Abstract
The microbiome affects eukaryotic host cells via many metabolites, including the well-studied queuine as substrate for host tRNA queuosine modification. The microbial metabolite pre-queuosine 1 (preQ1) is produced in the bacterial tRNA queuosine biosynthesis pathway, with unknown effects on host cell biology. Here we show that preQ1 strongly represses cell proliferation in both human and mouse cells. Queuine reverses this effect by competing with preQ1 to modify the same tRNA. PreQ1 is detectable in the plasma and tissues of mice, and its injection suppresses tumour growth in a mouse cancer model. Mechanistically, preQ1 reduces cognate tRNA levels specifically, as well as codon-dependent translation of housekeeping genes. We identify the endoplasmic reticulum-localized inositol-requiring enzyme 1 (IRE1) ribonuclease as the enzyme responsible for the selective degradation of preQ1-modified tRNAs on translating ribosomes. Our results identify two microbial metabolites competing for host tRNA modification, which elicits translation quality control and impacts cell proliferation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All sequencing data generated during this study, including tRNA-seq with or without queuine/preQ1 treatment, input/polysome mRNA-seq with or without preQ1 treatment, ribo-seq, tRNA-seq with or without PNK, tRNA-seq with or without IRE1 inhibitor 4µ8C and human stool PAQS-seq, are available at the Gene Expression Omnibus (GEO) under accession code GSE233846. The human genome GRCh38 and genomic tRNA database (https://gtrnadb.ucsc.edu) was used to map the sequencing data. Source data are provided with this paper.
Code availability
All custom scripts are available on GitHub (https://github.com/ckatanski/preQ1 and https://github.com/ckatanski/Q_paper).
References
Fergus, C., Barnes, D., Alqasem, M. A. & Kelly, V. P. The queuine micronutrient: charting a course from microbe to man. Nutrients 7, 2897–2929 (2015).
Sarid, L. et al. Queuine salvaging in the human parasite Entamoeba histolytica. Cells 11, 2509 (2022).
Patel, B. I., Heiss, M., Samel-Pommerencke, A., Carell, T. & Ehrenhofer-Murray, A. E. Queuosine salvage in fission yeast by Qng1-mediated hydrolysis to queuine. Biochem. Biophys. Res. Commun. 624, 146–150 (2022).
Hung, S. H. et al. Structural basis of Qng1-mediated salvage of the micronutrient queuine from queuosine-5′-monophosphate as the biological substrate. Nucleic Acids Res. 51, 935–951 (2023).
Yuan, Y. et al. Discovery of novel bacterial queuine salvage enzymes and pathways in human pathogens. Proc. Natl Acad. Sci. USA 116, 19126–19135 (2019).
Burtnyak, L. et al. The oncogene SLC35F2 is a high-specificity transporter for the micronutrients queuine and queuosine. Proc. Natl Acad. Sci. USA 122, e2425364122 (2025).
Boland, C., Hayes, P., Santa-Maria, I., Nishimura, S. & Kelly, V. P. Queuosine formation in eukaryotic tRNA occurs via a mitochondria-localized heteromeric transglycosylase. J. Biol. Chem. 284, 18218–18227 (2009).
Kessler, A. C. et al. Retrograde nuclear transport from the cytoplasm is required for tRNA(Tyr) maturation in T. brucei. RNA Biol. 15, 528–536 (2018).
Hegedusova, E., Kulkarni, S., Burgman, B., Alfonzo, J. D. & Paris, Z. The general mRNA exporters Mex67 and Mtr2 play distinct roles in nuclear export of tRNAs in Trypanosoma brucei. Nucleic Acids Res. 47, 8620–8631 (2019).
Harada, F. & Nishimura, S. Possible anticodon sequences of tRNA His, tRNA Asm and tRNA Asp from Escherichia coli B. Universal presence of nucleoside Q in the first postion of the anticondons of these transfer ribonucleic acids. Biochemistry 11, 301–308 (1972).
Nishimura, S. Structure, biosynthesis, and function of queuosine in transfer RNA. Prog. Nucleic Acid Res. Mol. Biol. 28, 49–73 (1983).
Zhao, X. et al. Glycosylated queuosines in tRNAs optimize translational rate and post-embryonic growth. Cell 186, 5517–5535.e24 (2023).
Wang, X. et al. Queuosine modification protects cognate tRNAs against ribonuclease cleavage. RNA 24, 1305–1313 (2018).
Meier, F., Suter, B., Grosjean, H., Keith, G. & Kubli, E. Queuosine modification of the wobble base in tRNAHis influences ‘in vivo’ decoding properties. EMBO J. 4, 823–827 (1985).
Zaborske, J. M. et al. A nutrient-driven tRNA modification alters translational fidelity and genome-wide protein coding across an animal genus. PLoS Biol. 12, e1002015 (2014).
Kulkarni, S. et al. Preferential import of queuosine-modified tRNAs into Trypanosoma brucei mitochondrion is critical for organellar protein synthesis. Nucleic Acids Res. 49, 8247–8260 (2021).
