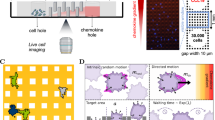
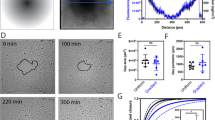
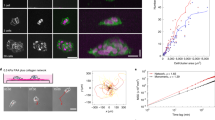
Abstract
Haptotaxis is the process of directed cell migration along gradients of extracellular matrix density and is central to morphogenesis, immune responses and cancer invasion. It is commonly assumed that cells respond to these gradients by migrating directionally towards the regions of highest ligand density. In contrast with this view, here we show that cells exposed to micropatterned fibronectin gradients exhibit a wide range of complex trajectories, including directed haptotactic migration up the gradient but also linear oscillations and circles with extended periods of migration down the gradient. To explain this behaviour, we developed a biophysical model of haptotactic cell migration based on a coarse-grained molecular clutch model coupled to persistent stochastic polarity dynamics. Although initial haptotactic migration is explained by the differential friction at the front and back of the cell, the observed complex trajectories over longer timescales arise from the interplay between differential friction, persistence and physical confinement. Overall, our study reveals that confinement and persistence modulate the ability of cells to sense and respond to haptotactic cues and provides a framework for understanding how cells navigate complex environments.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available via the CORA Research Data Repository at https://doi.org/10.34810/data2489.
Code availability
All analysis procedures and codes are available from the corresponding authors. The code for the model is available via GitHub at https://github.com/dbrueckner/Haptotaxis.
References
Van Helvert, S., Storm, C. & Friedl, P. Mechanoreciprocity in cell migration. Nat. Cell Biol. 20, 8–20 (2018).
Barriga, E. H. & Theveneau, E. In vivo neural crest cell migration is controlled by ‘mixotaxis’. Front. Physiol. 11, 586432 (2020).
SenGupta, S., Parent, C. A. & Bear, J. E. The principles of directed cell migration. Nat. Rev. Mol. Cell Biol. 22, 529–547 (2021).
Fortunato, I. C. & Sunyer, R. The forces behind directed cell migration. Biophysica 2, 548–563 (2022).
Janetopoulos, C. & Firtel, R. A. Directional sensing during chemotaxis. FEBS Lett. 582, 2075–2085 (2008).
Swaney, K. F., Huang, C.-H. & Devreotes, P. N. Eukaryotic chemotaxis: a network of signaling pathways controls motility, directional sensing, and polarity. Annu. Rev. Biophys. 39, 265–289 (2010).
Carter, S. B. Principles of cell motility: the direction of cell movement and cancer invasion. Nature 208, 1183–1187 (1965).
Carter, S. B. Haptotaxis and the mechanism of cell motility. Nature 213, 256–260 (1967).
Dertinger, S. K. W., Jiang, X., Li, Z., Murthy, V. N. & Whitesides, G. M. Gradients of substrate-bound laminin orient axonal specification of neurons. Proc. Natl Acad. Sci. USA 99, 12542–12547 (2002).
DeLong, S. A., Gobin, A. S. & West, J. L. Covalent immobilization of RGDS on hydrogel surfaces to direct cell alignment and migration. J. Control. Release 109, 139–148 (2005).
Lu, J. et al. Pancreatic stellate cells promote hapto-migration of cancer cells through collagen I-mediated signalling pathway. Br. J. Cancer 110, 409–420 (2014).
Wen, J. H. et al. Haptotaxis is cell type specific and limited by substrate adhesiveness. Cell. Mol. Bioeng. 8, 530–542 (2015).
Moreno-Arotzena, O., Borau, C., Movilla, N., Vicente-Manzanares, M. & García-Aznar, J. M. Fibroblast migration in 3D is controlled by haptotaxis in a non-muscle myosin II-dependent manner. Ann. Biomed. Eng. 43, 3025–3039 (2015).
Autenrieth, T. J. et al. Actomyosin contractility and RhoGTPases affect cell-polarity and directional migration during haptotaxis. Integr. Biol. 8, 1067–1078 (2016).
King, S. J. et al. Lamellipodia are crucial for haptotactic sensing and response. J. Cell Sci. 129, 2329–2342 (2016).
Luo, X. et al. Lymphocytes perform reverse adhesive haptotaxis mediated by LFA-1 integrins. J. Cell Sci. 133, jcs242883 (2020).
Weber, M. et al. Interstitial dendritic cell guidance by haptotactic chemokine gradients. Science 339, 328–332 (2013).
Oudin, M. J. et al. Tumor cell–driven extracellular matrix remodeling drives haptotaxis during metastatic progression. Cancer Discov. 6, 516–531 (2016).
