Abstract
Gastrointestinal colonization by the nosocomial pathogen vancomycin-resistant Enterococcus faecium (VREfm) can lead to bloodstream infections with high mortality rates. Shifts in VREfm lineages found within healthcare settings occur, but reasons underlying these changes are not understood. Here we sequenced 710 VREfm clinical isolates collected between 2017 and 2022 from a large tertiary care centre. Genomic analyses revealed a polyclonal VREfm population, although 46% of isolates formed genetically related clusters, suggesting a high transmission rate. Comparing these data to a global collection of 15,631 publicly available VREfm genomes collected between 2002 and 2022 identified replacement of the sequence type (ST) 17 VREfm lineage by emergent ST80 and ST117 lineages at the local and global level. Comparative genomic and functional analyses revealed that emergent lineages encoded bacteriocin T8, which conferred a competitive advantage over bacteriocin T8-negative strains in vitro and upon colonization of the mouse gut. Bacteriocin T8 carriage was also strongly associated with strain emergence in the global genome collection. These data suggest that bacteriocin T8-mediated competition may have contributed to VREfm lineage replacement.
Similar content being viewed by others
Main
Enterococcus faecium is a gastrointestinal tract commensal that can also cause serious infections, most commonly bloodstream and urinary tract infections, especially in immunocompromised and hospitalized patients1. Hospitalized patients are often exposed to high levels of antibiotics, which decrease the diversity of commensals in the gastrointestinal (GI) tract and facilitate the overgrowth of multidrug-resistant organisms such as vancomycin-resistant E. faecium (VREfm)2,3,4. VREfm overgrowth within the intestinal tract predisposes patients to invasive bloodstream infections2,4,5,6,7. Furthermore, increased VREfm GI tract burdens cause patients to shed VREfm into the environment, facilitating transmission to other patients mainly through the faecal–oral route8,9,10.
Whole-genome sequencing facilitates the surveillance and characterization of VREfm population structure and transmission dynamics within healthcare settings. Multilocus sequence typing allows tracking of VREfm lineages both within and between healthcare facilities and on both local and global scales11,12,13. Sequence types (STs) with similar genotypes, defined as having four or more identical loci, can be grouped into clonal complexes. VREfm lineages most often belong to clonal complex 17 (CC17), which phylogenetically resides within hospital-associated E. faecium clade A1. CC17 strains frequently encode antimicrobial resistance genes, mobile genetic elements and genes that enable the metabolism of amino sugars found on GI epithelia and mucin, probably contributing to the success of CC17 strains in healthcare settings12,13,14,15,16,17,18. This success is exemplified by CC17 lineages being identified as responsible for widespread outbreaks and increased rates of invasive infection14,15,17,19. Although several previous studies have investigated VREfm population structure and dynamics within healthcare settings, we know little about the factors that drive the emergence and persistence of particular VREfm lineages in the hospital.
In this study, we characterized the population structure and dynamics of VREfm within a single hospital using whole-genome sequencing-based surveillance and functional characterization of genes associated with nosocomial emergence. We systematically collected 710 VREfm clinical isolates over a 6-year period and used both genomic analysis and phenotypic testing to investigate factors contributing to population shifts observed within the facility. In addition, we compared local findings with a global collection of 15,631 publicly available VREfm genomes isolated from human sources from 2002 to 2022. We found that a bacteriocin produced by emergent VREfm lineages provided a strong competitive advantage, highlighting an adaptive mechanism that probably contributes to lineage replacement of VREfm on both local and global scales.
Results
Population structure of VREfm at a single hospital
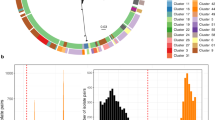
Between 2017 and 2022, the Enhanced Detection System for Hospital-Associated Transmission (EDS-HAT) whole-genome sequencing surveillance programme collected 710 healthcare-associated VREfm isolates, that is, isolates collected from patients with hospital stays >2 days or before 30-day healthcare exposures at the University of Pittsburgh Medical Center (UPMC). The most common isolate sources were urine (42%), blood (24%) and wound sites (19%) (Supplementary Dataset 1). We investigated the genomic diversity of this collection through multilocus sequence typing, which identified 42 different STs. All isolates belonged to hospital-adapted lineages within CC17, including ST17 (23%), ST117 (13%), ST1471 (11%) and ST80 (10%) (Fig. 1a and Supplementary Dataset 1). To characterize the population structure of our collection, a core-genome phylogenetic tree was constructed based on 1,604 core genes (Extended Data Fig. 1a). Despite being entirely composed of CC17 strains, the VREfm population showed variable genetic diversity within and between STs and showed evidence that some isolates were closely related to one another. To assess genetic relatedness among the collected isolates, we performed Split Kmer Analysis to cluster isolates that had fewer than 10 single nucleotide polymorphisms (SNPs) in pairwise genome-wide comparisons. This analysis revealed 112 putative transmission clusters that contained 2–9 isolates each and encompassed 46% of the collection (Fig. 1a). Despite a high degree of clustering among all isolates, the proportion of isolates residing in putative transmission clusters was variable between STs. Although ST17 was the most prevalent lineage, it had the lowest percentage of clustered isolates (33%, 54 of 165 isolates, P = 0.0008). On the other hand, ST1478 showed a significantly higher percentage of clustered isolates (69%, 38 of 55 isolates, P = 0.0002) (Fig. 1b).
a, Cluster network diagram of 710 sequenced VREfm genomes constructed using Gephi v0.10. Isolates are grouped and coloured by multilocus ST. Isolates that fall within putative transmission clusters (≤10 SNPs) are connected with grey lines. b, Prevalence of cluster isolates within different STs. Asterisks mark STs that show a higher or lower percentage of clustered isolates compared with the total collection (one-sided, single-proportion hypothesis test). P values were adjusted for multiple comparisons (*P < 0.004): ST1478, P = 0.0002; ST17, P = 0.0008. c, Biannual distribution of STs over the study period. Q1–Q2 = January–June; Q3–Q4 = July–December. The number of samples within each sample period is also noted. Emergent lineages are noted with a black star.
VREfm lineage replacement
We next investigated how the VREfm population changed over time within our centre by characterizing the ST distribution over the collection period in 6-month intervals (Fig. 1c). Before 2020, ST17 was the most frequently sampled ST, making up 34% of the collection between 2017 and 2019. However, during 2020, the emergence of ST1478 (23%) coincided with the decline of ST17 (17%). For the remainder of the collection period, the presence of ST17 continued to decline, and this lineage was not detected during the second half of 2022 (Fig. 1c). By contrast, lineages ST80 and ST117 were not detected in 2017, but together rose to 81% by the end of 2022, effectively replacing ST17 and other lineages that were previously detected. We therefore designated ST80, ST117 and ST1478 as emergent lineages at our centre.