Muller, M. et al. Queuine links translational control in eukaryotes to a micronutrient from bacteria. Nucleic Acids Res. 47, 3711–3727 (2019).
Tuorto, F. et al. Queuosine-modified tRNAs confer nutritional control of protein translation. EMBO J 37, e99777 (2018).
Cirzi, C. et al. Queuosine-tRNA promotes sex-dependent learning and memory formation by maintaining codon-biased translation elongation speed. EMBO J. 42, e112507 (2023).
Chen, D. W. & Garud, N. R. Rapid evolution and strain turnover in the infant gut microbiome. Genome Res. 32, 1124–1136 (2022).
Garud, N. R., Good, B. H., Hallatschek, O. & Pollard, K. S. Evolutionary dynamics of bacteria in the gut microbiome within and across hosts. PLoS Biol. 17, e3000102 (2019).
Jiang, X. et al. Invertible promoters mediate bacterial phase variation, antibiotic resistance and host adaptation in the gut. Science 363, 181–187 (2019).
Zhao, S. et al. Adaptive evolution within gut microbiomes of healthy people. Cell Host Microbe 25, 656–667 e8 (2019).
Fergus, C. et al. The human tRNA-guanine transglycosylase displays promiscuous nucleobase preference but strict tRNA specificity. Nucleic Acids Res. 49, 4877–4890 (2021).
Bessler, L. et al. Functional integration of a semi-synthetic azido-queuosine derivative into translation and a tRNA modification circuit. Nucleic Acids Res. 50, 10785–10800 (2022).
Zhang, W., Foo, M., Eren, A. M. & Pan, T. tRNA modification dynamics from individual organisms to metaepitranscriptomics of microbiomes. Mol. Cell 82, 891–906 (2022).
Yan, L. L. & Zaher, H. S. How do cells cope with RNA damage and its consequences? J. Biol. Chem. 294, 15158–15171 (2019).
Probst-Rud, S. et al. Synergistic effect of UV-A and UV-C light is traced to UV-induced damage of the transfer RNA. Water Res. 252, 121189 (2024).
Sun, C., Limbach, P. A. & Addepalli, B. Characterization of UVA-induced alterations to transfer RNA sequences. Biomolecules 10, 1527 (2020).
Kladwang, W., Hum, J. & Das, R. Ultraviolet shadowing of RNA can cause significant chemical damage in seconds. Sci. Rep. 2, 517 (2012).
Simms, C. L. & Zaher, H. S. Quality control of chemically damaged RNA. Cell. Mol. Life Sci. 73, 3639–3653 (2016).
Zhang, H. X. et al. An integrated approach to evaluate acetamiprid-induced oxidative damage to tRNA in human cells based on oxidized nucleotide and tRNA profiling. Environ. Int. 178, 108038 (2023).
Thompson, D. M., Lu, C., Green, P. J. & Parker, R. tRNA cleavage is a conserved response to oxidative stress in eukaryotes. RNA 14, 2095–2103 (2008).
Wurtmann, E. J. & Wolin, S. L. RNA under attack: cellular handling of RNA damage. Crit. Rev. Biochem. Mol. Biol. 44, 34–49 (2009).
Tanaka, T., Ohashi, S. & Kobayashi, S. Roles of YB-1 under arsenite-induced stress: translational activation of HSP70 mRNA and control of the number of stress granules. Biochim. Biophys. Acta 1840, 985–992 (2014).
Graille, M. & Seraphin, B. Surveillance pathways rescuing eukaryotic ribosomes lost in translation. Nat. Rev. Mol. Cell Biol. 13, 727–735 (2012).
Kervestin, S. & Jacobson, A. NMD: a multifaceted response to premature translational termination. Nat. Rev. Mol. Cell Biol. 13, 700–712 (2012).
Shoemaker, C. J. & Green, R. Translation drives mRNA quality control. Nat. Struct. Mol. Biol. 19, 594–601 (2012).
Doma, M. K. & Parker, R. Endonucleolytic cleavage of eukaryotic mRNAs with stalls in translation elongation. Nature 440, 561–564 (2006).
Monaghan, L., Longman, D. & Caceres, J. F. Translation-coupled mRNA quality control mechanisms. EMBO J. 42, e114378 (2023).
Frischmeyer, P. A. et al. An mRNA surveillance mechanism that eliminates transcripts lacking termination codons. Science 295, 2258–2261 (2002).
van Hoof, A., Frischmeyer, P. A., Dietz, H. C. & Parker, R. Exosome-mediated recognition and degradation of mRNAs lacking a termination codon. Science 295, 2262–2264 (2002).
Saito, S., Hosoda, N. & Hoshino, S. The Hbs1-Dom34 protein complex functions in non-stop mRNA decay in mammalian cells. J. Biol. Chem. 288, 17832–17843 (2013).
Copela, L. A., Fernandez, C. F., Sherrer, R. L. & Wolin, S. L. Competition between the Rex1 exonuclease and the La protein affects both Trf4p-mediated RNA quality control and pre-tRNA maturation. RNA 14, 1214–1227 (2008).
