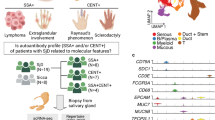
Abstract
Viral infections are implicated in the pathogenesis of autoimmune diseases, including Sjögren’s disease (SjD), but the mechanisms linking viral antigens to disease development remain poorly understood. To address this, we conducted shotgun metagenomic sequencing of saliva samples from 35 patients with SjD and 25 healthy controls. The salivary virome of the patients with SjD, particularly those with high disease activity, had an expansion of Siphoviridae bacteriophages and increased eukaryotic viral sequences, including Vientovirus. This virus was associated with lacrimal gland dysfunction and elevated anti-SSA/Ro52 autoantibody levels. Alignment analysis and cross-blocking assay identified molecular mimicry between the Vientovirus capsid protein and the autoantigen SSA/Ro52. Mice immunized with a Vientovirus capsid peptide developed anti-SSA/Ro52 antibodies and showed immunological features resembling those of patients with SjD. These findings highlight distinct virome profiles in SjD and provide mechanistic evidence supporting the role of Vientovirus in triggering autoimmunity through molecular mimicry.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The sequence data generated in this study can be found in the Sequence Read Archive (SRA) of NCBI under BioProject accession numbers PRJNA1223632 and PRJNA1272653. Source data are provided with this paper.
Change history
12 September 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41564-025-02143-z
References
Baldini, C., Fulvio, G., La Rocca, G. & Ferro, F. Update on the pathophysiology and treatment of primary Sjögren syndrome. Nat. Rev. Rheumatol. 20, 473–491 (2024).
Mariette, X. & Criswell, L. A. Primary Sjögren’s syndrome. N. Engl. J. Med. 378, 931–939 (2018).
Seror, R., Nocturne, G. & Mariette, X. Current and future therapies for primary Sjögren syndrome. Nat. Rev. Rheumatol. 17, 475–486 (2021).
Wang, B. et al. Targeted therapy for primary Sjögren’s syndrome: where are we now? BioDrugs 35, 593–610 (2021).
Ramos-Casals, M. et al. EULAR recommendations for the management of Sjögren’s syndrome with topical and systemic therapies. Ann. Rheum. Dis. 79, 3–18 (2020).
Nocturne, G. & Mariette, X. Advances in understanding the pathogenesis of primary Sjögren’s syndrome. Nat. Rev. Rheumatol. 9, 544–556 (2013).
Crow, M. K., Olferiev, M. & Kirou, K. A. Type I interferons in autoimmune disease. Annu. Rev. Pathol. 14, 369–393 (2019).
Jonsson, R., Theander, E., Sjostrom, B., Brokstad, K. & Henriksson, G. Autoantibodies present before symptom onset in primary Sjögren syndrome. JAMA 310, 1854–1855 (2013).
Getts, D. R., Chastain, E. M., Terry, R. L. & Miller, S. D. Virus infection, antiviral immunity, and autoimmunity. Immunol. Rev. 255, 197–209 (2013).
Vinuesa, C. G., Grenov, A. & Kassiotis, G. Innate virus-sensing pathways in B cell systemic autoimmunity. Science 380, 478–484 (2023).
Virgin, H. W., Wherry, E. J. & Ahmed, R. Redefining chronic viral infection. Cell 138, 30–50 (2009).
Maslinska, M. & Kostyra-Grabczak, K. The role of virus infections in Sjögren’s syndrome. Front. Immunol. 13, 823659 (2022).
Maslinska, M. The role of Epstein–Barr virus infection in primary Sjögren’s syndrome. Curr. Opin. Rheumatol. 31, 475–483 (2019).
Nakamura, H. et al. Reevaluation for clinical manifestations of HTLV-I-seropositive patients with Sjögren’s syndrome. BMC Musculoskelet. Disord. 16, 335 (2015).
Fleck, M., Kern, E. R., Zhou, T., Lang, B. & Mountz, J. D. Murine cytomegalovirus induces a Sjögren’s syndrome-like disease in C57Bl/6-lpr/lpr mice. Arthritis Rheum. 41, 2175–2184 (1998).
Stathopoulou, E. A., Routsias, J. G., Stea, E. A., Moutsopoulos, H. M. & Tzioufas, A. G. Cross-reaction between antibodies to the major epitope of Ro60 kD autoantigen and a homologous peptide of Coxsackie virus 2B protein. Clin. Exp. Immunol. 141, 148–154 (2005).
Ranger-Rogez, S., Vidal, E., Liozon, F. & Denis, F. Primary Sjögren’s syndrome and antibodies to human herpesvirus type 6. Clin. Infect. Dis. 19, 1159–1160 (1994).