To identify factors contributing to lineage replacement, we investigated the frequency of non-susceptibility to the clinically relevant, last-line antibiotics linezolid and daptomycin (Extended Data Fig. 1a and Supplementary Dataset 1). The emergent lineages (ST80, ST117, ST1478) did not show a higher frequency of non-susceptible isolates, with non-susceptibility rates of 0–5% (linezolid) and 6–20% (daptomycin). We then investigated the distribution of genomic features such as genome length, antimicrobial resistance genes (ARGs), virulence factors and plasmid replicons among the different lineages (Extended Data Fig. 1b–e). We observed variation in the number of ARGs and virulence genes within the emergent lineages, with ST117 (mean = 15.6 ARGs) and ST1478 (mean = 16.0 ARGs) having more ARGs compared with ST17 (mean = 14.1, P < 0.0001) (Extended Data Fig. 1c). The macrolide efflux transporter mefH was found in nearly all ST117 and ST1478 isolates and was identified only in 4 other isolates (Supplementary Dataset 2). Similarly, the aminoglycoside nucleotidyltransferase ant(6)-Ia was highly enriched in these two lineages, being present in 99% and 93% of ST117 and ST1478 isolates, compared with 52% of other isolates. ST117 also had more virulence genes (mean = 4.0) compared with ST17 (mean = 3.7, P < 0.0001) (Extended Data Fig. 1d). Virulence genes enriched (>98%) in ST117 genomes included the colonization factors acm, fss3, ecbA and sgrA (Supplementary Dataset 2). ST1478 and ST117 genomes also encoded more plasmid replicons compared with the historical lineage ST17 (P < 0.0001) (Extended Data Fig. 1e). We further investigated the distribution of replicons among lineages and found that the rep11a replicon was present at higher frequencies in the emergent lineages ST80 (64%), ST117 (75%) and ST1478 (95%), versus only 15% of other isolates (Supplementary Dataset 2). Similarly, the repUS15_2 family replicon was enriched in ST117 (95%) and ST1478 (98%) but was seen at a lower prevalence in the remaining isolates (28%), including ST80 (10%) (Supplementary Dataset 2). Together, these data suggest that emergent lineages possess genomic features that might facilitate their emergence within the hospital.
Emergent isolates with bacteriocin T8 inhibit growth
To determine other factors contributing to lineage replacement, we investigated whether emergent lineage VREfm isolates inhibited the in vitro growth of historical lineage isolates. We performed a pairwise spot killing assay using the earliest available isolates from the historical lineage ST17 and the emergent lineage ST117. We found that the ST117 isolate was able to inhibit growth of the historical ST17 isolate, causing a large zone of inhibition in the ST17 isolate lawn surrounding the ST117 isolate spot (Fig. 2a). We then conducted pairwise spot killing assays using isolates from each of the 11 lineages having ≥10 isolates in the dataset (Fig. 2b and Supplementary Dataset 3). We found that isolates from all three emergent lineages caused growth inhibition of isolates from other lineages. Interestingly, we also observed low-level inhibition when emergent strains were spotted onto lawns of other emergent strains, possibly due to a high abundance of an antibacterial factor produced by the spot strain. Bacteriocins are antimicrobial peptides that have been widely studied in Enterococcus due to their ability to inhibit the growth of other bacteria and their potential role as probiotics20,21,22,23. We screened the genomes in the dataset for predicted bacteriocins and found one bacteriocin, called T8, that was differentially present and found in 36% of isolates (Extended Data Fig. 2a and Supplementary Dataset 2). Bacteriocin T8 is identical to two other enterococcal bacteriocins, named hiracin JM79 (ref. 21) and bacteriocin 43 (ref. 22), and all three names have been used in previous studies to describe what is now known to be the same bacteriocin. We chose to refer to this bacteriocin as T8 because this is how it was initially and most frequently described in the literature. To assess the association between bacteriocin T8 with growth inhibition, we screened 28 VREfm isolates representing 11 STs against the same ST17 reference isolate to assess growth inhibition and found that bacteriocin T8 presence was strongly associated with growth inhibition (P < 0.0001) (Fig. 2c and Supplementary Dataset 4). A single isolate, called VRE36503, lacked bacteriocin T8 but still showed growth inhibition of the ST17 lawn. While no predicted bacteriocins were identified in the VRE36503 genome using the BAGEL4 prediction tool, an additional search for secondary metabolites identified a gene cluster with homology to the carnobacteriocin XY biosynthetic gene cluster24, suggesting that growth inhibition by VRE36503 might be independent of bacteriocin T8.
a, Pairwise spot killing assay with representative ST17 (historical lineage) and ST117 (emergent lineage) isolates. The dashed circle shows where the ST17 isolate was spotted onto the ST117 lawn but did not grow. b, Pairwise spot killing assay with reference isolates from each of the 11 main lineages within the UPMC collection. The inhibition zones (mm) are shaded from highest inhibition (burgundy) to no inhibition (grey). Inhibition zone values were averaged from three biological replicates. The midpoint-rooted phylogenetic tree was constructed using RAxML with 100 bootstraps based on a single-copy core genome alignment produced by Roary. The scale bar represents nucleotide substitutions per site. c, Spot killing assay results of 28 VREfm isolates spotted onto a lawn, once per isolate, of the bacteriocin T8-negative, ST17 VRE32530 representative isolate. Isolates are grouped by presence (burgundy) or absence (grey) of bacteriocin T8 in their genomes. The P value indicates significance from a two-tailed Mann–Whitney test (unadjusted, P ≤ 0.0001). d, Abundance of bacteriocin T8 within main STs at UPMC. Emergent lineages are noted with a black star.
To investigate whether bacteriocin T8 was encoded by a plasmid, we performed long-read sequencing and hybrid genome assembly on a bacteriocin T8-positive ST117 isolate. We found that bacteriocin T8 and the corresponding immunity factor were carried on a 6,173-bp rep11a-family plasmid that also encoded mobilization genes mobABC, allowing plasmid transfer, and was similar to plasmids previously described20,22 (Extended Data Fig. 2b). Of all bacteriocin T8-positive isolates (n = 253), rep11a was found in 236 isolates (93%) based on short-read assembly data, and 211 of these isolates encoded both bacteriocin T8 and rep11a on the same contig. We also characterized the distribution of bacteriocin T8 among the main lineages in the dataset, and identified a high prevalence among the emergent lineages ST80 (70%), ST117 (74%) and ST1478 (96%) (Fig. 2d). Bacteriocin T8 was found only in 16% of the remaining isolates in the collection, most of which belonged to ST17 (25%). Due to the enrichment of bacteriocin T8 in emergent lineage genomes, we hypothesized that it might provide a competitive advantage to VREfm during colonization and infection of hospitalized patients.
