Abstract
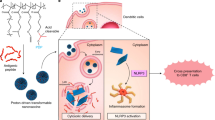
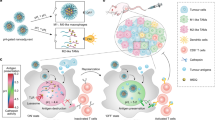
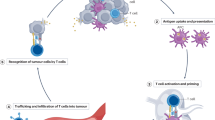
Autophagosome cancer vaccines can promote cross-presentation of multiple tumour antigens and induce cross-reactive T cell responses. However, so far, there is no effective method for obtaining a highly immunogenic autophagosomal cancer vaccine because autophagosomes, once formed, quickly fuse with lysosomes and cannot easily escape from cells. Here we report a functional Ti2NX nanodot that caps the autophagosome membrane lipid phosphatidylinositol-4-phosphate, blocking the fusion of autophagosomes with lysosomes and producing stable nanodot-coated autophagosomes in tumours. The formed nanodot-coated autophagosomes can escape from cancer cells to lymph nodes, where they activate tumour-specific T cells. We show that our approach reduces tumour burden and provide long-term immune surveillance protection for cured mice. This work provides a method for the direct formation of personalized autophagosome-based cancer vaccines in vivo, offering a promising strategy for tumour treatment.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All relevant data of this study are available within the paper and its Supplementary Information files. Raw data are publicly available on figshare and can be accessed through https://doi.org/10.6084/m9.figshare.23723583. Source data are provided with this paper.
References
Kaiser, J. Personalized tumour vaccines keep cancer in check. Science 356, 122 (2017).
Pulendran, B. & Davis, M. M. The science and medicine of human immunology. Science 369, eaay4014 (2020).
Ci, T. et al. Cryo-shocked cancer cells for targeted drug delivery and vaccination. Sci. Adv. 6, eabc3013 (2020).
Jiang, Y. et al. Engineered cell-membrane-coated nanoparticles directly present tumor antigens to promote anticancer immunity. Adv. Mater. 32, e2001808 (2020).
Guo, J. et al. Cancer vaccines from cryogenically silicified tumour cells functionalized with pathogen-associated molecular patterns. Nat. Biomed. Eng. 6, 19–31 (2022).
Harari, A., Graciotti, M., Bassani-Sternberg, M. & Kandalaft, L. E. Antitumour dendritic cell vaccination in a priming and boosting approach. Nat. Rev. Drug Discov. 19, 635–652 (2020).
Marar, C., Starich, B. & Wirtz, D. Extracellular vesicles in immunomodulation and tumor progression. Nat. Immunol. 22, 560–570 (2021).
Cheng, L. & Hill, A. F. Therapeutically harnessing extracellular vesicles. Nat. Rev. Drug Discov. 21, 379–399 (2022).
Jhunjhunwala, S., Hammer, C. & Delamarre, L. Antigen presentation in cancer: insights into tumour immunogenicity and immune evasion. Nat. Rev. Cancer 21, 298–312 (2021).
Roth, G. A. et al. Designing spatial and temporal control of vaccine responses. Nat. Rev. Mater. 7, 174–195 (2022).
Ma, L. et al. Immunotherapy and prevention of cancer by nanovaccines loaded with whole-cell components of tumor tissues or cells. Adv. Mater. 33, e2104849 (2021).
Saxena, M., van der Burg, S. H., Melief, C. J. M. & Bhardwaj, N. Therapeutic cancer vaccines. Nat. Rev. Cancer 21, 360–378 (2021).
Zhang, X., Cui, H., Zhang, W., Li, Z. & Gao, J. Engineered tumor cell-derived vaccines against cancer: the art of combating poison with poison. Bioact. Mater. 22, 491–517 (2023).
Page, D. B. et al. Glimpse into the future: harnessing autophagy to promote antitumour immunity with the DRibbles vaccine. J. Immunother. Cancer 4, 25 (2016).
Wenger, T. et al. Autophagy inhibition promotes defective neosynthesized proteins storage in ALIS, and induces redirection toward proteasome processing and MHCI-restricted presentation. Autophagy 8, 350–363 (2012).
Yi, Y. et al. Autophagy-assisted antigen cross-presentation: autophagosome as the argo of shared tumour-specific antigens and DAMPs. Oncoimmunology 1, 976–978 (2012).