Lee, S. J. et al. Detection of HTLV-1 in the labial salivary glands of patients with Sjögren’s syndrome: a distinct clinical subgroup? J. Rheumatol. 39, 809–815 (2012).
Hudson, E. et al. Evidence that autoantibody production may be driven by acute Epstein–Barr virus infection in Sjögren’s disease. Ann. Rheum. Dis. (2024). https://doi.org/10.1136/ard-2024-226226
Koonin, E. V., Kuhn, J. H., Dolja, V. V. & Krupovic, M. Megataxonomy and global ecology of the virosphere. ISME J. 18, wrad042 (2024).
Virgin, H. W. The virome in mammalian physiology and disease. Cell 157, 142–150 (2014).
Liang, G. & Bushman, F. D. The human virome: assembly, composition and host interactions. Nat. Rev. Microbiol. 19, 514–527 (2021).
Hou, X. et al. Using artificial intelligence to document the hidden RNA virosphere. Cell 187, 6929–6942.e16 (2024).
Abbas, A. A. et al. Redondoviridae, a family of small, circular DNA viruses of the human oro-respiratory tract associated with periodontitis and critical illness. Cell Host Microbe 25, 719–729.e4 (2019).
Norman, J. M. et al. Disease-specific alterations in the enteric virome in inflammatory bowel disease. Cell 160, 447–460 (2015).
Tun, H. M. et al. Gut virome in inflammatory bowel disease and beyond. Gut 73, 350–360 (2024).
Chen, B. et al. Disturbed gut virome with potent interferonogenic property in systemic lupus erythematosus. Sci. Bull. 68, 295–304 (2023).
Tomofuji, Y. et al. Whole gut virome analysis of 476 Japanese revealed a link between phage and autoimmune disease. Ann. Rheum. Dis. 81, 278–288 (2022).
Wook Kim, K. et al. Distinct gut virome profile of pregnant women with type 1 diabetes in the ENDIA study. Open Forum Infect. Dis. 6, ofz025 (2019).
Hu, F. et al. Rheumatoid arthritis patients harbour aberrant enteric bacteriophages with autoimmunity-provoking potential: a paired sibling study. Ann. Rheum. Dis. 83, 1677–1690 (2024).
Abeles, S. R. et al. Human oral viruses are personal, persistent and gender-consistent. ISME J. 8, 1753–1767 (2014).
Ho, S. X., Min, N., Wong, E. P. Y., Chong, C. Y. & Chu, J. J. H. Characterization of oral virome and microbiome revealed distinctive microbiome disruptions in paediatric patients with hand, foot and mouth disease. npj Biofilms Microbiomes 7, 19 (2021).
Edlund, A., Santiago-Rodriguez, T. M., Boehm, T. K. & Pride, D. T. Bacteriophage and their potential roles in the human oral cavity. J. Oral. Microbiol 7, 27423 (2015).
Lemon, K. P. et al. Comparative analyses of the bacterial microbiota of the human nostril and oropharynx. mBio 1, e00129-10 (2010).
Keeler, E. L. et al. Widespread, human-associated redondoviruses infect the commensal protozoan Entamoeba gingivalis. Cell Host Microbe 31, 58–68.e5 (2023).
Abbas, A., Taylor, L. J., Collman, R. G., Bushman, F. D. & Ictv Report, C. ICTV virus taxonomy profile: Redondoviridae. J. Gen. Virol. 102, jgv001526 (2021).
Abbas, A. A. et al. Redondoviridae, a family of small, circular DNA viruses of the human oro-respiratory tract associated with periodontitis and critical illness. Cell Host Microbe 26, 297 (2019).
Cui, L. et al. Identification and genetic characterization of a novel circular single-stranded DNA virus in a human upper respiratory tract sample. Arch. Virol. 162, 3305–3312 (2017).
Taylor, L. J. et al. Redondovirus diversity and evolution on global, individual, and molecular scales. J. Virol. 95, e0081721 (2021).
Zhang, Y., Wang, C., Feng, X., Chen, X. & Zhang, W. Redondoviridae and periodontitis: a case-control study and identification of five novel redondoviruses from periodontal tissues. Virus Evol. 7, veab033 (2021).
Spezia, P. G. et al. Redondovirus DNA in human respiratory samples. J. Clin. Virol. 131, 104586 (2020).
Lazaro-Perona, F. et al. Metagenomic detection of two vientoviruses in a human sputum sample. Viruses 12, 327 (2020).
Merenstein, C. et al. Signatures of COVID-19 severity and immune response in the respiratory tract microbiome. mBio 12, e0177721 (2021).
Munz, C., Lunemann, J. D., Getts, M. T. & Miller, S. D. Antiviral immune responses: triggers of or triggered by autoimmunity? Nat. Rev. Immunol. 9, 246–258 (2009).