Bacteriocin T8 expression provides a competitive advantage
We confirmed that bacteriocin T8 caused growth inhibition by transforming a bacteriocin T8-negative clinical E. faecium isolate belonging to ST203 and called DVT705 with pBAC (plasmid containing bacteriocin T8 and immunity factor) or pEV (empty vector). To test whether pBAC conferred growth inhibition, we performed a pairwise spot killing assay and found that the pBAC strain caused a large zone of inhibition on a lawn of the pEV strain (Fig. 3a). Next, we quantified the competitive advantage of the pBAC strain by performing a liquid competition assay. We independently competed the pBAC and pEV strains against the parent strain at 50:50 and 10:90 starting ratios and quantified the abundance of each strain in the mixture after 24 h and 48 h. At both ratios and timepoints, the pBAC strain was able to outcompete the parent strain to a much greater extent compared with the pEV strain (P < 0.01) (Fig. 3b and Supplementary Dataset 5). We then evaluated whether the competitive advantage conferred by bacteriocin T8 in vitro translated to the mammalian gut. To assess this, we pretreated C57BL/6 mice with vancomycin to deplete their endogenous Enterococcus population before orally gavaging mice with the pBAC or pEV strains for 2 days. We monitored the abundance of each strain in stool for 8 days following the initiation of infection (Fig. 3c and Supplementary Dataset 6A). On day 1, there was no difference in GI burden between the two groups, indicating that mice received similar inocula of each strain. However, at all subsequent timepoints, the pBAC strain was detected at a significantly higher abundance compared with the pEV strain (P < 0.05) (Fig. 3c). Next, to directly evaluate whether a bacteriocin T8-encoding emergent lineage strain could outcompete a historical lineage strain in the gut, we performed a competition experiment with a bacteriocin T8-positive ST117 strain and a bacteriocin T8-negative ST17 strain. To model strain emergence, mice were co-inoculated with ST117 and ST17 strains at a 10:90 ratio, with the emergent ST117 strain at an initial disadvantage (Fig. 3d and Supplementary Dataset 6B). Using culture-enriched metagenomic sequencing, we observed that mice were initially colonized on day 0 with 10.26% ST117 strain and 89.74% ST17 strain. However, at 4 days postinfection, the ST117 strain was detected at an average of 90% frequency, with half of the animals having only the bacteriocin T8-positive ST117 strain detected (P < 0.0001). These findings further suggest that bacteriocin T8 provides a competitive advantage to VREfm in the mammalian GI tract.
a, Pairwise spot killing assay of strains pBAC, carrying bacteriocin T8 and the corresponding immunity gene, and pEV, carrying an empty vector. The dashed circle shows where the pEV strain was spotted onto the pBAC strain lawn but did not grow. b, Liquid competition assay. The pBAC and pEV strains were independently competed against the parent strain at 50:50 and 10:90 ratios. Samples were taken after 24 h and 48 h to calculate CFU ml−1. Assays were performed in three biological replicates each consisting of three technical replicates. Instances in which the parent strain fell below the limit of detection (LOD, shown with the grey dashed line) were not included in statistical analyses. Competitive indexes were summarized using the geometric mean and a 95% confidence interval (error bars) for each timepoint and ratio70. The distribution of competitive index values at each timepoint and ratio was compared between pBAC and pEV strains using a two-tailed Mann–Whitney test (adjusted, *P < 0.005, **P < 0.001); 50:50 24 h (P = 0.0028), 50:50 48 h (P = 0.0002), 10:90 24 h (P = 0.0008) and 10:90 48 h (P = 0.0004). Black dashed line corresponds to a competitive index of 1.0. c, Colonization of pBAC and pEV strains in the mouse gut. Mice were orally gavaged with either the pBAC (n = 10) or pEV (n = 10) strain for 2 days. Stool samples were collected starting on day 1 after the initiation of infection to quantify CFU g−1 of each strain over time. Data were summarized using the mean with error bars representing standard error measurement. A two-tailed multiple Mann–Whitney test was used and assessed CFU g−1 distribution between pBAC and pEV strains (adjusted, *P < 0.05; NS, non-significant); day 1 (P = 0.6839), day 2 (P = 0.0230), day 3 (P = 0.0084), day 4 (P = 0.0026), day 5 (P = 0.0120), day 6 (P = 0.0410), day 7 (P = 0.0001) and day 8 (P = 0.0061). d, Competition between a bacteriocin T8-positive ST117 strain and a bacteriocin T8-negative ST17 strain within the mouse gut. Data show the percentages of ST117 in the inoculum on day 0 and at 4 days postinfection. Proportions of ST117 on day 4 and in the inoculum for each animal were compared using a one-sided difference in proportion hypothesis test (adjusted, ***P ≤ 0.0001) for each mouse.
Global VREfm lineage replacement linked with bacteriocin T8
We next sought to determine whether the lineage replacement we observed at our local centre was reflective of global VREfm population dynamics. To investigate this question, we gathered 15,631 publicly available VREfm genomes collected from human sources between 2002 and 2022 (Supplementary Dataset 7). This collection consisted of genomes from 53 countries; however, the majority of isolates were from the United States (23%), Denmark (23%) and Australia (20%) (Extended Data Fig. 3 and Supplementary Dataset 7). To investigate VREfm global genomic diversity, we performed sequence typing on this collection and examined the distributions of STs by continent (Fig. 4a). Europe and Asia had relatively clonal populations, while the populations in North America and Australia were more diverse. ST80 was the single most prevalent ST (20%) and was mainly found in Europe (30%) and Asia (36%). ST117 was the second most prevalent ST (12%) and had the highest prevalence in Europe (18%) and North America (12%). ST17 accounted for 7% of the global population and was sampled predominantly in North America (18%). To characterize global population dynamics of VREfm, we investigated the prevalence of STs over the 20-year global collection period (Fig. 4b and Supplementary Dataset 7). Before 2010, ST17 and ST18 were the most prevalent lineages, while ST80 and ST117 were detected very infrequently. After 2010, however, ST117 and ST80 rose to 60% by the end of 2022, effectively replacing ST17 (3%) and ST18 (0.2%). These data suggest that the emergence of ST80 and ST117 that we observed locally was reflective of global trends.
a, Geographic distribution of 15,631 global VREfm isolates across continents. EU, Europe; NA, North America; AUS, Australia; AS, Asia; SA, South America; AF, Africa. Isolates are coloured by ST. b, Global ST distribution between 2002 and 2022. Isolates are coloured by ST. Emergent lineages are noted with a black star. c, Abundance of bacteriocin T8 within main global STs. The bars are separated into emergent and historical lineages based on trends seen within the UPMC collection. Bacteriocin T8 presence is shaded in burgundy and absence in grey. d, Prevalence of bacteriocin T8 over time at UPMC (burgundy) and in the global collection (light burgundy).
To investigate whether bacteriocin T8 was similarly enriched in emergent lineages, we examined the distribution of bacteriocin T8 among the STs sampled in the global collection of VREfm isolated from human sources (Fig. 4c). Bacteriocin T8 was enriched in emergent lineages ST80, ST117 and ST1478 globally, with more than 79% of isolates in each ST predicted to encode the bacteriocin. Similar to our local prevalence (25%), bacteriocin T8 was found in only 30% of isolates in the previously dominant lineage ST17. We also investigated whether bacteriocin T8 was increasing over time within both collections (Fig. 4d). Within our local collection, bacteriocin T8 presence rose from 8% in 2017 to 62% in 2022. Similarly, we observed a 67% increase in the prevalence of bacteriocin T8 between 2002 and 2022 in the global collection. Within both collections, the increase in bacteriocin T8 prevalence was associated with the replacement of the historical ST17 lineage with emergent lineages ST80 and ST117. Taken together, these findings suggest that bacteriocin T8 may be a driving feature of global VREfm strain emergence and persistence in healthcare settings.