Hou, W. et al. Strange attractors: DAMPs and autophagy link tumor cell death and immunity. Cell Death Dis. 4, e966 (2013).
Yamamoto, K. et al. Autophagy promotes immune evasion of pancreatic cancer by degrading MHC-I. Nature 581, 100–105 (2020).
MacNabb, B. W. et al. Dendritic cells can prime antitumour CD8+ T cell responses through major histocompatibility complex cross-dressing. Immunity 55, 982–997.e8 (2022).
Dersh, D., Holly, J. & Yewdell, J. W. A few good peptides: MHC class I-based cancer immunosurveillance and immunoevasion. Nat. Rev. Immunol. 21, 116–128 (2021).
Li, Y. et al. Tumor-derived autophagosome vaccine: mechanism of cross-presentation and therapeutic efficacy. Clin. Cancer Res. 17, 7047–7057 (2011).
Li, Y. et al. Efficient cross-presentation depends on autophagy in tumor cells. Cancer Res. 68, 6889–6895 (2008).
Ye, Z. et al. Manipulation of PD-L1 endosomal trafficking promotes anticancer immunity. Adv. Sci. 10, e2206411 (2022).
Raudenska, M., Balvan, J. & Masarik, M. Crosstalk between autophagy inhibitors and endosome-related secretory pathways: a challenge for autophagy-based treatment of solid cancers. Mol. Cancer 20, 140 (2021).
Wen, Z. F. et al. Tumor cell-released autophagosomes (TRAPs) promote immunosuppression through induction of M2-like macrophages with increased expression of PD-L1. J. Immunother. Cancer 6, 151 (2018).
Sanborn, R. E. et al. A pilot study of an autologous tumor-derived autophagosome vaccine with docetaxel in patients with stage IV non-small cell lung cancer. J. Immunother. Cancer 5, 103 (2017).
Diao, L. & Liu, M. Rethinking antigen source: cancer vaccines based on whole tumor cell/tissue lysate or whole tumor cell. Adv. Sci. 10, e2300121 (2023).
Wang, H. et al. GABARAPs regulate PI4P-dependent autophagosome:lysosome fusion. Proc. Natl Acad. Sci. USA 112, 7015–7020 (2015).
Sun, H. Q. et al. PI4P-dependent targeting of ATG14 to mature autophagosomes. Biochemistry 61, 722–729 (2022).
Cebollero, E. et al. Phosphatidylinositol-3-phosphate clearance plays a key role in autophagosome completion. Curr. Biol. 22, 1545–1553 (2012).
van Niel, G., D’Angelo, G. & Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 19, 213–228 (2018).
Martens, S., Nakamura, S. & Yoshimori, T. Phospholipids in autophagosome formation and fusion. J. Mol. Biol. 428, 4819–4827 (2016).
Zhao, Y. G., Codogno, P. & Zhang, H. Machinery, regulation and pathophysiological implications of autophagosome maturation. Nat. Rev. Mol. Cell Biol. 22, 733–750 (2021).
Shinoda, S. et al. Syntaxin 17 recruitment to mature autophagosomes is temporally regulated by PI4P accumulation. eLife 12, RP92189 (2024).
Laczkó-Dobos, H. et al. PtdIns4P is required for the autophagosomal recruitment of STX17 (syntaxin 17) to promote lysosomal fusion. Autophagy 20, 1639–1650 (2024).
Chen, D. et al. A mammalian autophagosome maturation mechanism mediated by TECPR1 and the Atg12-Atg5 conjugate. Mol. Cell 45, 629–641 (2012).
Nakamura, S. & Yoshimori, T. New insights into autophagosome–lysosome fusion. J. Cell Sci. 130, 1209–1216 (2017).
Johnson, D., Qiao, Z., Uwadiunor, E. & Djire, A. Holdups in nitride MXene’s development and limitations in advancing the field of MXene. Small 18, e2106129 (2022).
Marino, G., Niso-Santano, M., Baehrecke, E. H. & Kroemer, G. Self-consumption: the interplay of autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 15, 81–94 (2014).