Kadaba, S., Wang, X. & Anderson, J. T. Nuclear RNA surveillance in Saccharomyces cerevisiae: Trf4p-dependent polyadenylation of nascent hypomethylated tRNA and an aberrant form of 5S rRNA. RNA 12, 508–521 (2006).
Ozanick, S. G. et al. Rex1p deficiency leads to accumulation of precursor initiator tRNAMet and polyadenylation of substrate RNAs in Saccharomyces cerevisiae. Nucleic Acids Res. 37, 298–308 (2009).
Vanacova, S. et al. A new yeast poly(A) polymerase complex involved in RNA quality control. PLoS Biol. 3, e189 (2005).
Alexandrov, A. et al. Rapid tRNA decay can result from lack of nonessential modifications. Mol. Cell 21, 87–96 (2006).
Chernyakov, I., Whipple, J. M., Kotelawala, L., Grayhack, E. J. & Phizicky, E. M. Degradation of several hypomodified mature tRNA species in Saccharomyces cerevisiae is mediated by Met22 and the 5′-3′ exonucleases Rat1 and Xrn1. Genes Dev. 22, 1369–1380 (2008).
Wilusz, J. E., Whipple, J. M., Phizicky, E. M. & Sharp, P. A. tRNAs marked with CCACCA are targeted for degradation. Science 334, 817–821 (2011).
Whipple, J. M., Lane, E. A., Chernyakov, I., D’Silva, S. & Phizicky, E. M. The yeast rapid tRNA decay pathway primarily monitors the structural integrity of the acceptor and T-stems of mature tRNA. Genes Dev. 25, 1173–1184 (2011).
Fedry, J. et al. Visualization of translation reorganization upon persistent ribosome collision stress in mammalian cells. Mol. Cell 84, 1078–1089.e4 (2024).
Yip, M. C. J. & Shao, S. Detecting and rescuing stalled ribosomes. Trends Biochem. Sci. 46, 731–743 (2021).
Pan, X. et al. Development, validation and application of an LC-MS/MS method quantifying free forms of the micronutrients queuine and queuosine in human plasma using a surrogate matrix approach. Anal. Bioanal. Chem. 416, 5711–5719 (2024).
Katanski, C. D. et al. Analysis of queuosine and 2-thio tRNA modifications by high throughput sequencing. Nucleic Acids Res. 50, e99 (2022).
Zhang, W., Xu, R., Matuszek, Z., Cai, Z. & Pan, T. Detection and quantification of glycosylated queuosine modified tRNAs by acid denaturing and APB gels. RNA 26, 1291–1298 (2020).
Amalric, A. et al. Mass spectrometry-based pipeline for identifying RNA modifications involved in a functional process: application to cancer cell adaptation. Anal. Chem. 96, 1825–1833 (2024).
Quaiyum, S. et al. Queuosine salvage in Bartonella henselae Houston 1: a unique evolutionary path. Microbiol. (Reading) 170, 001490 (2024).
Watkins, C. P., Zhang, W., Wylder, A. C., Katanski, C. D. & Pan, T. A multiplex platform for small RNA sequencing elucidates multifaceted tRNA stress response and translational regulation. Nat. Commun. 13, 2491 (2022).
Randerath, E., Agrawal, H. P. & Randerath, K. Specific lack of the hypermodified nucleoside, queuosine, in hepatoma mitochondrial aspartate transfer RNA and its possible biological significance. Cancer Res. 44, 1167–1171 (1984).
Suzuki, T. et al. Complete chemical structures of human mitochondrial tRNAs. Nat. Commun. 11, 4269 (2020).
Schmidt, E. K., Clavarino, G., Ceppi, M. & Pierre, P. SUnSET, a nonradioactive method to monitor protein synthesis. Nat. Methods 6, 275–277 (2009).
Acosta-Alvear, D. et al. The unfolded protein response and endoplasmic reticulum protein targeting machineries converge on the stress sensor IRE1. eLife 7, e43036 (2018).
Cox, J. S. & Walter, P. A novel mechanism for regulating activity of a transcription factor that controls the unfolded protein response. Cell 87, 391–404 (1996).
Calfon, M. et al. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415, 92–96 (2002).
Yoshida, H., Matsui, T., Yamamoto, A., Okada, T. & Mori, K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107, 881–891 (2001).
Korennykh, A. V. et al. Structural and functional basis for RNA cleavage by Ire1. BMC Biol. 9, 47 (2011).
Jin, H. et al. 5′-tRNA(Gly(GCC)) halves generated by IRE1α are linked to the ER stress response. Nat. Commun. 15, 9273 (2024).
Cross, B. C. et al. The molecular basis for selective inhibition of unconventional mRNA splicing by an IRE1-binding small molecule. Proc. Natl Acad. Sci. USA 109, E869–E878 (2012).