Discussion
In this study, we examined the population structure and dynamics of 710 VREfm clinical isolates collected over 6 years from a single hospital. A strength of our study lies in the use of a systematic collection of hospital-associated VREfm isolates collected over a multi-year period. In addition, by comparing our findings to a large global collection of over 15,000 VREfm isolates from human sources, we confirmed that many of our findings were generalizable to other settings worldwide. Our data show the emergence of ST80 and ST117 both locally and globally, highlighting the strong competitive advantage of these lineages and identifying bacteriocin T8 as a likely contributor to VREfm lineage replacement.
Similar to other studies, we found that the VREfm population at our hospital was polyclonal, with the majority (57%) of isolates belonging to 4 prevalent lineages: ST17, ST117, ST80 and ST1471 (refs. 12,13,25,26). These lineages belong to the hospital-associated clade A1 of E. faecium and also belong to CC17, which is known to be highly epidemic within healthcare systems and the cause of widespread outbreaks13,14,15,17,27. While previous studies have reported nosocomial VREfm transmission rates ranging from 60% to 80% (refs. 17,28,29,30), we found that only 46% of isolates in our dataset belonged to putative transmission clusters. This difference is probably due to our use of a 10-SNP cut-off for clustering and not including sampling of VREfm from GI tract colonization, which might limit our ability to detect transmission31. We did, however, note differences in the percentage of isolates residing within putative transmission clusters among different lineages, with lineage ST1478 showing a significantly higher proportion of clustered isolates. Previous literature has found that the ST117 lineage was responsible for numerous VREfm hospital outbreaks12,14,32, and that the ST1478 lineage has previously been detected in the United States, Canada and Europe13,19,33. Taken together, these findings suggest that some VREfm lineages might be more efficient than others at transmitting between patients in the hospital.
Through phenotypic screening and comparative genomic analysis, we found that the antimicrobial peptide bacteriocin T8 was enriched in emergent lineages both locally and globally, and that it conferred a growth advantage to E. faecium both in vitro and in vivo. The enrichment of bacteriocin T8 in emergent lineages and increasing prevalence over time suggest that acquisition of this bacteriocin is highly advantageous. Bacteriocins have been shown to facilitate expansion of bacterial populations by killing susceptible bacteria, thereby carving out a stable environment for the expansion of bacteriocin-expressing bacteria34,35,36,37,38,39. In polymicrobial settings that experience strong selection and associated population bottlenecks, such as within the GI tract of hospitalized patients, strains must compete with one another to dominate and persist in the population. A previous study investigated the competitive advantage conferred by bacteriocin-21 to Enterococcus faecalis in the mammalian GI tract34. Similar to our findings, bacteriocin production in that study was associated with increased GI tract colonization and conferred a competitive advantage in the mouse gut. Furthermore, the study found that the production of bacteriocin-21 was able to clear a vancomycin-resistant E. faecalis strain from the GI tract34. Due to the strong inhibitory activity of bacteriocins, they are an attractive avenue for development as new antimicrobial interventions, such as inclusion in probiotics and food preservation36,40. A recent study showed that a genetically engineered probiotic Escherichia coli strain containing three bacteriocins, including bacteriocin T8 (referred to as hiracin JM79), was able to clear vancomycin-resistant E. faecium and E. faecalis in a mouse model of enterococcal colonization36. Although this result is exciting, it is somewhat troubling that based on our global findings the vast majority of VREfm isolates sequenced in 2022 already encoded bacteriocin T8 and the associated immunity gene, suggesting that they would be resistant to bacteriocin T8 activity.
Bacteriocin T8 is a class IIa, secretion-dependent, heat-stable bacteriocin20. It was isolated in 2006 from an E. faecium strain called T8 that was sampled from patients with human immunodeficiency virus20. E. faecium appears to be the natural host for bacteriocin T8, although a single report documented a bacteriocin T8-encoding Enterococcus hirae strain isolated from mallard ducks21. Bacteriocin T8 causes bacterial cell death by irreversibly binding to the mannose phosphotransferase system resulting in pore formation41,42. Inhibitory activity has been reported against Enterococcus spp., Listeria spp. and Lactobacillus spp., and even some Staphylococcus aureus and Clostridium spp. strains20,21,22. Resistance to class IIa bacteriocins appears to occur largely through decreased expression of the target mannose phosphotransferase system43,44. Bacteriocins are typically found on mobile genetic elements and are transmitted to compatible strains through horizontal gene transfer45. The bacteriocin T8-encoding plasmid that we characterized in this study is highly similar to plasmids described in the early 2000s, suggesting widespread conservation and propagation of this mobile element20,22.
Our study had several limitations. Within our local collection, we investigated only VREfm isolates collected from clinical infections that were suspected to be healthcare- associated. Isolates not meeting inclusion criteria, including potentially community-associated VREfm, were not included. It is very likely that we undersampled the full VREfm population diversity within our centre because many hospitalized patients have asymptomatic GI tract colonization31. The global collection that we analysed was also biased towards countries with high rates of VREfm infection and with the infrastructure and capacity to perform next-generation sequencing, which resulted in some continents, such as South America and Africa, to be greatly undersampled. Additionally, we focused on bacteriocin T8 as a contributor to lineage success; however, this may not be the only factor driving the lineage replacement that we observed. We did not investigate other potential adaptations among emergent lineages, such as virulence factors that were enriched in the ST117 lineage. Furthermore, it is important to note that E. faecium virulence factors in general are poorly characterized, limiting their identification across our collection. Moreover, additional uncharacterized mutations within the emergent lineages could contribute to antimicrobial resistance or tolerance, potentially aiding in lineage replacement. In addition, in the mouse model, we focused on the impact of bacteriocin production on enterococci, potentially overlooking other microbiome disruptions that could be explored through additional metagenomic sequencing.
In summary, we characterized the local and global population structure and temporal dynamics of VREfm using comparative genomics and functional analyses. By investigating VREfm populations sampled over 6 years at our healthcare centre and over 20 years globally, we identified lineage replacement associated with the spread of strains encoding bacteriocin T8. Phenotypic characterization showed that bacteriocin T8 probably contributes to VREfm lineage replacement by conferring a strong competitive advantage that is observed both in vitro and in vivo. Although we identified bacteriocin T8 production as a potential adaptive mechanism directing VREfm lineage replacement, this study prompts further investigation into other features driving the evolutionary dynamics in this important and difficult-to-treat pathogen.
Methods
Study setting
The study was approved by the Institutional Review Board at the University of Pittsburgh (STUDY21040126). This was a retrospective observational study of VREfm collected from patients at UPMC by the EDS-HAT46. UPMC is an adult tertiary care hospital with 699 beds (including 134 critical care beds) and performs >400 solid organ transplants each year. A total of 710 VREfm clinical isolates were collected from patients with a hospital admission date ≥2 days previously or with a recent healthcare exposure within 30 days before the culture date, from January 2017 to December 2022. Available daptomycin and linezolid minimal inhibitory concentration data were collected from patient records and interpreted using Clinical and Laboratory Standards Institute M100 guidelines.