Debnath, J., Gammoh, N. & Ryan, K. M. Autophagy and autophagy-related pathways in cancer. Nat. Rev. Mol. Cell Biol. 24, 560–575 (2023).
Clarke, A. J. & Simon, A. K. Autophagy in the renewal, differentiation and homeostasis of immune cells. Nat. Rev. Immunol. 19, 170–183 (2019).
Zhao, J. et al. In situ growth of nanoantioxidants on cellular vesicles for efficient reactive oxygen species elimination in acute inflammatory diseases. Nano Today 40, 101282 (2021).
Murshid, A., Gong, J., Stevenson, M. A. & Calderwood, S. K. Heat shock proteins and cancer vaccines: developments in the past decade and chaperoning in the decade to come. Expert Rev. Vaccines 10, 1553–1568 (2011).
Lhuillier, C. et al. Radiotherapy-exposed CD8+ and CD4+ neoantigens enhance tumor control. J. Clin. Invest. 131, e138740 (2021).
Kreiter, S. et al. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature 520, 692–696 (2015).
Qin, H. et al. Development of a cancer vaccine using in vivo click-chemistry-mediated active lymph node accumulation for improved immunotherapy. Adv. Mater. 33, e2006007 (2021).
Song, T. et al. Engineering the deformability of albumin-stabilized emulsions for lymph-node vaccine delivery. Adv. Mater. 33, e2100106 (2021).
Gong, T., Liu, L., Jiang, W. & Zhou, R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol. 20, 95–112 (2020).
Marichal, T. et al. DNA released from dying host cells mediates aluminum adjuvant activity. Nat. Med. 17, 996–1002 (2011).
Gou, S. et al. Engineered nanovaccine targeting Clec9a+ dendritic cells remarkably enhances the cancer immunotherapy effects of STING agonist. Nano Lett. 21, 9939–9950 (2021).
Xu, J. et al. A general strategy towards personalized nanovaccines based on fluoropolymers for postsurgical cancer immunotherapy. Nat. Nanotechnol. 15, 1043–1052 (2020).
Zhao, J. et al. A minimalist binary vaccine carrier for personalized postoperative cancer vaccine therapy. Adv. Mater. 34, e2109254 (2022).
Lin, Z. J., Zhuo, M. J., Li, M. S., Wang, J. Y. & Zhou, Y. C. Synthesis and microstructure of layered-ternary Ti2AlN ceramic. Scr. Mater. 56, 1115–1118 (2007).
Acknowledgements
This work was financially supported by the National Key R&D Program of China (grant number 2020YFA0710700 to Y.-Z.Y.), the National Natural Science Foundation of China (grant number 52021002 to Y.-Z.Y., grant number 52131305 to Y.-Z.Y., grant number 22131010 to C.-Y.H., grant number 52322301 to Z.Z., grant number 52303211 to G.C., grant number 52203196 to X.N. and grant number 51873202 to Y.-Z.Y.), the Fundamental Research Funds for the Central Universities (grant number YD2060002016 to Y.-Z.Y.) and the Postdoctoral Fellowship Program of CPSF (grant number GZC20241622 to W.-Q.H.). We are deeply appreciative of the assistance provided by Y. Guan and Y. Liu from the BL07W beamline at the Hefei Light Source of the National Synchrotron Radiation Laboratory for their assistance in nano-CT and cryo-soft X-ray tomography experiments.
Author information
Authors and Affiliations
Contributions
Y.-Z.Y., W.-Q.H., Y.-Q.Z., L.-H.W., C.-Y.H. and G.C. designed and performed the conceptualization and methodology. F.G., Y.-Q.Z., W.-Q.H., Z.Z., X.N. and G.C. performed the investigation. W.-Q.H., W.Y., Y.-Q.Z., Y.-Z.Y., Q.S. and Z.-Z.W. performed the visualization. Y.-Z.Y., J.X., L.-H.W., G.C. and C.-Y.H. supervised all the experiments. Y.-Z.Y., W.-Q.H., W.Y., Y.-Q.Z. and G.C. wrote the original paper. Y.-Z.Y., L.-H.W., G.C., Z.Z. and C.-Y.H. revised the final paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Xiaoyuan Chen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Characterization of NCAPs.