Bonner, M. et al. Detection of the amoeba Entamoeba gingivalis in periodontal pockets. Parasite 21, 30 (2014).
Bao, X., Wiehe, R., Dommisch, H. & Schaefer, A. S. Entamoeba gingivalis causes oral inflammation and tissue destruction. J. Dent. Res. 99, 561–567 (2020).
Bao, X., Weiner, J. 3rd, Meckes, O., Dommisch, H. & Schaefer, A. S. Entamoeba gingivalis exerts severe pathogenic effects on the oral mucosa. J. Dent. Res. 100, 771–776 (2021).
Damania, B., Kenney, S. C. & Raab-Traub, N. Epstein–Barr virus: biology and clinical disease. Cell 185, 3652–3670 (2022).
Young, L. S. & Rickinson, A. B. Epstein–Barr virus: 40 years on. Nat. Rev. Cancer 4, 757–768 (2004).
Xuan, J. et al. Serological evidence for the association between Epstein–Barr virus infection and Sjögren’s syndrome. Front. Immunol. 11, 590444 (2020).
McClain, M. T. et al. Early events in lupus humoral autoimmunity suggest initiation through molecular mimicry. Nat. Med. 11, 85–89 (2005).
Kimura, K. et al. A reverse-transcription loop-mediated isothermal amplification technique to detect tomato mottle mosaic virus, an emerging tobamovirus. Viruses 15, 1688 (2023).
Zhang, T. et al. RNA viral community in human feces: prevalence of plant pathogenic viruses. PLoS Biol. 4, e3 (2006).
Gibbs, M. J. & Weiller, G. F. Evidence that a plant virus switched hosts to infect a vertebrate and then recombined with a vertebrate-infecting virus. Proc. Natl Acad. Sci. USA 96, 8022–8027 (1999).
Shiboski, C. H. et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren’s syndrome. Ann. Rheum. Dis. 76, 9 (2017).
Seror, R. et al. EULAR Sjogren’s syndrome disease activity index: development of a consensus systemic disease activity index for primary Sjögren’s syndrome. Ann. Rheum. Dis. 69, 1103–1109 (2010).
Seror, R. et al. Accurate detection of changes in disease activity in primary Sjögren’s syndrome by the European League Against Rheumatism Sjögren’s Syndrome Disease Activity Index. Arthritis Care Res. 62, 551–558 (2010).
Lim, E. S. et al. Early life dynamics of the human gut virome and bacterial microbiome in infants. Nat. Med. 21, 1228–1234 (2015).
Hyatt, D. et al. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics 11, 119 (2010).
Buchfink, B., Xie, C. & Huson, D. H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 12, 59–60 (2015).
Chen, G., Tang, X., Shi, M. & Sun, Y. VirBot: an RNA viral contig detector for metagenomic data. Bioinformatics 39, 1688 (2023).
Guo, J. et al. VirSorter2: a multi-classifier, expert-guided approach to detect diverse DNA and RNA viruses. Microbiome 9, 37 (2021).
Acknowledgements
This study was supported by the National Natural Science Foundation of China (82471833, Y. Liu; 82101841, X.Z.; 82171779 and 82371802, G.S.), the Natural Science Foundation of Fujian Province (2023J06055, Y. Liu), the Fujian Health and Family Planning Commission (2024GGB20, X.Z.) and the Scientific and Technological Projects of Xiamen City (2022XMSLCYX01, G.S.).
Author information
Authors and Affiliations
Contributions
Y. Liu, G.S. and X.Z. conceptualized the project. X.Z., Y. Li (0000-0003-3020-6424) and Y.Q. designed the methodology. Y.Q., Z.L. and Y.C. collected the specimens. X.Z., Y. Li (0000-0003-3020-6424), C.D. and H.Q. conducted the investigations. Y. Li (0000-0003-3020-6424) and X.Z. wrote the original draft of the paper. Y. Li (0000-0002-5587-2784), G.S., S.C., Y.H. and Y.Q. reviewed and edited the paper. G.S., Y. Liu and X.Z. acquired funding and resources.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Microbiology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Differences in salivary virome composition of SjD patients.