Whole-genome sequencing and bioinformatic analyses
Genomic DNA was extracted using a DNeasy Blood and Tissue Kit (Qiagen) from VREfm isolates that were grown overnight at 37 °C on blood agar plates. Following DNA extraction, next-generation sequencing libraries were generated using the Illumina DNA Prep protocol and then sequenced (2 × 150 bp, paired end) on NextSeq500, NextSeq2000 or MiSeq. The resulting reads were assembled using SPAdes v3.15.5 (ref. 47). Assembly quality was determined using QUAST v5.2.0 (ref. 48). Assemblies passed quality control if the coverage was >35× and the assembly had <350 contigs. Species were identified and possible contamination was detected using Kraken2 (v2.0.8-β)49. Multilocus STs were identified using the PubMLST database with mlst v2.11 (refs. 50,51). Isolates with undefined STs were uploaded to the PubMLST server, and if their ST was a single locus variant of a known ST, they were grouped with the latter. Clusters of genetically related isolates were identified using Split Kmer Analysis v1.0 (ref. 52) with average linkage clustering and a 10-SNP cut-off, similar to previously used thresholds27,53,54. Genomes were annotated using PROKKA v1.14.5 (ref. 55). The bacteriocin T8-encoding plasmid was annotated with Bakta v1.9.2 (ref. 56). A cluster network diagram was visualized using Gephi v0.10 with the Fruchterman–Reingold layout57. Phylogenetic trees were built using core genome alignments produced by Roary v3.13.0 (ref. 58). Gaps in the core genome alignment were masked using Geneious (Geneious Biologics 2024, https://www.geneious.com/biopharma). Trees were constructed using RAxML HPC v8.2.12 with 100 bootstraps59. In the UPMC collection, bacteriocins were identified using BAGEL4 with ≥95% coverage and identity60. antiSMASH v7.1.0 was used to further identify secondary metabolites in the VRE36503 genome61. To identify bacteriocin T8 in the global genome collection, a custom database consisting of the nucleotide sequence for bacteriocin T8 and the corresponding immunity factor was built using ABRicate v1.01, and gene presence was defined as hits with ≥95% coverage and identity62. Antimicrobial resistance genes were identified with AMRFinderPlus v3.12.8 (ref. 63). The presence of plasmid replicons and virulence factors was determined using ABRicate v1.0.01 (ref. 62) with the PlasmidFinder64 and VFDB65 databases, respectively. Gene presence was defined as hits with ≥90% coverage and identity. The bacteriocin T8-encoding plasmid was resolved by using Unicycler v0.5.0 (ref. 66) to hybrid assemble Illumina and MinION sequencing data collected from isolate VRE38098. A MinION device with R9.4.1 flow cells (Oxford Nanopore Technologies) was used, with base calling performed by Dorado v 0.7.2 (ref. 67).
Spot killing assay
Test strains were cultivated for 16–18 h at 37 °C in brain heart infusion (BHI) broth. Subsequently, 5 ml of a BHI top agar lawn (containing 0.35% agar) was prepared by mixing the molten agar with 100 μl of a 1:100 dilution of the overnight culture. This mixture was poured on top of 25 ml of solid BHI agar. Competing strains were spotted (5 μl undiluted overnight culture) onto solidified top agar lawns. Inhibition zones were measured (in mm) after 16–18 h of incubation at 37 °C.
Cloning and expression of bacteriocin T8
Bacteriocin T8 and the corresponding immunity factor were cloned into the expression vector pMSP3535, which was modified to encode the chloramphenicol resistance gene cat as a positive selectable maker68. The insert sequence was amplified from VRE38098 genomic DNA by PCR using primers 5′-AGA CCG GCC TCG AGT CTA GAA TGG GAC TGA TGA ATC AGA ATTG-3′ and 5′-GCG AGC TCG TCG ACA GCG CTC AGG CGT TAC TTG GTA GTA TAC-3′. The vector was amplified by PCR using primers 5′-AGC GCT GTC GAC GAG CTC GCAT-3′ and 5′-TCT AGA CTC GAG GCC GGT CTCC-3′. The amplified insert and vector were purified using a PCR Purification Kit (Qiagen), and Gibson assembly was conducted using a HiFi DNA Assembly Cloning Kit (New England Biolabs)69. The Gibson product was then transformed into NEB 5-alpha-competent E. coli, and transformants were selected on BHI agar containing 10 µg ml−1 chloramphenicol. Plasmids were amplified in 200-ml cultures, collected by Maxiprep and sequenced to confirm their identity. The bacteriocin T8-encoding vector (pBAC) or pMSP3535 empty vector (pEV) were then transformed into the bacteriocin T8-negative E. faecium ST203 strain DVT705, which is a plasmid-cured, vancomycin-susceptible derivative of the 10-10-S strain7. Successful transformation was confirmed with PCR using pMSP3535 backbone-specific primers 5′-CAA TAC GCA AAC CGC CTCTC-3′ and 5′-TGG CAC TCG GCA CTT AATGG-3′. Inhibitory activity of DVT705 transformed with pBAC was confirmed by a pairwise spot killing assay against DVT705 transformed with pEV.
Liquid competition assay
pBAC and pEV strains were competed individually against the parent DVT705 strain at two ratios, 50:50 and 10:90. For each ratio, three biological replicates each consisting of three technical replicates were performed. Before the competition, each strain was grown separately overnight and cultures were normalized to optical density (OD)600 = 0.5. Strains were mixed together at the above starting ratios, and the mixture was then diluted 1:100 into 5 ml of BHI and grown shaking at 37 °C. Samples were taken at 24-h and 48-h timepoints. Samples were track diluted onto BHI, and the BHI was supplemented with 10 µg ml−1 of chloramphenicol to calculate colony forming units per ml (CFU ml−1). The abundance of the parental strain was calculated by subtracting the CFU ml−1 on the BHI plate by the CFU ml−1 on the chloramphenicol plate. Parent measurements that fell below the limit of detection, 1,000 CFU ml−1, were not included in competitive index calculations. The competitive index was calculated as below, and results were summarized using the geometric mean and a 95% confidence interval for each timepoint and ratio70.
Mouse colonization and competition experiments
Animal experiments were approved by the Animal Care and Use Committees of the Children’s Hospital of Philadelphia (IAC 18–001316). Five-week-old C57BL/6 male mice were purchased from Jackson Laboratories and given 1 week to equilibrate their microbiota before experimentation. All experimental procedures were performed in a biosafety level two laminar flow hood. Mice were housed in a 12-h light–dark cycle with ambient temperature and humidity maintained at 68–74 °F and 30–70%, respectively. No statistical methods were used to predetermine sample sizes; however, our sample sizes are similar to those reported in previous publications2,34,36. Mice were given vancomycin (1 mg ml−1) in drinking water ad libitum for 5 days followed by a 2-day recovery period71,72. Mice were then infected with 5 × 108 E. faecium cells by oral gavage twice a day for 2 days. Mice were randomly assigned to experimental groups. Data collection and analysis were not performed blind to the conditions of the experiments.