a, SEM images of PBS-treated and Ti2NX nanodot-treated CT26 cells at 8 h. The experiments were repeated three times. Scale bar, 1 μm. b, SEM images of the control group and NCAP, NCAP escaped from Ti2NX nanodot-treated CT26 cells at 24 h. The experiments were repeated three times. Scale bar in control, 5 μm; Scale bar in Ti2NX group (24 h), 2 μm. c, SEM EDS analysis of NCAP. d, Size distribution of NCAP. e, Three-dimensional tomographic images showing the location of Ti2NX nanodots (40 μg/mL) in 4T1 tumour cells at 8 h, observed by soft transmission X-ray microscopy with nano-CT. f, Ti elemental distribution, determined by dual-energy (465 eV and 455 eV) contrast imaging of soft X-ray protection images. NCAPs were removed from Ti2NX nanodot-treated tumour cells for in situ observation (the concentration of the Ti2NX nanodot was 80 μg/mL). Suspension cells were first deposited onto a nickel EM grid, grown, and then incubated with Ti2NX nanodots. Hydrated NCAPs were directly cryopreserved via plunge freezing for cryo-soft X-ray imaging. The experiments were repeated three times. Scale bar, 200 nm.
Extended Data Fig. 2 The mechanism for Ti2NX nanodots blocking the fusion of autophagosome and lysosome.
a, Representative confocal images of cellular PI4P (marked with an anti-PI4P antibody) and LC3B (marked with an anti-LC3B antibody) in 4T1 cancer cells after 1 h of starvation or 8 h of Ti2NX nanodot treatment. The white arrowheads indicate PI4P-positive autophagosomes. The cells were starved for 1 h with Hank’s balanced salt solution (HBSS). The fluorescence intensity was plotted along the blue line. The experiments were repeated 7 times. b, Analysis of PI4P+ LC3B+ puncta within LC3B+ puncta per cell after different treatments, n = 7. P = 4 × 10−5. c, Representative confocal images of 4T1 tumour cells transfected with the STX17-FLAG lentivirus (MOI = 200) and subjected to various treatments. Under starvation conditions, the tumour cells were incubated in HBSS. For Ti2NX nanodot treatment, the tumour cells were incubated with 80 μg/mL Ti2NX nanodots. The tumour cells were then stained with an anti-FLAG antibody and an anti-LC3B antibody. The experiments were repeated 8 times. Scale bar = 10 µm. Arrows indicate STX17-FLAG+ LC3B+ autophagosomes. d, Quantification of the proportion of punctate structures positive for both STX17 and LC3B on punctate structures positive for LC3B at the indicated times after starvation, n = 8. P = 4 × 10−8. e, 4T1 tumour cells were transfected with SNAP29-FLAG lentivirus (MOI = 300). After different treatments (starvation conditions: tumour cells were incubated in HBSS for 1 h, Ti2NX nanodots: tumour cells were incubated with 80 μg/mL Ti2NX nanodots for 8 h), and tumour cells were stained with an anti-FLAG antibody and an anti-LC3B antibody. Scale bar = 10 µm. Arrows indicate SNAP29-FLAG+ LC3B+ autophagosomes. The experiments were repeated 8 times. f, Quantification of the proportion of punctate structures positive for both SNAP29 and LC3B among the punctate structures positive for LC3B at the indicated times after starvation; n = 8. P = 9 × 10−5. g, Confocal images of the extracellular space of 4T1 cancer cells transfected with STX17-FLAG were incubated with 80 μg/mL Ti2NX nanodots for 24 h and stained with an anti-FLAG antibody and an anti-LC3B antibody. Scale bar = 10 µm. h, Confocal images of the extracellular space of 4T1 tumour cells transfected with SNAP29-FLAG were incubated with 80 μg/mL Ti2NX nanodots for 24 h and stained with anti-FLAG and anti-LC3B antibodies. Scale bar = 10 µm. The experiments of (g) and (h) were repeated 8 times. Error bars represent the mean ± s.d. The data in (b), (d) and (f) were analysed by two-tailed, unpaired Student’s t test.
