 7.6 to ensure consistency with the buffer composition of the Ad5XBB.1.5 adenovirus stock solution. Next, add the adenovirus stock solution to achieve final concentrations of 5×1010 VPs/ml and 100 μg/ml, or 1.0×1011 VPs/ml and 200 μg/ml. To prevent adenovirus aggregation during vaccine preparation, mix the adenovirus slowly as it is added. Schematic illustration in b was created in BioRender. Wang, Y. (2025) https://BioRender.com/1jokmtk. c, Size distribution of the Ad5XBB.1.5 and Ad5XBB.1.5 + RBDXBB.1.5-HR vaccine preparations, assessed using dynamic light scattering (DLS). d, Viral infectivity of adenoviruses in the Ad5XBB.1.5 and Ad5XBB.1.5 + RBDXBB.1.5-HR vaccine preparations (n = 3 vaccine preparation samples). e, Stability assay of the Ad5XBB.1.5 + RBDXBB.1.5-HR vaccine stored at -20°C for one year. The assay monitored the viral infectivity of Ad5XBB.1.5, the antigen content of RBDXBB.1.5-HR, as well as the pH and osmotic pressure of the Ad5XBB.1.5 + RBDXBB.1.5-HR vaccine over the year. Data are presented as mean with SEM in d. P values in d were conducted by two-tailed Unpaired Student’s t-tests. ns, not significant.
Extended Data Fig. 2 Immune responses following low-dose administration of Ad5XBB.1.5 + RBDXBB.1.5-HR vaccine.
a, BALB/c mice were intranasally immunized with the following formulations: 5×107 VPs of Ad5XBB.1.5, 5×107 VPs of Ad5XBB.1.5 combined with 5 μg of RBDXBB.1.5-HR, 5×108 VPs of Ad5XBB.1.5, and 5×108 VPs of Ad5XBB.1.5 combined with 5 μg of RBDXBB.1.5-HR. Immunizations were conducted three times at 0, 4, and 8 weeks. Schematic illustration in a was created in BioRender. Wang, Y. (2025) https://BioRender.com/1jokmtk. b-c, Endpoint titers of anti-RBD binding IgG in sera (b) and IgA in BALF (c) collected at 11 weeks. d, Sera neutralizing antibodies against XBB.1.5 pseudovirus at 11 weeks. e-f, IFN-γ-spot-forming cells in BALF (e) and spleen (f) samples after stimulation with peptide pools for SARS-CoV-2 XBB.1.5 spike. n = 6 mice per group in b-f. Data are presented as geometric mean values with SD in b-d, and as mean with SEM in e-f. P values were conducted by two-tailed Unpaired Student’s t-tests in b-c, and e-f. ns, not significant.
Extended Data Fig. 3 The intranasal Ad5XBB.1.5 + RBDXBB.1.5-HR vaccine induces a viral antigen-responsive TRM cell response in the lung.
Representative flow cytometry data show that CD8+ TRM cells in the lungs of mice receiving intranasal immunization with Ad5XBB.1.5 + RBDXBB.1.5-HR are antigen-specific, demonstrating IFN-γ secretion after stimulation with Spike peptide pools.
Extended Data Fig. 4 Characterization of the adenovirus carrying BA.5 spike protein (Ad5BA.5) and recombinant trimeric RBDBA.5-HR protein.
a, The schematic representation of the design of full-length BA.5 spike protein-carrying adenovirus (top). Western blotting of spike protein expression in A549 cells in the absence or presence of Ad5BA.5 (bottom). SP signal peptide, NTD N-terminal domain, RBD receptor binding domain, HR1 and HR2 heptad repeats 1 and 2, TM transmembrane domain, CP cytoplasmic domain. b, RBDBA.5-HR protein includes RBD derived from the BA.5 variant and HR1 and HR2 domains in subunit S2 of the spike protein. The representative elution chromatographs of the recombinant RBDBA.5-HR protein using a calibrated Superdex 200 Increase column (bottom right). The SDS-PAGE analysis of the eluted protein is shown. M, marker; 1-3, the eluted samples of the ascending part and descending part of the peak. a-b are representative of two independent experiments with similar results. c, The schematic representations of the preparation of Ad5BA.5 plus RBDBA.5-HR. Schematic illustrations in a-c were created in BioRender. Wang, Y. (2025) https://BioRender.com/1jokmtk.