Gonzalez, T. N. & Walter, P. Ire1p: a kinase and site-specific endoribonuclease. Methods Mol. Biol. 160, 25–36 (2001).
Shamu, C. E. & Walter, P. Oligomerization and phosphorylation of the Ire1p kinase during intracellular signaling from the endoplasmic reticulum to the nucleus. EMBO J. 15, 3028–3039 (1996).
Sundaram, A., Plumb, R., Appathurai, S. & Mariappan, M. The Sec61 translocon limits IRE1à signaling during the unfolded protein response. eLife 6, e27187 (2017).
Sundaram, A., Appathurai, S., Plumb, R. & Mariappan, M. Dynamic changes in complexes of IRE1α, PERK and ATF6α during endoplasmic reticulum stress. Mol. Biol. Cell 29, 1376–1388 (2018).
Amin-Wetzel, N. et al. A J-protein co-chaperone recruits BiP to monomerize IRE1 and repress the unfolded protein response. Cell 171, 1625–1637.e13 (2017).
Belyy, V., Zuazo-Gaztelu, I., Alamban, A., Ashkenazi, A. & Walter, P. Endoplasmic reticulum stress activates human IRE1α through reversible assembly of inactive dimers into small oligomers. eLife 11, e74342 (2022).
Ali, M. M. et al. Structure of the Ire1 autophosphorylation complex and implications for the unfolded protein response. EMBO J. 30, 894–905 (2011).
Itzhak, D. et al. Multiple autophosphorylations significantly enhance the endoribonuclease activity of human inositol requiring enzyme 1α. BMC Biochem. 15, 3 (2014).
Lin, J. H. et al. IRE1 signaling affects cell fate during the unfolded protein response. Science 318, 944–949 (2007).
Yang, L. et al. A Phos-tag-based approach reveals the extent of physiological endoplasmic reticulum stress. PLoS ONE 5, e11621 (2010).
Volmer, R., van der Ploeg, K. & Ron, D. Membrane lipid saturation activates endoplasmic reticulum unfolded protein response transducers through their transmembrane domains. Proc. Natl Acad. Sci. USA 110, 4628–4633 (2013).
Guydosh, N. R., Kimmig, P., Walter, P. & Green, R. Regulated Ireÿ1-dependent mRNA decay requires no-go mRNA degradation to maintain endoplasmic reticulum homeostasis in S. pombe. eLife 6, e29216 (2017).
Simms, C. L., Yan, L. L. & Zaher, H. S. Ribosome collision is critical for quality control during no-go decay. Mol. Cell 68, 361–373.e5 (2017).
Navickas, A. et al. No-go decay mRNA cleavage in the ribosome exit tunnel produces 5′-OH ends phosphorylated by Trl1. Nat. Commun. 11, 122 (2020).
Powers, K. T., Szeto, J. A. & Schaffitzel, C. New insights into no-go, non-stop and nonsense-mediated mRNA decay complexes. Curr. Opin. Struct. Biol. 65, 110–118 (2020).
Wu, C. C., Peterson, A., Zinshteyn, B., Regot, S. & Green, R. Ribosome collisions trigger general stress responses to regulate cell fate. Cell 182, 404–416.e14 (2020).
Juszkiewicz, S. et al. ZNF598 is a quality control sensor of collided ribosomes. Mol. Cell 72, 469–481.e7 (2018).
Schaefer, M. et al. RNA methylation by Dnmt2 protects transfer RNAs against stress-induced cleavage. Genes Dev. 24, 1590–1595 (2010).
Muller, M. et al. Dynamic modulation of Dnmt2-dependent tRNA methylation by the micronutrient queuine. Nucleic Acids Res. 43, 10952–10962 (2015).
Rakovich, T. et al. Queuosine deficiency in eukaryotes compromises tyrosine production through increased tetrahydrobiopterin oxidation. J. Biol. Chem. 286, 19354–19363 (2011).
Zhang, J. et al. tRNA queuosine modification enzyme modulates the growth and microbiome recruitment to breast tumors. Cancers (Basel) 12, 628 (2020).
Dixit, S. et al. Dynamic queuosine changes in tRNA couple nutrient levels to codon choice in Trypanosoma brucei. Nucleic Acids Res. 49, 12986–12999 (2021).
Johannsson, S. et al. Structural insights into the stimulation of S. pombe Dnmt2 catalytic efficiency by the tRNA nucleoside queuosine. Sci. Rep. 8, 8880 (2018).
Huber, S. M. et al. Arsenite toxicity is regulated by queuine availability and oxidation-induced reprogramming of the human tRNA epitranscriptome. Proc. Natl Acad. Sci. USA 119, e2123529119 (2022).
Marks, T. & Farkas, W. R. Effects of a diet deficient in tyrosine and queuine on germfree mice. Biochem. Biophys. Res. Commun. 230, 233–237 (1997).
Hayes, P. et al. Queuine micronutrient deficiency promotes Warburg metabolism and reversal of the mitochondrial ATP synthase in Hela cells. Nutrients 12, 871 (2020).
