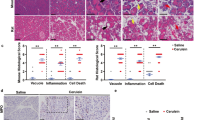
Abstract
Acute pancreatitis (AP) is associated with high mortality rates and is characterized by increased cell death of acinar cells, with the premature release and activation of digestive enzymes. In its acute phase, AP is accompanied by increased efferocytosis, to clear phagocytic apoptotic cells; annexin A1 (Anxa1) is key to efferocytosis, but its role in AP is still unknown. Here we show that Anxa1 deficiency abrogates the efferocytosis of pancreatic macrophages, resulting in the accumulation of apoptotic acinar cells and necrosis. Moreover, we showed that nano-liposomes loaded with Anxa1 mRNA alleviate AP pathology by suppressing the cGAMP-cGAS-STING pathway and restoring efferocytosis in macrophages. Our results reveal the crucial function of Anxa1 in the efferocytosis of macrophages during AP and illustrate a novel nanotechnology treatment approach for AP that may be of potential therapeutic value in humans.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Data supporting the findings of this study are available in the article and its Supplementary Information. Bulk RNA-seq data have been deposited in Gene Expression Omnibus under accession no. GSE249714. The proteomics data have been deposited in iProX database consortium under accession no. IPX0007564002. Source data are provided with this paper.
References
Mederos, M. A., Reber, H. A. & Girgis, M. D. Acute pancreatitis: a review. JAMA 325, 382–390 (2021).
Jaber, S. et al. Guidelines for the management of patients with severe acute pancreatitis, 2021. Anaesth. Crit. Care Pain. Med 41, 101060 (2022).
Banks, P. A. et al. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut 62, 102–111 (2013).
Mareninova, O. A. et al. Cell death in pancreatitis: caspases protect from necrotizing pancreatitis. J. Biol. Chem. 281, 3370–3381 (2006).
Louhimo, J., Steer, M. L. & Perides, G. Necroptosis is an important severity determinant and potential therapeutic target in experimental severe pancreatitis. Cell Mol. Gastroenterol. Hepatol. 2, 519–535 (2016).
Cao, Y., Adhikari, S., Clément, M. V., Wallig, M. & Bhatia, M. Induction of apoptosis by crambene protects mice against acute pancreatitis via anti-inflammatory pathways. Am. J. Pathol. 170, 1521–1534 (2007).
Zhu, Q. et al. The TRIM28/miR133a/CD47 axis acts as a potential therapeutic target in pancreatic necrosis by impairing efferocytosis. Mol. Ther. 32, 3025–3041 (2024).
Tashiro, M., Schäfer, C., Yao, H., Ernst, S. A. & Williams, J. A. Arginine induced acute pancreatitis alters the actin cytoskeleton and increases heat shock protein expression in rat pancreatic acinar cells. Gut 49, 241–250 (2001).
Hoque, R., Malik, A. F., Gorelick, F. & Mehal, W. Z. Sterile inflammatory response in acute pancreatitis. Pancreas 41, 353–357 (2012).
Zhao, Q., Wei, Y., Pandol, S. J., Li, L. & Habtezion, A. STING signaling promotes inflammation in experimental acute pancreatitis. Gastroenterology 154, 1822–1835.e2 (2018).
Sendler, M. et al. NLRP3 inflammasome regulates development of systemic inflammatory response and compensatory anti-inflammatory response syndromes in mice with acute pancreatitis. Gastroenterology 158, 253–269.e14 (2020).
Nagata, S. Apoptosis and clearance of apoptotic cells. Annu Rev. Immunol. 36, 489–517 (2018).
Arandjelovic, S. & Ravichandran, K. S. Phagocytosis of apoptotic cells in homeostasis. Nat. Immunol. 16, 907–917 (2015).
Vago, J. P. et al. Proresolving actions of synthetic and natural protease inhibitors are mediated by annexin A1. J. Immunol. 196, 1922–1932 (2016).
Shen, X., Zhang, S., Guo, Z., Xing, D. & Chen, W. The crosstalk of ABCA1 and ANXA1: a potential mechanism for protection against atherosclerosis. Mol. Med 26, 84 (2020).
McArthur, S. et al. Annexin A1: a central player in the anti-inflammatory and neuroprotective role of microglia. J. Immunol. 185, 6317–6328 (2010).