For colonization experiments, the pBAC and pEV strains were prepared by growing them to stationary phase in liquid culture and washing them with cold PBS immediately before infection. Four cages, each containing five mice, were independently infected with either strain, with two cages assigned to each strain. Stool samples were collected daily for quantification of bacterial CFUs. Samples were diluted and homogenized in PBS, and serially plated onto either bile esculin azide (BEA) agar to quantify the total enterococcal population or BEA agar with chloramphenicol (10 μg ml−1) to quantify the pBAC and pEV strains.
For competitive colonization experiments, the bacteriocin T8-positive ST117 VRE38098 and bacteriocin T8-negative ST17 VRE32530 strains were plated onto BEA agar and grown overnight at 37 °C. Colonies of each strain were inoculated into 4 ml of BHI media and grown shaking at 37 °C until OD600 values reached 0.6. Strains were washed with cold PBS and mixed at a 10:90 ratio of ST117:ST17 immediately before infection. Stool samples were collected daily for quantification of bacterial CFUs. Samples were diluted and homogenized in PBS and were then serially plated onto either BEA agar to quantify the total enterococcal population or BEA agar with vancomycin (50 μg ml−1) to quantify the ST17 and ST117 strains. From the vancomycin-supplemented plates, 100–1,000 colonies were pooled and sequenced at deep coverage (>100×) on the Illumina platform. The initial day 0 inoculum and samples from six mice (three per cage) collected 4 days postinfection were collected and sequenced. The resulting reads were mapped to the sequences of the seven multilocus ST alleles using breseq73 in population mode to assess the relative abundance of ST17 and ST117 strains.
Global isolate collection
All E. faecium genomes deposited in National Center for Biotechnology Information (NCBI) were downloaded on 23 May 2024. E. faecium genomes with collection dates between 2002 and 2022 and for which the ‘host’ in the BioSample metadata was listed as ‘Homo sapiens’, ‘Homo sapiens sapiens’, ‘hospitalized patient’ and ‘Human being’ were included. Genomes encoding the vanA or vanB operon, as identified using AMRFinderPlus, were retained for analysis.
Statistical analyses
A single-proportion hypothesis test was performed to assess the enrichment of cluster isolates within ST groups, setting the proportion of cluster isolates in the total collection as the null value, with data distribution assumed to be normal although not formally tested. The association of bacteriocin T8 presence with growth inhibition, liquid competitive advantage of pBAC versus pEV strains relative to the parent strain, and mouse GI tract colonization differences between the pBAC and pEV strains were assessed using a two-tailed Mann–Whitney test71. To assess the difference in the proportion of the ST117 strain on day 4 of the competitive colonization experiment compared with the initial inoculum, a one-sided difference in proportion hypothesis test was conducted using the average fold coverage for each sample as the sample size. Bacteriocin T8 enrichment in different STs was assessed with a single-proportion hypothesis test, using the overall proportion of bacteriocin T8-positive isolates as the null value. Differences in the number of antimicrobial resistance genes, plasmid replicons and virulence genes between lineages were assessed using two-sided t-tests, with data distribution assumed to be normal but not formally tested. Statistical significance was determined with an α = 0.05, and a Bonferroni correction for multiple comparisons was applied when appropriate. Assumptions were met for analyses unless noted otherwise.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Genomic sequences for all 710 VREfm isolates can be found in BioProject PRJNA475751 with accession numbers listed in Supplementary Dataset 1. Sequencing reads for the mouse competition experiment can be found in BioProject PRJNA1217837 with accession numbers listed in Supplementary Dataset 6B.
References
Arias, C. A. & Murray, B. E. The rise of the Enterococcus: beyond vancomycin resistance. Nat. Rev. Microbiol. 10, 266–278 (2012).
Ubeda, C. et al. Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J. Clin. Invest. 120, 4332–4341 (2010).
Paterson, D. L. et al. Acquisition of rectal colonization by vancomycin-resistant Enterococcus among intensive care unit patients treated with piperacillin–tazobactam versus those receiving cefepime-containing antibiotic regimens. Antimicrob. Agents Chemother. 52, 465–469 (2008).
Sjlund, M., Wreiber, K., Andersson, D. I., Blaser, M. J. & Engstrand, L. Long-term persistence of resistant Enterococcus species after antibiotics to eradicate Helicobacter pylori. Ann. Intern. Med. 139, 483–487 (2003).
Van Tyne, D. & Gilmore, M. S. Friend turned foe: evolution of enterococcal virulence and antibiotic resistance. Annu. Rev. Microbiol. 68, 337–356 (2014).
Stellfox, M. E. & Van Tyne, D. Last bacteria standing: VREfm persistence in the hospitalized gut. mBio 13, e0067022 (2022).
Chilambi, G. S. et al. Evolution of vancomycin-resistant Enterococcus faecium during colonization and infection in immunocompromised pediatric patients. Proc. Natl Acad. Sci. USA 117, 11703–11714 (2020).
Austin, D. J., Bonten, M. J. M., Weinstein, R. A., Slaughter, S. & Anderson, R. M. Vancomycin-resistant enterococci in intensive-care hospital settings: transmission dynamics, persistence, and the impact of infection control programs. Proc. Natl Acad. Sci. USA 96, 6908–6913 (1999).
Correa-Martinez, C. L. et al. Risk factors for long-term vancomycin-resistant enterococci persistence—a prospective longitudinal study. Microorganisms 7, 400 (2019).
Lebreton, F. et al. Tracing the enterococci from Paleozoic origins to the hospital. Cell 169, 849–861.e13 (2017).
Talaga-Ćwiertnia, K. & Bulanda, M. Analysis of the world epidemiological situation among vancomycin-resistant Enterococcus faecium infections and the current situation in Poland. Przegl Epidemiol. 72, 3–15 (2018).
Falgenhauer, L. et al. Near-ubiquitous presence of a vancomycin-resistant Enterococcus faecium ST117/CT71/vanB–clone in the Rhine-Main metropolitan area of Germany. Antimicrob. Resist. Infect. Control 8, 128 (2019).
Egan, S. A. et al. Genomic analysis of 600 vancomycin-resistant Enterococcus faecium reveals a high prevalence of ST80 and spread of similar vanA regions via IS1216E and plasmid transfer in diverse genetic lineages in Ireland. J. Antimicrob. Chemother. 77, 320–330 (2022).
Lisotto, P. et al. Molecular characterisation of vancomycin-resistant Enterococcus faecium isolates belonging to the lineage ST117/CT24 causing hospital outbreaks. Front. Microbiol. 12, 728356 (2021).
Jozefíková, A., Valček, A., Šoltys, K., Nováková, E. & Bujdáková, H. Persistence and multi-ward dissemination of vancomycin-resistant Enterococcus faecium ST17 clone in hospital settings in Slovakia 2017–2020. Int. J. Antimicrob. Agents 59, 106561 (2022).
McCracken, M. et al. Emergence of pstS-Null vancomycin-resistant Enterococcus faecium clone ST1478, Canada, 2013–2018. Emerg. Infect. Dis. 26, 2247–2250 (2020).