Nagaraja, S. et al. Queuine is a nutritional regulator of Entamoeba histolytica response to oxidative stress and a virulence attenuator. mBio 12, e03549-20 (2021).
Belkaid, Y. & Hand, T. W. Role of the microbiota in immunity and inflammation. Cell 157, 121–141 (2014).
Zheng, D., Liwinski, T. & Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 30, 492–506 (2020).
Wu, H. J. & Wu, E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 3, 4–14 (2012).
Lazar, V. et al. Aspects of gut microbiota and immune system interactions in infectious diseases, immunopathology and cancer. Front. Immunol. 9, 1830 (2018).
Liu, Y., Wang, J. & Wu, C. Modulation of gut microbiota and immune system by probiotics, pre-biotics and post-biotics. Front. Nutr. 8, 634897 (2021).
Stadler, M. & Fire, A. Wobble base-pairing slows in vivo translation elongation in metazoans. RNA 17, 2063–2073 (2011).
Chevance, F. F., Le Guyon, S. & Hughes, K. T. The effects of codon context on in vivo translation speed. PLoS Genet. 10, e1004392 (2014).
Grosjean, H. & Westhof, E. An integrated, structure- and energy-based view of the genetic code. Nucleic Acids Res. 44, 8020–8040 (2016).
Laursen, B. S., Sorensen, H. P., Mortensen, K. K. & Sperling-Petersen, H. U. Initiation of protein synthesis in bacteria. Microbiol. Mol. Biol. Rev. 69, 101–123 (2005).
Kudla, G., Murray, A. W., Tollervey, D. & Plotkin, J. B. Coding-sequence determinants of gene expression in Escherichia coli. Science 324, 255–258 (2009).
Rodnina, M. V. The ribosome in action: tuning of translational efficiency and protein folding. Protein Sci. 25, 1390–1406 (2016).
Reeve, B., Hargest, T., Gilbert, C. & Ellis, T. Predicting translation initiation rates for designing synthetic biology. Front. Bioeng. Biotechnol. 2, 1 (2014).
Brooks, A. F., Garcia, G. A. & Showalter, H. D. H. A short, concise synthesis of queuine. Tetrahedron Lett. 51, 4163–4165 (2010).
Das, S. et al. Selective catalytic reductions of amides and nitriles to amines. Top. Catal. 53, 979–984 (2010).
Migawa, M. T., Hinkley, J. M., Hoops, G. C. & Townsend, L. B. A two step synthesis of the nucleoside Q precursor 2-amino-5-cyanopyrrolo[2,3-d]pyrimidin-4-one (PreQ0). Synth. Commun. 26, 3317–3322 (1996).
Gerber, H. D. & Klebe, G. Concise and efficient syntheses of preQ1 base, Q base, and (ent)-Q base. Org. Biomol. Chem. 10, 8660–8668 (2012).
Levic, J. & Micura, R. Syntheses of 15N-labeled pre-queuosine nucleobase derivatives. Beilstein J. Org. Chem. 10, 1914–1918 (2014).
Zoetendal, E. G. et al. Isolation of RNA from bacterial samples of the human gastrointestinal tract. Nat. Protoc. 1, 954–959 (2006).
Alexander, R. J. & Raicht, R. F. Purification of total RNA from human stool samples. Dig. Dis. Sci. 43, 2652–2658 (1998).
Towbin, H., Staehelin, T. & Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl Acad. Sci. USA 76, 4350–4354 (1979).
Mahmood, T. & Yang, P. C. Western blot: technique, theory and trouble shooting. N. Am. J. Med. Sci. 4, 429–434 (2012).
Alegria-Schaffer, A., Lodge, A. & Vattem, K. Performing and optimizing western blots with an emphasis on chemiluminescent detection. Methods Enzymol. 463, 573–599 (2009).
Pandey, S. et al. A whole-tissue RNA-seq toolkit for organism-wide studies of gene expression with PME-seq. Nat. Protoc. 15, 1459–1483 (2020).
Kadoki, M. et al. Organism-level analysis of vaccination reveals networks of protection across tissues. Cell 171, 398–413 e321 (2017).
Pandey, S. et al. Pairwise stimulations of pathogen-sensing pathways predict immune responses to multi-adjuvant combinations. Cell Syst. 11, 495–508 e410 (2020).
Ingolia, N. T., Brar, G. A., Rouskin, S., McGeachy, A. M. & Weissman, J. S. The ribosome profiling strategy for monitoring translation in vivo by deep sequencing of ribosome-protected mRNA fragments. Nat. Protoc. 7, 1534–1550 (2012).
McGlincy, N. J. & Ingolia, N. T. Transcriptome-wide measurement of translation by ribosome profiling. Methods 126, 112–129 (2017).
Han, P. et al. Genome-wide survey of ribosome collision. Cell Rep. 31, 107610 (2020).
Mito, M., Mishima, Y. & Iwasaki, S. Protocol for disome profiling to survey ribosome collision in humans and zebrafish. STAR Protoc. 1, 100168 (2020).