Tzelepis, F. et al. Annexin1 regulates DC efferocytosis and cross-presentation during Mycobacterium tuberculosis infection. J. Clin. Invest. 125, 752–768 (2015).
da Rocha, G. H. O. et al. Control of expression and activity of peroxisome proliferated-activated receptor γ by annexin A1 on microglia during efferocytosis. Cell Biochem. Funct. 37, 560–568 (2019).
Sun, L., Wu, J., Du, F., Chen, X. & Chen, Z. J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339, 786–791 (2013).
Gao, D. et al. Cyclic GMP-AMP synthase is an innate immune sensor of HIV and other retroviruses. Science 341, 903–906 (2013).
Wu, J. et al. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science 339, 826–830 (2013).
Wang, Z. et al. Development and applications of mRNA treatment based on lipid nanoparticles. Biotechnol. Adv. 65, 108130 (2023).
Patel, S. K. et al. Hydroxycholesterol substitution in ionizable lipid nanoparticles for mRNA delivery to T cells. J. Control. Release 347, 521–532 (2022).
Bitounis, D., Jacquinet, E., Rogers, M. A. & Amiji, M. M. Strategies to reduce the risks of mRNA drug and vaccine toxicity. Nat. Rev. Drug Discov. 23, 281–300 (2024).
Kon, E., Ad-El, N., Hazan-Halevy, I., Stotsky-Oterin, L. & Peer, D. Targeting cancer with mRNA-lipid nanoparticles: key considerations and future prospects. Nat. Rev. Clin. Oncol. 20, 739–754 (2023).
Kumari, A., Kaur, A. & Aggarwal, G. The emerging potential of siRNA nanotherapeutics in treatment of arthritis. Asian J. Pharm. Sci. 18, 100845 (2023).
Li, Z. et al. Synergistic dual cell therapy for atherosclerosis regression: ROS-responsive bio-liposomes co-loaded with geniposide and emodin. J. Nanobiotechnol. 22, 129 (2024).
Nasra, S., Bhatia, D. & Kumar, A. Targeted macrophage re-programming: synergistic therapy with methotrexate and RELA siRNA folate-liposome in RAW264.7 cells and arthritic rats. Adv. Health. Mater. 13, e2400679 (2024).
Ma, J., Ding, L., Peng, X., Jiang, L. & Liu, G. Recent advances of engineered cell membrane-based nanotherapeutics to combat inflammatory diseases. Small 20, e2308646 (2024).
Dai, Q. et al. Quantifying the ligand-coated nanoparticle delivery to cancer cells in solid tumors. ACS Nano 12, 8423–8435 (2018).
Yan, H. et al. Engineering cell membrane-based nanotherapeutics to target inflammation. Adv. Sci. 6, 1900605 (2019).
Melendez, E. et al. Natural killer cells act as an extrinsic barrier for in vivo reprogramming. Development 149, dev200361 (2022).
Wu, J. et al. Macrophage phenotypic switch orchestrates the inflammation and repair/regeneration following acute pancreatitis injury. eBioMedicine 58, 102920 (2020).
Manohar, M. et al. Novel circulating and tissue monocytes as well as macrophages in pancreatitis and recovery. Gastroenterology 161, 2014–2029 (2021).
Wu, L. et al. Annexin A1 alleviates kidney injury by promoting the resolution of inflammation in diabetic nephropathy. Kidney Int. 100, 107–121 (2021).
Liu, X. et al. USP25 deficiency exacerbates acute pancreatitis via up-regulating TBK1-NF-κB signaling in macrophages. Cell Mol. Gastroenterol. Hepatol. 14, 1103–1122 (2022).
Wan, J. C. M. et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat. Rev. Cancer 17, 223–238 (2017).
Maltbaek, J. H., Cambier, S., Snyder, J. M. & Stetson, D. B. ABCC1 transporter exports the immunostimulatory cyclic dinucleotide cGAMP. Immunity 55, 1799–1812 (2022).
Zhou, Y. et al. Blockade of the phagocytic receptor MerTK on tumor-associated macrophages enhances P2X7R-dependent STING activation by tumor-derived cGAMP. Immunity 52, 357–373.e9 (2020).
Lee, G. Y. et al. Hyaluronic acid nanoparticles for active targeting atherosclerosis. Biomaterials 53, 341–348 (2015).
Gao, C. et al. Treatment of atherosclerosis by macrophage-biomimetic nanoparticles via targeted pharmacotherapy and sequestration of proinflammatory cytokines. Nat. Commun. 11, 2622 (2020).