Fang, H., Fröding, I., Ullberg, M. & Giske, C. G. Genomic analysis revealed distinct transmission clusters of vancomycin-resistant Enterococcus faecium ST80 in Stockholm, Sweden. J. Hosp. Infect. 107, 12–15 (2021).
Lebreton, F. et al. Emergence of epidemic multidrug-resistant Enterococcus faecium from animal and commensal strains. mBio 4, e00534-13 (2013).
Kleinman, D. R. et al. Vancomycin-resistant Enterococcus sequence type 1478 spread across hospitals participating in the Canadian Nosocomial Infection Surveillance Program from 2013 to 2018. Infect. Control Hosp. Epidemiol. 44, 17–23 (2023).
De Kwaadsteniet, M., Fraser, T., Van Reenen, C. A. & Dicks, L. M. T. Bacteriocin T8, a novel class IIa sec-dependent bacteriocin produced by Enterococcus faecium T8, isolated from vaginal secretions of children infected with human immunodeficiency virus. Appl. Environ. Microbiol. 72, 4761–4766 (2006).
Sánchez, J. et al. Amino acid and nucleotide sequence, adjacent genes, and heterologous expression of hiracin JM79, a sec-dependent bacteriocin produced by Enterococcus hirae DCH5, isolated from mallard ducks (Anas platyrhynchos). FEMS Microbiol. Lett. 270, 227–236 (2007).
Todokoro, D., Tomita, H., Inoue, T. & Ike, Y. Genetic analysis of bacteriocin 43 of vancomycin-resistant Enterococcus faecium. Appl. Environ. Microbiol. 72, 6955–6964 (2006).
Herranz, C. et al. Enterocin P selectively dissipates the membrane potential of Enterococcus faecium T136. Appl. Environ. Microbiol. 67, 1689–1692 (2001).
Britton, A. P., van der Ende, S. R., van Belkum, M. J. & Martin‐Visscher, L. A. The membrane topology of immunity proteins for the two‐peptide bacteriocins carnobacteriocin XY, lactococcin G, and lactococcin MN shows structural diversity. Microbiologyopen 9, e00957 (2019).
Shokoohizadeh, L. et al. High frequency distribution of heterogeneous vancomycin resistant Enterococcous faecium (VREfm) in Iranian hospitals. Diagn. Pathol. 8, 163 (2013).
van Hal, S. J. et al. Polyclonal emergence of vanA vancomycin-resistant Enterococcus faecium in Australia. J. Antimicrob. Chemother. 72, 998–1001 (2017).
Sundermann, A. J. et al. Outbreak of vancomycin-resistant Enterococcus faecium in interventional radiology: detection through whole-genome sequencing-based surveillance. Clin. Infect. Dis. 70, 2336–2343 (2020).
Sherry, N. L. et al. Multi-site implementation of whole genome sequencing for hospital infection control: a prospective genomic epidemiological analysis. Lancet Reg. Health West. Pac. 23, 100446 (2022).
Eichel, V. et al. Challenges in interpretation of WGS and epidemiological data to investigate nosocomial transmission of vancomycin-resistant Enterococcus faecium in an endemic region: incorporation of patient movement network and admission screening. J. Antimicrob. Chemother. 75, 1716–1721 (2020).
Gouliouris, T. et al. Quantifying acquisition and transmission of Enterococcus faecium using genomic surveillance. Nat. Microbiol. 6, 103–111 (2021).
Sundermann, A. J. et al. Genomic sequencing surveillance of patients colonized with vancomycin-resistant Enterococcus (VRE) improves detection of hospital-associated transmission. Preprint at medRxiv https://doi.org/10.1101/2024.05.01.24306710 (2024).
Weber, A., Maechler, F., Schwab, F., Gastmeier, P. & Kola, A. Increase of vancomycin-resistant Enterococcus faecium strain type ST117 CT71 at Charité - Universitätsmedizin Berlin, 2008 to 2018. Antimicrob. Resist. Infect. Control 9, 109 (2020).
McInnes, R. S. et al. Integration of vanHAX downstream of a ribosomal RNA operon restores vancomycin resistance in a susceptible Enterococcus faecium strain. NPJ Antimicrob. Resist. 2, 2 (2024).
Kommineni, S. et al. Bacteriocin production augments niche competition by enterococci in the mammalian GI tract. Nature 526, 719–722 (2015).
Corr, S. C. et al. Bacteriocin production as a mechanism for the antiinfective activity of Lactobacillus salivarius UCC118. Proc. Natl Acad. Sci. USA 104, 7617–7621 (2007).
Geldart, K. G. et al. Engineered E. coli Nissle 1917 for the reduction of vancomycin-resistant Enterococcus in the intestinal tract. Bioeng. Transl. Med. 3, 197–208 (2018).
Wagner, T. M. et al. Interactions between commensal Enterococcus faecium and Enterococcus lactis and clinical isolates of Enterococcus faecium. FEMS Microbes 5, xtae009 (2024).
Ríos Colombo, N. S. et al. Impact of bacteriocin-producing strains on bacterial community composition in a simplified human intestinal microbiota. Front. Microbiol. 14, 1290697 (2023).
Garretto, A., Dawid, S. & Woods, R. Increasing prevalence of bacteriocin carriage in a 6-year hospital cohort of E. faecium. J. Bacteriol. 206, e00294–24 (2024).
Davies, E. A., Bevis, H. E. & Delves-Broughton, J. The use of the bacteriocin, nisin, as a preservative in ricotta-type cheeses to control the food-borne pathogen Listeria monocytogenes. Lett. Appl. Microbiol. 24, 343–346 (1997).
Kjos, M., Salehian, Z., Nes, I. F. & Diep, D. B. An extracellular loop of the mannose phosphotransferase system component IIC is responsible for specific targeting by class IIa bacteriocins. J. Bacteriol. 192, 5906–5913 (2010).
Kjos, M. et al. Target recognition, resistance, immunity and genome mining of class II bacteriocins from Gram-positive bacteria. Microbiology 157, 3256–3267 (2011).
Geldart, K. & Kaznessis, Y. N. Characterization of class IIa bacteriocin resistance in Enterococcus faecium. Antimicrob. Agents Chemother. 61, e02033-16 (2017).
Kjos, M., Nes, I. F. & Diep, D. B. Mechanisms of resistance to bacteriocins targeting the mannose phosphotransferase system. Appl. Environ. Microbiol. 77, 3335–3342 (2011).
Arbulu, S. & Kjos, M. Revisiting the multifaceted roles of bacteriocins. Microb. Ecol. 87, 41 (2024).
Sundermann, A. J. et al. Whole-genome sequencing surveillance and machine learning of the electronic health record for enhanced healthcare outbreak detection. Clin. Infect. Dis. 75, 476–482 (2022).
Prjibelski, A., Antipov, D., Meleshko, D., Lapidus, A. & Korobeynikov, A. Using SPAdes De Novo Assembler. Curr. Protoc. Bioinformatics 70, e102 (2020).
Gurevich, A., Saveliev, V., Vyahhi, N. & Tesler, G. QUAST: quality assessment tool for genome assemblies. Bioinformatics 29, 1072–1075 (2013).