Wittig, I., Braun, H. P. & Schagger, H. Blue native PAGE. Nat. Protoc. 1, 418–428 (2006).
Huerta-Cepas, J., Serra, F. & Bork, P. ETE 3: reconstruction, analysis and visualization of phylogenomic data. Mol. Biol. Evol. 33, 1635–1638 (2016).
Chan, P. P. & Lowe, T. M. GtRNAdb 2.0: an expanded database of transfer RNA genes identified in complete and draft genomes. Nucleic Acids Res. 44, D184–D189 (2016).
Girardot, C., Scholtalbers, J., Sauer, S., Su, S. Y. & Furlong, E. E. Je, a versatile suite to handle multiplexed NGS libraries with unique molecular identifiers. BMC Bioinformatics 17, 419 (2016).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Robinson, J. T. et al. Integrative genomics viewer. Nat. Biotechnol. 29, 24–26 (2011).
Li, H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Andrews, S. FastQC: a quality control tool for high throughput sequence data. v0.11.9 (Babraham Bioinformatics, 2010); http://www.bioinformatics.babraham.ac.uk/projects/fastqc/
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Li, B. & Dewey, C. N. RSEM: accurate transcript quantification from RNA-seq data with or without a reference genome. BMC Bioinformatics 12, 323 (2011).
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Ashburner, M. et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 25, 25–29 (2000).
Gene Ontology Consortium The Gene Ontology resource: enriching a GOld mine. Nucleic Acids Res. 49, D325–D334 (2021).
Zhang, P. et al. Genome-wide identification and differential analysis of translational initiation. Nat. Commun. 8, 1749 (2017).
Frye, M. & Bornelov, S. CONCUR: quick and robust calculation of codon usage from ribosome profiling data. Bioinformatics 37, 717–719 (2021).
Acknowledgements
T.P. was supported by NIH grants nos. RM1HG008935 and R33CA272357, and a pilot from the Univ. Chicago CIID Centre (P30 DK42086). N.C. was supported by NIH grants nos. DP2-AI145100 and U01-AI160418, the Chan-Zuckerberg Initiative, the UCCCC Janet D. Rowley Discovery Fund, the Univ. Chicago CIID centre (P30 DK42086) and the Chicago Immunoengineering Innovation Center and Pritzker School of Molecular Engineering. A. David was supported by Occitanie Region/FEDER (PPRi, SMART project), INCa (RPT20001FFA–INCA 2020-116) and Ligue Contre le Cancer (no. AAPARN 2022.LCC/AID). All other authors were supported by grants awarded to corresponding principal investigators.
Author information
Authors and Affiliations
Contributions
W.Z., N.C., A. David and T.P. conceived the project and wrote the paper. W.Z. performed cell culture preQ1 experiments, polysome profiling, ribo-seq, ribosome collision and quality-control checks. W.Z. and D.R. performed IRE1 and tRNA cleavage characterizations. M.S. performed the translation inhibitor experiment. K.L., H.G., A.A., F.M. and J.F. developed LC/MS/MS methods for queuosine and preQ1sine measurements, performed extraction and MS measurements and analysis of the cells and mouse tissues. D.G. and D.V. synthesized the queuosine and preQ1sine nucleosides. D.C., A.M.S. and N.C. designed and performed all mouse preQ1 and tumour experiments. O.Z. and K.J. performed BMDC experiments. C.D.K., C.P.W., H.C. and M.A. performed MSR-seq and PAQS-seq experiments. A. Djiane and C.H. provided guidance on LC/MS/MS experiments. S.H., L.R.F. and C.D.K. performed RNA-seq data analysis. W.Z., A. David and T.P. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cell Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Additional data for Fig. 1.
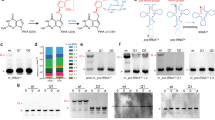
ns: not significant, * p < 0.05, ** p < 0.01, *** p < 10−3, **** p < 10−4. Mann-Whitney U test, two sided. Data are presented as mean values ± SD. a) Queuine, preQ1, queuosine (Q) and N15-labeled q (queuineN15) MS calibration curves with linear fits of the log10 values. Additional calibration curves (used for group 2 samples) used the MS matrix optimized for plasma. The limit of detection is ~0.1 nM for queuine and preQ1. b) Short retrosynthetic chemical description of the different synthetic routes that led to the MS standard compounds used in this work. c) Deletion rate of PAQS-seq of Q-modifiable tRNAs of genus roseburia in the human stool sample with and without periodate (IO) treatment. d) Proliferation measurement by absorbance at 460 nm, HEK293T cells. Arrow indicates the time of addition of preQ1 and/or queuine. n = 8 biological replicates. e) Proliferation measurement by absorbance at 460 nm, HEK293T cells. Arrow indicates the time of addition of preQ1 and/or queuine. n = 8 biological replicates. f) Proliferation measurement by absorbance at 460 nm, HEK293T cells. The starting cells were mixtures of 0Q and 100Q cells. Arrow indicates the time of addition of preQ1. n = 8 biological replicates. g) Proliferation measurement by absorbance at 460 nm, MEF cells. Arrow indicates the time of addition of preQ1 and/or queuine. n = 8 biological replicates. h) Relative cell count of proliferation measurement, BMDC cells. Arrow indicates the time of addition of preQ1 and/or queuine. Cell counts are normalized to 0Q cells at t = 0. n = 5 replicates for each condition. i) Flow cytometry of Murine BMDCs. From left to right: the myeloid cell population is identified by forward and side scatter, doublets are excluded, and live cells are quantified by selecting the DAPI low population. n = 5 biological replicates. j) Normalized cell count or proliferation of mouse BMDC cells under indicated preQ1 and queuine concentrations. All data normalized to the average of 0,0 condition at day 1. n = 5 replicates for each condition. p values from left to right: 0.202, 6.09e-3, 6.09e-3. Source numerical data are available in.