Zhou, M. et al. Targeted delivery of hyaluronic acid-coated solid lipid nanoparticles for rheumatoid arthritis therapy. Drug Deliv. 25, 716–722 (2018).
Sousa de Almeida, M. et al. Understanding nanoparticle endocytosis to improve targeting strategies in nanomedicine. Chem. Soc. Rev. 50, 5397–5434 (2021).
Pan, Y. et al. Synergistic inhibition of pancreatic cancer with anti-PD-L1 and c-Myc inhibitor JQ1. Oncoimmunology 8, e1581529 (2019).
Pan, Y. et al. Development of a novel model of hypertriglyceridemic acute pancreatitis in mice. Sci. Rep. 7, 40799 (2017).
Sendler, M. et al. Cathepsin B-mediated activation of trypsinogen in endocytosing macrophages increases severity of pancreatitis in mice. Gastroenterology 154, 704–718.e10 (2018).
Deng, L. et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity 41, 843–852 (2014).
Ahn, J., Xia, T., Rabasa Capote, A., Betancourt, D. & Barber, G. N. Extrinsic phagocyte-dependent STING signaling dictates the immunogenicity of dying cells. Cancer Cell 33, 862–873.e5 (2018).
Ablasser, A. et al. Cell intrinsic immunity spreads to bystander cells via the intercellular transfer of cGAMP. Nature 503, 530–534 (2013).
Luteijn, R. D. et al. SLC19A1 transports immunoreactive cyclic dinucleotides. Nature 573, 434–438 (2019).
Zhou, C. et al. Transfer of cGAMP into bystander cells via LRRC8 volume-regulated anion channels augments STING-mediated interferon responses and anti-viral immunity. Immunity 52, 767–781.e6 (2020).
Kosicka, A. et al. Attenuation of plasma annexin A1 in human obesity. FASEB J. 27, 368–378 (2013).
De Ponti, F. F. & Scott, C. L. Modulating hepatic macrophages with annexin A1 in non-alcoholic steatohepatitis. Clin. Sci. 136, 1111–1115 (2022).
Locatelli, I. et al. Endogenous annexin A1 is a novel protective determinant in nonalcoholic steatohepatitis in mice. Hepatology 60, 531–544 (2014).
Morioka, S. et al. Efferocytosis induces a novel SLC program to promote glucose uptake and lactate release. Nature 563, 714–718 (2018).
Pan, Y. et al. Single-cell RNA sequencing reveals compartmental remodeling of tumor-infiltrating immune cells induced by anti-CD47 targeting in pancreatic cancer. J. Hematol. Oncol. 12, 124 (2019).
Chondronasiou, D. et al. Deciphering the roadmap of in vivo reprogramming toward pluripotency. Stem Cell Rep. 17, 2501–2517 (2022).
He, R. et al. SULF2 enhances GDF15-SMAD axis to facilitate the initiation and progression of pancreatic cancer. Cancer Lett. 538, 215693 (2022).
Li, L. et al. Integrative proteogenomic characterization of early esophageal cancer. Nat. Commun. 14, 1666 (2023).
Hamilton, A. G. et al. Ionizable lipid nanoparticles with integrated immune checkpoint inhibition for mRNA CAR T cell engineering. Adv. Health. Mater. 12, e2301515 (2023).
Flores, A. M. et al. Pro-efferocytic nanoparticles are specifically taken up by lesional macrophages and prevent atherosclerosis. Nat. Nanotechnol. 15, 154–161 (2020).
Acknowledgements
This study was supported by the Joint Funds for the innovation of Science and Technology, Fujian province (2024Y9277 to Y.P.), Excellent Young Scholars Cultivation Project of Fujian Medical University Union Hospital (2022XH025 to Y.P.) and National Natural Science Foundation of China (no. 82103310 to Y.P.). J.C. acknowledges the Fundação Ciência e Tecnologia, IP national support, through UID/04923 - Comprehensive Health Research Centre.