Extended Data Fig. 3 Allo-NCAP activates immune cells in lymph nodes.
a, and b, Activation of BMDCs after BMDC incubation with Allo-NCAP for 24 h. n = 3 biological replicates. P = 1 × 10−6. c, Proinflammatory cytokine concentrations in BMDC supernatants after incubation with Allo-NCAP for 24 h. d, Allo-NCAP stimulates DC maturation within lymph nodes in vivo. n = 3 biological replicates. e, Lymph node sections after staining with CD11c and CD4 antibodies and DAPI, indicating the numbers and regional distribution of DCs (green) and CD4 T cells (red), respectively. The experiments were repeated three times. Scale bar, 500 μm. f, Statistical analysis of M1-like macrophages (F4/80+ CD80+) in the lymph nodes in vivo. n = 3 biological replicates. g, Representative flow cytometry analysis of M1-like macrophages (F4/80+ CD80+) in the lymph nodes in vivo. h, 4T1 tumour sections after staining with CD206 antibodies and DAPI; M2 macrophages are indicated by CD206. The experiments were repeated three times. Scale bar, 100 μm. Error bars represent the mean ± s.d. The data in (b), (d) and (f) were analysed by two-tailed, unpaired Student’s t test.
Extended Data Fig. 4 Antitumour ability of the Allo-NCAP vaccine.
a, Schematic illustration of the preparations of Con-AP by increasing the pH in lysosome in vitro, and the Allo-NCAPs by Ti2NX nanodots in vitro. b, Tumour growth curves after different treatments in the 4T1 tumour model. n = 7. P = 7 × 10−14. c, Survival curves of mice after various treatments. d, Individual tumour growth curves of the PBS, Con-AP, and Allo-NCAP groups after treatment; n = 7 mice per group. e, Schematic illustration of the experimental design with the luc-4T1 hematogenous metastasis model. f, In vivo bioluminescence imaging of 4T1-luc pulmonary metastases. g, Average tumour burden (photons/second; p/s) of each group on day 17; n = 4 mice per group. h, Inflammatory cytokines in the serum were detected using an ELISA kit. i, Photographs of lung and H&E-stained lung sections (metastatic tumour area, white dash) on day 22. j, Images of lung sections after staining with CD206 and GzmB antibodies and DAPI, CD206 (red) and GzmB (green). The experiments were repeated three times. Scale bar, 50 μm. Error bars represent the mean ± s.d. The data in (b) were analysed by two-way ANOVA, and the data in (g) were analysed by two-tailed Student’s t test.
Extended Data Fig. 5 Systemic i.v. administration of Ti2NX nanodots eliminated established tumours.
a, An orthotopic luc-4T1 murine breast cancer model was established; Ti2NX nanodot injections during intravenous administration were administered on day 0, 3, and 6; in the intratumoural injection group, three tumour-bearing mice were treated with a single injection, while the 4th received a second injection on the 6th day. b, Tumour growth curves after different treatments in the 4T1 tumour model, n = 4. c, In vivo bioluminescence imaging of orthotopic 4T1 murine breast cancer, n = 4. d, Schematic illustration of the experimental design. The 4T1 lung metastatic tumour model was established. e, Quantification of 4T1 metastatic nodules in the lungs; n = 4 mice per group. P = 5 × 10−5. f, Representative photograph of lungs collected from mice after different treatments. g, Images of H&E-stained lungs collected from mice after different treatments; n = 4 mice per group. Error bars represent the mean ± s.d. The data in (e), were analysed by two-tailed, unpaired Student’s t test.
Supplementary information
Supplementary Information
Supplementary Figs. 1–58, Table 1 and discussion.
Source data
Source Data Fig. 2
Unprocessed western blots.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, WQ., You, W., Zhu, YQ. et al. Autophagosomes coated in situ with nanodots act as personalized cancer vaccines. Nat. Nanotechnol. 20, 451–462 (2025). https://doi.org/10.1038/s41565-024-01826-8
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41565-024-01826-8
This article is cited by
-
Targeting phosphatidylserine in tumor cell membranes with a zinc-containing molecule to efficiently combat tumor metastasis
Journal of Nanobiotechnology (2025)