Wood, D. E., Lu, J. & Langmead, B. Improved metagenomic analysis with Kraken 2. Genome Biol. 20, 257 (2019).
Jolley, K. A., Bray, J. E. & Maiden, M. C. J. Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. Wellcome Open Res. 3, 124 (2018).
Seemann, T. mlst: scan contig files against PubMLST typing schemes. GitHub https://github.com/tseemann/mlst (2015).
Harris, S. R. SKA: Split kmer analysis toolkit for bacterial genomic epidemiology. GitHub https://doi.org/10.1101/453142 (2018).
Maechler, F. et al. Split k-mer analysis compared to cgMLST and SNP-based core genome analysis for detecting transmission of vancomycin-resistant enterococci: results from routine outbreak analyses across different hospitals and hospitals networks in Berlin, Germany. Microb. Genom. 9, mgen000937 (2023).
Liese, J. et al. Expansion of vancomycin-resistant Enterococcus faecium in an academic tertiary hospital in southwest Germany: a large-scale whole-genome-based outbreak investigation. Antimicrob. Agents Chemother. 63, e01978-18 (2019).
Seemann, T. Prokka: rapid prokaryotic genome annotation. Bioinformatics 30, 2068–2069 (2014).
Schwengers, O. et al. Bakta: rapid and standardized annotation of bacterial genomes via alignment-free sequence identification. Microb. Genom. 7, 000685 (2021).
Bastian, M., Heymann, S. & Jacomy, M. Gephi: an open source software for exploring and manipulating networks. In Proc. 3rd International AAAI Conf. on Web and Social Media 361–362 (Association for the Advancement of Artificial Intelligence, 2009).
Page, A. J. et al. Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics 31, 3691–3693 (2015).
Stamatakis, A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313 (2014).
van Heel, A. J. et al. BAGEL4: a user-friendly web server to thoroughly mine RiPPs and bacteriocins. Nucleic Acids Res. 46, W278–W281 (2018).
Medema, M. H. et al. antiSMASH: rapid identification, annotation and analysis of secondary metabolite biosynthesis gene clusters in bacterial and fungal genome sequences. Nucleic Acids Res. 39, W339–W346 (2011).
Seemann, T. Abricate: mass screening of contigs for antimicrobial and virulence genes. GitHub https://github.com/tseemann/abricate (2015).
Feldgarden, M. et al. AMRFinderPlus and the Reference Gene Catalog facilitate examination of the genomic links among antimicrobial resistance, stress response, and virulence. Sci. Rep. 11, 12728 (2021).
Carattoli, A. et al. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob. Agents Chemother. 58, 3895–3903 (2014).
Chen, L., Zheng, D., Liu, B., Yang, J. & Jin, Q. VFDB 2016: hierarchical and refined dataset for big data analysis—10 years on. Nucleic Acids Res. 44, D694–D697 (2016).
Wick, R. R., Judd, L. M., Gorrie, C. L. & Holt, K. E. Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 13, e1005595 (2017).
Nanoporetech dorado: Oxford Nanopore’s Basecaller. GitHub https://github.com/nanoporetech/dorado (2022).
Kim, J.-H. & Mills, D. A. Improvement of a nisin-inducible expression vector for use in lactic acid bacteria. Plasmid 58, 275–283 (2007).
Gibson, D. G. et al. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 6, 343–345 (2009).
Dammann, A. N. et al. Group B Streptococcus capsular serotype alters vaginal colonization fitness. J. Infect. Dis. 225, 1896–1904 (2021).
Smith, A. B. et al. Enterococci enhance Clostridioides difficile pathogenesis. Nature 611, 780–786 (2022).
Smith, A. B. et al. Liberation of host heme by Clostridioides difficile-mediated damage enhances Enterococcus faecalis fitness during infection. mBio 15, e0165623 (2023).
Deatherage, D. E. & Barrick, J. E. Identification of mutations in laboratory evolved microbes from next-generation sequencing data using breseq. Methods Mol. Biol. 1151, 165–188 (2014).
Acknowledgements
We gratefully acknowledge Y. Li, K. Waggle, H. Coyle and H. Manzer for their helpful contributions to this study, and V. Cooper and A. Rokes for assistance in performing whole-genome sequencing of EDS-HAT isolates. The study was funded by National Institutes of Health grants R01AI165519 (to D.V.T.), R01AI127472 (to L.H.H.) and R35GM138369 (to J.P.Z.). The funders had no role in study design, data collection and analysis, preparation of the paper or decision to publish.
Author information
Authors and Affiliations
Contributions
E.G.M., L.H.H., J.P.Z. and D.V.T. designed the study. E.G.M., A.B.S., M.P.G., K.H., L.P. and A.J.S. performed the experiments and collected data. E.G.M., J.P.Z. and D.V.T. analysed and interpreted the data. E.G.M. and D.V.T. drafted the paper and figures. All authors reviewed and edited the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Microbiology thanks Morten Kjos and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Genetic relatedness and genomic features of 710 VREfm isolates from UPMC.
(A) The midpoint-rooted phylogenetic tree was constructed from a single-copy core genome alignment. The scale bar represents nucleotide substitutions per site. Sequence types (ST) and isolation source are colored as indicated. Emergent lineages are noted by a black star. Antimicrobial susceptibility testing (AST) results for daptomycin (DAP) and linezolid (LNZ) were interpreted as resistant (R), intermediate (I), or susceptible (S). (B) Genome length, (C) presence of antimicrobial resistance genes, (D) presence of virulence genes, and (E) presence of plasmid replicons within main VREfm lineages. Averages of genomic features within each ST were compared to the average seen in the previously dominant ST17 lineage using a two-sided t-test. Asterisks indicate p-values < 0.0045. Horizontal lines represent the average and error bars show the standard deviation for each group.
Extended Data Fig. 2 Bacteriocin prevalence and genomic context of bacteriocin T8.
(A) Distribution of bacteriocins within 710 VREfm isolates from UPMC. Bacteriocins were identified using BAGEL4 with sequence identity and coverage thresholds of ≥ 95%. (B) Bacteriocin T8-encoding rep11a plasmid from the ST117 isolate VRE38098. Bacteriocin T8 and corresponding immunity factor genes are colored in burgundy.
Extended Data Fig. 3 Global population structure of 15,631 VREfm genomes from human sources collected between 2002-2022.
Geographical distribution of VREfm genomes pulled from NCBI. The number of genomes from each country is shown from lowest (light grey) to highest (purple). Countries with >300 genomes are highlighted with the distribution of STs and vancomycin resistance alleles.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mills, E.G., Hewlett, K., Smith, A.B. et al. Bacteriocin production facilitates nosocomial emergence of vancomycin-resistant Enterococcus faecium. Nat Microbiol 10, 871–881 (2025). https://doi.org/10.1038/s41564-025-01958-0
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41564-025-01958-0
This article is cited by
-
E. faecium gains an edge from bacteriocin
Nature Reviews Microbiology (2025)
-
A superbug rose to the top after gaining a chemical weapon
Nature (2025)