Author information
Authors and Affiliations
Contributions
H.F.: methodology, data analysis and investigation. P.Y.: software, methodology, data analysis and investigation. S.L.: methodology, data analysis, investigation and writing—original draft. Y.W.: investigation, methodology, data analysis and methodology. J.L.: methodology, investigation, software and data analysis. Z.H.: conceptualization, methodology and investigation. F. Liang: methodology, investigation and data analysis. C.C.: methodology and investigation. Z.W.: methodology and investigation. L.C.: methodology and investigation. S.Z.: methodology and investigation. X.C.: methodology and investigation. K.Z.: methodology and data analysis. F. Lu: methodology and investigation. M.P.: data analysis, methodology, paper editing and revision. Y.Z.: paper editing and revision. C.Y.: conceptualization, methodology, investigation and writing—original draft. J.C.: conceptualization, methodology, investigation and writing—original draft. H.H.: conceptualization, methodology, investigation and writing—original draft. Y.P.: conceptualization, methodology, data analysis, investigation, writing—original draft—and revision. All authors have given approval to the final version of the paper.
Corresponding authors
Ethics declarations
Competing interests
J.C. is a co-founder and shareholder of TargTex S.A. Targeted therapeutics for Glioblastoma Multiforme. J.C. is also a member of the Global Burden Disease (GBD) consortium from Institute for Health Metrics and Evaluation (IHME), University of Washington. Y.P., F. Liang and P.Y. are authors of a provisional patent (202411349874.8, China) based on this paper. The other authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Mansoor Amiji and Matthias Sendler for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Myeloid Anxa1 expression in the mouse pancreas during AP.
(a) RNA-seq of pancreas in AP mice with different time points. (b) Anxa1 mRNA expression in the pancreas in AP mice at different time points. * indicates compared with control; # indicates compared with 24 h; NS, not significant as compared with control. (c) Monocyte abundance of pancreas in AP mice with different time points. * indicates compared with control; (d) Macrophage abundance in the pancreas in AP mice at different time points. * indicates compared with control; NS, not significant as compared with control. (e) Correlation between monocyte/macrophage abundance and the expression of Anxa1. (f) UMAP plot showing 6 immune cell clusters in the pancreas of AP mice. (g) Fraction of 6 immune cell clusters in each sample. (h) Dot plot depicting percent expression and average expression of Anxa1 in CER and control groups. (i) Flow cytometry analysis of Anxa1 expression in CD45+F4/80+ cells from the mouse pancreas during AP and recovery. *P < 0.01 as compared with 0 h; #P < 0.05 as compared with 12 h; NS, not significant as compared with 0 h. (j) Flow cytometry analysis of Anxa1 expression in CD45+F4/80+ and CD45+F4/80− cells from CER mice. NS, not significant as compared with control. Data are presented as means ± SD of n = 3 (b–d), n = 5 (i), or n = 10 (j) biologically independent samples per group. Data are presented as means ± SD of n ≥ 3 (b–d), n = 5 (i), or n = 10 (j) biologically independent samples per group. P values were determined by one-way ANOVA with Dunnett’s post-hoc test (b–d), Spearman’s correlation (e), unpaired two-tailed Student’s t-test (j) or one-way ANOVA with Tukey’s post-hoc test (i). Illustrations in a created with Figdraw.com.
Extended Data Fig. 2 The type I IFN response induced by Anxa1-deficient mice requires ATP-binding cassette transporter ABCG2.
(a) Scheme for KO-143 treatment of Anxa1CKO AP mice induced with 7 hourly injections of cerulein. (b) Pancreas histology scores of PBS- and KO-143-treated saline-control (Control) or AP mice. (c) Immunoblot analysis of ABCG2, c-Casp3, IFN-β, p-TBK1, TBK1, p-IRF3, IRF3, p-P65, and P65 in pancreas tissues of PBS- and KO-143-treated AP mice. (d) Anxa1CKO Abcg+/+ and Anxa1CKO Abcg2−/− mice were induced with 7 hourly injections of cerulein. (e) Immunoblot analysis of ABCG2, c-Casp3, IFN-β, p-TBK1, TBK1, p-IRF3, IRF3, p-P65, and P65 in pancreas tissues of cerulein-treated (AP) Anxa1CKO Abcg+/+ and Anxa1CKO Abcg2−/− mice. (f) Pancreas histology scores for Anxa1CKO Abcg+/+ and Anxa1CKO Abcg2−/−, saline-treated (NaCl) or AP mice. (g) Schematic sketch. Up: Anxa1 (A1) binds with high affinity to phosphatidylserine (PS) on the surface of apoptotic cells in a calcium (Ca+)-dependent manner, and contributes to the engulfment of apoptotic cells by efferocytosis of macrophages. Down: The deficiency of Anxa1 in macrophages impairs the clearance of apoptotic cells, triggering increased cGAMP production in the apoptotic or necrotic acinar cells which in turn enters the macrophages through ABCG2 transporter and activates STING/TBK1/NFκB signaling to induce the production of type I IFN. Data are presented as means ± SD of n = 5 (b, d) biologically independent samples per group. P values were determined by one-way ANOVA with Tukey’s post-hoc test (B.D). Illustrations in a and d created with Figdraw.com.