Extended Data Fig. 2 Additional data for Fig. 2.
ns: not significant, * p < 0.05, ** p < 0.01, *** p < 10−3. Panel (a, c): Mann-Whitney U test, two sided. Panel (e): Tukey’s Honestly Significant Difference test (HSD), two-sided. Data are presented as mean values ± SD. a) Proliferation measurement by absorbance at 460 nm, shRNA-QTRT1 knockdown, and control HEK293T cells. Arrow indicates the time of addition of preQ1 and/or queuine. Error bar corresponds to the range of n = 8 biological replicates. b) Western blot showing shRNA knockdown of the QTRT2 protein. CycB is the loading control. c) Same setup as panel (a), except for shRNA-QTRT2 and control cells. d) Western blot of QTRT1 protein in HEK293T, MEF, and BMDC cells. Vinculin is the loading control. e) Quantification of QTRT1 level in panel (d). n = 3 biological replicates. p values from left to right: 0.097, 8.0e-4, 0.007. f) Reaction scheme of the preQ1-modified tRNA with NHS ester for Northern blot analysis. Source numerical data and unprocessed blots are available in.
Extended Data Fig. 3 Additional data for Fig. 3.
ns: not significant, * p < 0.05, ** p < 0.01, *** p < 10−3, **** p < 10−4. Mann-Whitney U test, two sided. Data are presented as mean values ± SD. a) LC-MS/MS of mouse feces showing queuine and preQ1 metabolites and queuosine, and preQ1sine nucleosides. The Y axis represents the concentrations of preQ1, queuine, queuosine and preQ1sine. Mean values on top of graph. n = 2 biological replicates. b) Quantitation of Q-modification levels from Northern blot results of preQ1 injected liver and kidney tRNAHis and tRNAAsn. n = 4 biological replicates. p values from left to right: 0.0152, 0.0152, 0.0152, 0.0152. c) Proliferation measurement by absorbance at 460 nm, B16 cells. Arrow indicates the time of addition of preQ1 and/or queuine. Error bar corresponds to the range of n = 8 biological replicates. Source numerical data are available in.
Extended Data Fig. 4 Additional data for Figs. 4 and 5.
a) tRNA-seq replicate data for HEK293T cells related to Fig. 4a–d. Pearson’s values for comparing the fraction tRNA reads for the biological replicates under different preQ1 and queuine treatments. b) MEF cells, Northern blots for Q-modifiable tRNAs: tRNATyr, tRNAAsp, tRNAHis, and tRNAAsn. 5S rRNA is the loading control. Lines indicated preQ1 and queuine treatments are replicates. c) Heatmap of expression of individual tRNA anticodon families, cognate tRNATyr/His/Asn/Asp are indicated by arrows. All tRNA normalized to 0Q samples without preQ1 treatment. mt: mitochondrial tRNA. d) mRNA-seq replicate data for HEK293T cells related to Fig. 4g–k. Pearson’s values for replicates of input and polysome samples. e) PreQ1 versus 0Q input mRNA (left) and polysome mRNA (right). Red, preQ1/0Q > 2. Blue, 0Q/preQ1 > 2. The 117 ribosomal protein genes (named RPL/RPS) are highlighted in brown. f) Gene ontology (GO) analysis for biological process and molecular function of genes with significant change in TE. Blue: TE < 0.2 in preQ1/0Q. Red: TE > 5 in preQ1/0Q. g) mRNA expression heatmap of 98 “blue” genes, normalized to 0Q input sample. h) mRNA expression heatmap of 154 “red” genes, normalized to 0Q input sample. i) Comparing codon usage of the 4 amino acids decoded by Q-modifiable tRNAs affected by preQ1 treatment. X-axis represents the codon usage (CU) of the blue transcripts in Fig. 4h, and y-axis represents the codon usage of the red transcripts in Fig. 4h. C-ending codons are in blue, U-ending codons are in green. j) Codon usage of genes in Fig. 4h. Blue: TE < 0.2 in preQ1/0Q. Red: TE > 5 in preQ1/0Q. Grey: all other genes. Unprocessed blots are available in source data.