Extended Data Fig. 5 Activation of the STING signaling pathway in lung dendritic cells following immunization with the two-component intranasal vaccine.
a, The t-distributed stochastic neighbor embedding (t-SNE) analysis revealed distinct annotated cell clusters derived from scRNA-seq data obtained from lung tissues of wild-type mice treated with PBS and those administered Ad5XBB.1.5 + RBDXBB.1.5-HR, along with Sting−/− mice receiving the same treatment (Three mice were merged in one group). b, A heatmap depicting canonical cell-type markers was generated to identify distinct cell populations. c, The expression profile of the Tmem173 gene across various cell populations was illustrated. d, Quantitative analysis of Tmem173 gene expression was performed in each subcellular population. e, The expression of the Tmem173 gene in CCR7+ DC, DC1, and plasmacytoid dendritic cell (pDC) populations from wild-type mice treated with PBS or Ad5XBB.1.5 + RBDXBB.1.5-HR, and Sting−/− mice receiving Ad5XBB.1.5 + RBDXBB.1.5-HR. f, Expression levels of genes (Adar, Ccl5, Ddx41, Ifi203, Ifit1, Irf7, Irf9, Isg15, Oas1a, and Trim21) associated with STING signaling pathway activation in each group. g, The expression of genes related to antigen processing and presentation in lung dendritic cells. h, The phagocytosis of FITC fluorescence-conjugated RBDXBB.1.5-HR proteins by DCs in the absence or presence of Ad5Empty or Ad5XBB.1.5. i, The scoring of plasma cell induction and B cell activation in lung tissues was based on scRNA-seq data analysis. The expression levels of the genes in e–f were analyzed across three dendritic cell subsets (CCR7⁺ DCs, DC1, and pDCs) in different sample groups. Bar plots represent the mean expression levels across biological replicates (three mice were merged in one group), with error bars indicating the SEM. P values in e-f were assessed using the Wilcoxon rank-sum test.
Extended Data Fig. 6 Three-component vaccine elicits cross-neutralizing responses and enhances immunity as a heterologous booster following mRNA vaccination.
a, The radar chart was generated using the Log10 GMT data presented in main Fig. 1g and Fig. 4d to compare the tropism of the sera neutralizing antibody response induced by the two-component and three-component vaccines. The numbers represent the fold difference of GMTs between the neutralizing antibodies induced by the three-component vaccine and those induced by the two-component vaccine. b, Sera Neutralizing antibodies induced by the three-component vaccines containing RBDXBB.1.5-HR and RBDBA.5-HR with different antigenic ratios. BALB/c mice were intranasally immunized three times with the three-component vaccine composed of RBDXBB.1.5-HR and RBDBA.5-HR at antigenic ratios of 3:1, 1:1, and 1:3, respectively. Sera were collected to determine the neutralizing antibody levels and compared with titers of neutralizing antibodies induced by the three-component vaccine with antigenic ratios of 3:1 (n = 6 mice per group). c-e, The percentage of TRM cells in lung tissues (c), Tfh (d) cells, and RBD-specific GC B cells (e) in mLNs after four doses of mRNA vaccine or 3 doses of mRNA followed by twice intranasal delivery of three-component vaccine (n = 6 mice per group). f-k, BALB/c mice were intramuscularly immunized with four doses of encapsulated liposome (LPX)/Spike-mRNA, or immunized with three doses of mRNA followed by once intranasal delivery of three-component vaccine (3×mRNA+1×Three-component vaccine) (n = 6 mice per group). The sera anti-RBD IgG (g), BALF anti-RBD IgG (h), BALF IgA antibody (i), BALF TRM cells (j), and Spike-specific T cells in lung tissue (k) were detected. Schematic illustration in f was created in BioRender. Wang, Y. (2025) https://BioRender.com/tp7lcm5. Data are presented as geometric mean values with SD in b, g-i, and as mean with SEM in c-e, and j-k. P values were calculated by two-tailed Unpaired Student’s t-tests in d-e and g-i, and by two-way ANOVA analysis followed by Sidak’s multiple comparisons test in c and j-k. ns, not significant.
Extended Data Fig. 7 Neutralizing activities in human participants receiving intranasal vaccine containing 1×1010 VPs of Ad5XBB.1.5 plus 20 μg of RBDXBB.1.5-HR protein.
Participants were intranasally administered Ad5XBB.1.5 (1×1010 VPs) plus RBDXBB.1.5-HR protein (20 μg) on days 0 and 14. The neutralizing antibodies in sera collected on days 14 and 28 against pseudoviruses including prototype, BA.5, XBB.1.5, XBB.1.6, XBB.1.16, XBB.1.9.1, and XBB.2.3 were determined (n = 20 participants). Data are presented as geometric mean values with SD.
Supplementary information
Source data
Source Data
Source data for Figs. 1–6 and Extended Data Figs. 1–7.
Source Data Fig. 1 and Extended Data Fig. 4
Original images of western blots and gels shown in Fig. 1a,b and Extended Data Fig. 4a,b.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hong, W., Cheng, P., Yang, J. et al. Intranasal vaccine combining adenovirus and trimeric subunit protein provides superior immunity against SARS-CoV-2 Omicron variant. Nat. Biomed. Eng (2025). https://doi.org/10.1038/s41551-025-01517-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41551-025-01517-2
This article is cited by
-
Working together: a multi-component intranasal vaccine provides synergistic protection against COVID-19
Signal Transduction and Targeted Therapy (2025)
-
Benchmarking matters
Nature Biomedical Engineering (2025)