Extended Data Fig. 3 Characterization and targeting capability of HA-M@Lip@Anxa1 NPs.
(a) The mRNA contents per µg for Lip@Anxa1 NPs at the feeding different ration (mRNA:phosphatidylcholine). (b) TEM of HA-M@Lip@Anxa1 NPs. Scale bars = 100 nm. (c) Hydrodynamic size distribution of different NPs. (d) ζ-potential measurement of different NPs. (e) Representative images of CLSM and (f) quantification analysis of RAW 264.7 cells with nuclei staining by Hoechst 33342 incubated with Lip NPs, M@Lip NPs, and HA-M@Lip NPs for 4 h after activation by LPS (+) or not. Inflammatory macrophages were treated with HA solution and HA-M@Lip NPs. Scale bars = 60 μm. (g) The schematic diagram showed that acinar cells were co-cultured with inflammatory macrophages in a transwell system for simulating plaque in vitro. (h) Phagocytosis of HA-M@Lip NPs in acinar cells and inflammatory macrophages in transwell. BF indicates bright field. Scale bars = 60μm. (i) Fluorescence quantitation of HA-M@Lip NPs (red). (j) RAW 264.7 cells without any treatment were used as control. Adding LPS as the model group. Western blot assay of Anxa1 expression in macrophages (LPS+) treatment with Anxa1, M@Lip@Anxa1, HA-M@Lip@Anxa1, and Lip@Anxa1. (K&L) Fluorescence images and quantitative analysis of mice after being treated with different formulations. ***P < 0.001. Data are presented as means ± SD of n = 3 biologically independent samples (a, d, f, i, j, l). P values were determined by unpaired two-tailed Student’s t-test (i) or one-way ANOVA with Tukey’s post-hoc test (f, l).
Extended Data Fig. 4 Myeloid Anxa1 deficiency exacerbates SAP.
(a) Pancreas histology scores in Anxa1f/f and Anxa1CKO mice that were saline-treated (NaCl group) or L-arginine-treated (SAP group). The proportion of apoptotic (b) and necrotic cells (c) in pancreas tissues. Pancreas IFN-β (d), TNF-α (e), IL-6 (f), and MCP-1 (g) in Anxa1f/f and Anxa1CKO NaCl or SAP mice. (h) (a) Representative immunoblot analysis of TLR4, STING, p-TBK1, TBK1, p-P65, P65, and NLRP3 in pancreas tissues from Anxa1f/f SAP and Anxa1CKO SAP mice. HA-M@Lip@Anxa1 NPs treatment of SAP mice induced with injection of L-arginine. The pancreas pathology score (i), phagocytic index (j), pancreas IFN-β (k), TNF-α (l), IL-6 (m), and MCP-1 (n) in phosphate buffer saline (PBS) and HA-M@Lip@Anxa1 NPs-treated saline control (Con) and SAP mice. (o) Immunoblot analysis of TLR4, STING, p-TBK1, TBK1, p-P65, P65, and NLRP3 in pancreas tissues of SAP mice after receiving PBS or HA-M@Lip@Anxa1 NPs treatment. Data are presented as means ± SD of n = 5 biologically independent samples (a–g, i–n). P values are determined by unpaired two-tailed Student’s t-test (b–g, j–n) or one-way ANOVA with Tukey’s post-hoc test (a and i).
Supplementary information
Supplementary Information
Supplementary Figs. 1–29, Tables 1–3 and Methods.
Supplementary Table 1
Source data for Supplementary figures.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Unprocessed western blots.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Unprocessed western blots.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Unprocessed western blots.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Unprocessed western blots.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Unprocessed western blots.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Unprocessed western blots.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Unprocessed western blots.
Source Data Extended Data Fig. 4
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fang, H., You, P., Lin, S. et al. Annexin A1 mRNA-loaded liposomes alleviate acute pancreatitis by suppressing STING pathway and promoting efferocytosis in macrophages. Nat. Nanotechnol. 20, 1514–1525 (2025). https://doi.org/10.1038/s41565-025-01979-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41565-025-01979-0