(a) Alpha diversity of eukaryotic RNA virome comparisons based on observed richness, Chao1 richness, Shannon diversity, and Simpson diversity of the HC (n = 25 biological replicates), ESSDAI-low SjD (n = 23 biological replicates) and ESSDAI-high SjD (n = 12 biological replicates) groups. One-way ANOVA and two-sided Mann‒Whitney U test was used. Each data point is shown, the boxplot represents the median and interquartile range values, and whiskers represent the minimum and maximum values. (b) Observed richness, Chao1 richness, Shannon diversity, and Simpson diversity the eukaryotic DNA virome of the HC (n = 25), ESSDAI-low SjD (n = 23) and ESSDAI-high SjD (n = 12) groups. One-way ANOVA and two-sided Mann‒Whitney U test was used. Each data point is shown, the boxplot represents the median and interquartile range values, and whiskers represent the minimum and maximum values. (c) Observed richness, Chao1 richness, Shannon diversity, and Simpson diversity of prokaryotic virome of the HC (n = 25), ESSDAI-low SjD (n = 23) and ESSDAI-high SjD (n = 12) groups. One-way ANOVA and two-sided Mann‒Whitney U test was used. Each data point is shown, the boxplot represents the median and interquartile range values, and whiskers represent the minimum and maximum values. (d) Multivariate sparse partial least-squares discriminant analysis (sPLS-DA) of relative abundance at the family level of the saliva virome, comparing the HC and SjD groups. Two-sided PERMANOVA was used. (e) sPLS-DA of relative abundance at the species level of the saliva virome, comparing the HC and SjD groups. Two-sided PERMANOVA was used. (f) sPLS-DA of the relative abundance of the saliva virome at the family level, comparing the HC, ESSDAI-low SjD and ESSDAI-high SjD groups. Two-sided PERMANOVA was used. (g) sPLS-DA of the relative abundance of the saliva virome at the species level, comparing the HC, ESSDAI-low SjD and ESSDAI-high SjD groups. Two-sided PERMANOVA was used. ESSDAI, EULAR Sjögren’s Syndrome Disease Activity Index.
Extended Data Fig. 2 Detection of RdRp gene of Tomato mosaic virus (ToMV) and indicated genes of EBV from oral swabs of SjD and HC subjects.
(a) Representative PCR bands of indicated genes from oral swabs. (b) Quantifications of relative band intensities normalized to ACTB and the semi-quantitative levels were shown in the heatmap. (c) Two-sided Spearman’s correlation between relative band intensity of ToMV and anti-SSA/Ro52 level was shown. (d) Two-sided Spearman’s correlation between the relative band intensity of ToMV and unstimulated salivary flow rate (uSFR) was shown. (e) Two-sided Spearman’s correlation between relative band intensity of EBV genes and indicated clinical parameter were shown in the heatmap. uSFR, unstimulated salivary flow rate; sSFR, stimulated salivary flow rate; ESSPRI, EULAR Sjögren’s Syndrome Patient Reported Index; ESSDAI, EULAR Sjögren’s Syndrome Disease Activity Index. *, P < 0.05, **, P < 0.01, ***, P < 0.001.
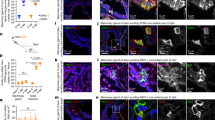
Extended Data Fig. 3 Salivary glands change in mice immunized with Vientovirus capsid peptide.
(a) Saliva flow rates were measured in BSA-, mimotope 1-, and mimotope 2-immunization groups. Dots plot with mean ± SD. n = 7 mice for the BSA group, n = 6 mice for the mimotope 1 group, n = 5 for mimotope 2 group. Statistical significances were determined by Two-way ANOVA test with two-sided Tukey’s multiple comparisons test. (b) The serum IgG against SG antigens was detected by ELISA. n = 7 mice for the BSA group, n = 6 mice for the mimotope 1 group, n = 5 for mimotope 2 group. Statistical significance was determined by two-way ANOVA with two-sided Tukey’s multiple comparisons test. Dots plot with mean ± SD. (c) Immune deposits were detected by immunofluorescence staining for IgM (green) and complement C3 (red) in a representative salivary gland section from three groups. Scale bars, 250 µm. (d) Histological evaluation of lymphocyte infiltration in three groups was performed on tissue sections of salivary glands with H&E staining. Scale bars, 250 µm (left panel),100 µm (right panel).
Supplementary information
Supplementary Information
Supplementary Figs. 1–6, Supplementary Tables 1–3 and 5–8, and uncropped scans of gels for Supplementary Fig. 3.
Supplementary Table 4
Sequence identity of vOTU_2333 and vOTU_3152 Rep sequences with other reference strains.
Supplementary Table 9
PCR primers used in this study.
Supplementary Data
Source data for supplementary figures.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 4
Unprocessed gels for Fig. 4b.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 2
Unprocessed gels for Extended Data Fig. 2a.
Source Data Extended Data Fig. 3
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, X., Li, Y., Qin, Y. et al. Vientovirus capsid protein mimics autoantigens and contributes to autoimmunity in Sjögren’s disease. Nat Microbiol 10, 2591–2602 (2025). https://doi.org/10.1038/s41564-025-02115-3
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41564-025-02115-3