Extended Data Fig. 5 Additional data for Fig. 6.
Panels (a–e, g–i) are from HEK293T cells. Lanes shown with lines are biological replicates. ns: not significant, * p < 0.05, ** p < 0.01, *** p < 10−3. Tukey’s Honestly Significant Difference test (HSD), two-sided. Data are presented as mean values ± SD. a) Q-modifiable tRNA levels by Northern blot with and without emetine inhibition of translation. b) Q-modifiable tRNA levels and preQ1-modification by Northern blot using NHS treatment method with and without cycloheximide inhibition of translation. c) SUnSET translation activity assay with puromycin antibody under mock, emetine, or cycloheximide conditions with and without preQ1 treatment. Left: Western blot using anti-puromycin and β-actin antibodies (top) and the same blot stained with Coomassie blue (bottom). Right: quantification of Western blots in left panel. β-actin is the loading control. n = 3 biological replicates. p values from left to right: 1.31e-8, 9.78e-9, 0.833, 5.45e-9, 4.21e-9. d) GO term of genes enriched in the polysome (red mRNA transcripts in Fig. 4h) with preQ1. e) Northern blot using NHS reaction method to detect preQ1-tRNA using preQ1 treated cells with and without 4µ8C, the inhibitor of IRE1 ribonuclease activity for different times as indicated. Total RNA without preQ1 and 4µ8C treatment was used as the no NHS reaction control. f) Conservation of IRE1 protein from Pfam. g) Western blot (left) and quantitation (right) of IRE1 phosphorylation under queuine/preQ1 and TG treatment. n = 3 biological replicates. Fisher’s Least Significant Difference (LSD) test, two-sided. p values from left to right: 4.75e-3, 0.516, 1.28e-3. h) Blue-Native gel followed by Western blot (left) and quantitation (right) of IRE1 oligomerization under queuine, preQ1, or TG treatment. n = 3 biological replicates. p values from left to right: 0.238, 0.891, 0.955. i) Western blot (top) and quantitation (bottom) of eIF2α phosphorylation with the indicated queuine, preQ1, and positive control thapsigargin (TG) treatments. n = 3 biological replicates. p values from left to right: 0.0862, 0.446, 7.72e-3, 1.77e-6. j) RT-PCR followed by PAGE gel electrophoresis (left) and quantitation (right) of XBP1 pre-mRNA splicing with the indicated queuine, preQ1, and positive control thapsigargin (TG) treatments. n = 3 biological replicates. p values from left to right: 0.0684, 0.946, 2.01e-12, 1.77e-6, 0.691, 3.23e-3, 2.04e-13. Source numerical data and unprocessed blots are available in.
Extended Data Fig. 6 Additional data for Figs. 7 and 8.
Panels (a–e) are from HEK293T cells. Lanes under the each indicated condition are replicates. a) Distribution of ZAKα across polysome fractions (fractions starting from monosome) related to Fig. 6a. ZAKα abundance in all fractions was normalized to the ZAKα abundance in the first 80S monosome fraction, the first underlined fraction in Fig. 6a. Polysome fractions were numbered from 1 for the first 80S monosome fraction to higher polysome fractions. b) Sucrose gradient polysome profiles without RNase digestion of HEK293T cells under intermediate concentration of emetine (1 µg/ml) treatment. Western blot of ZAKα and ZNF598 distribution in polysome fractions under indicated conditions. ZNF598 in higher polysome fractions are indicated by lines. c) Distribution of ZAKα across polysome fractions (fractions starting from monosome) related to panel b. ZAKα abundance in all fractions was normalized to the ZAKα abundance in the first 80S monosome fraction, the first underlined fraction in panel b. Polysome fractions were numbered from 1 for the first 80S monosome fraction to higher polysome fractions. d) Translation efficiency (TE) of preQ1 treated and control samples, 0Q versus preQ1 from ribo-seq. Highlighted are transcripts whose TE differs by >5-fold between mock and preQ1-treated cells. e) Difference in codon usage between genes with higher TE in preQ1 over 0Q (red transcripts in panel d) and genes with higher TE in 0Q over preQ1 (blue transcripts in panel d). Codon ends with A (red), C (blue), G (orange), U (green) indicated by colors. f) GO term of genes enriched in the ribosome (red mRNA transcripts in panel d) with preQ1. Gene Ontology enrichment analysis was performed using the GeneOntology.org tool with Fisher’s exact test (one-sided). Multiple comparisons were adjusted using the False Discovery Rate (FDR) method. Source numerical data and unprocessed blots are available in.
Supplementary information
Supplementary Tables
Mass spectrometer parameters, MRM transitions and Northern blot probes.
Source data
Source Data all figures
Statistical source data.
Source Data all figures
Unprocessed western blots and/or gels.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, W., Lahry, K., Cipurko, D. et al. Two microbiome metabolites compete for tRNA modification to impact mammalian cell proliferation and translation quality control. Nat Cell Biol (2025). https://doi.org/10.1038/s41556-025-01750-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41556-025-01750-6