Schwarz, J. et al. Dendritic cells interpret haptotactic chemokine gradients in a manner governed by signal-to-noise ratio and dependent on GRK6. Curr. Biol. 27, 1314–1325 (2017).
Roca-Cusachs, P., Sunyer, R. & Trepat, X. Mechanical guidance of cell migration: lessons from chemotaxis. Curr. Opin. Cell Biol. 25, 543–549 (2013).
Chan, C. E. & Odde, D. J. Traction dynamics of filopodia on compliant substrates. Science 322, 1687–1691 (2008).
Elosegui-Artola, A., Trepat, X. & Roca-Cusachs, P. Control of mechanotransduction by molecular clutch dynamics. Trends Cell Biol. 28, 356–367 (2018).
Kechagia, J. Z., Ivaska, J. & Roca-Cusachs, P. Integrins as biomechanical sensors of the microenvironment. Nat. Rev. Mol. Cell Biol. 20, 457–473 (2019).
Lin, C.-H. & Forscher, P. Growth cone advance is inversely proportional to retrograde F-actin flow. Neuron 14, 763–771 (1995).
Gardel, M. L. et al. Traction stress in focal adhesions correlates biphasically with actin retrograde flow speed. J. Cell Biol. 183, 999–1005 (2008).
Bangasser, B. L. et al. Shifting the optimal stiffness for cell migration. Nat. Commun. 8, 15313 (2017).
Elosegui-Artola, A. et al. Rigidity sensing and adaptation through regulation of integrin types. Nat. Mater. 13, 631–637 (2014).
Elosegui-Artola, A. et al. Mechanical regulation of a molecular clutch defines force transmission and transduction in response to matrix rigidity. Nat. Cell Biol. 18, 540–548 (2016).
Camley, B. A. et al. Polarity mechanisms such as contact inhibition of locomotion regulate persistent rotational motion of mammalian cells on micropatterns. Proc. Natl Acad. Sci. USA 111, 14770–14775 (2014).
Maiuri, P. et al. Actin flows mediate a universal coupling between cell speed and cell persistence. Cell 161, 374–386 (2015).
Ron, J. E., Monzo, P., Gauthier, N. C., Voituriez, R. & Gov, N. S. One-dimensional cell motility patterns. Phys. Rev. Res. 2, 033237 (2020).
Ricoult, S. G., Kennedy, T. E. & Juncker, D. Substrate-bound protein gradients to study haptotaxis. Front. Bioeng. Biotechnol. 3, 40 (2015).
Song, J. et al. A microfluidic device for studying chemotaxis mechanism of bacterial cancer targeting. Sci. Rep. 8, 6394 (2018).
Versaevel, M. et al. Collective migration during a gap closure in a two-dimensional haptotactic model. Sci. Rep. 11, 5811 (2021).
Hu, P. et al. Bacterial chemotaxis in static gradients quantified in a biopolymer membrane-integrated microfluidic platform. Lab Chip 22, 3203–3216 (2022).
Mallet, D. G. & Pettet, G. J. A mathematical model of integrin-mediated haptotactic cell migration. Bull. Math. Biol. 68, 231–253 (2006).
Wössner, V., Drozdowski, O. M., Ziebert, F. & Schwarz, U. S. Active gel model for one-dimensional cell migration coupling actin flow and adhesion dynamics. New J. Phys. 26, 073039 (2024).
Palamidessi, A. et al. Unjamming overcomes kinetic and proliferation arrest in terminally differentiated cells and promotes collective motility of carcinoma. Nat. Mater. 18, 1252–1263 (2019).
Cai, G. et al. Compressive stress drives adhesion-dependent unjamming transitions in breast cancer cell migration. Front. Cell Dev. Biol. 10, 933042 (2022).
Gilles, C. et al. Vimentin contributes to human mammary epithelial cell migration. J. Cell Sci. 112, 4615–4625 (1999).
Gross, S. M. et al. A multi-omic analysis of MCF10A cells provides a resource for integrative assessment of ligand-mediated molecular and phenotypic responses. Commun. Biol. 5, 1066 (2022).
Sunyer, R. et al. Collective cell durotaxis emerges from long-range intercellular force transmission. Science 353, 1157–1161 (2016).
d’Alessandro, J. et al. Cell migration guided by long-lived spatial memory. Nat. Commun. 12, 4118 (2021).
Baschieri, F. et al. Fibroblasts generate topographical cues that steer cancer cell migration. Sci. Adv. 9, eade2120 (2023).
Schreiber, C., Amiri, B., Heyn, J. C. J., Rädler, J. O. & Falcke, M. On the adhesion–velocity relation and length adaptation of motile cells on stepped fibronectin lanes. Proc. Natl Acad. Sci. USA 118, e2009959118 (2021).
Palecek, S. P., Loftus, J. C., Ginsberg, M. H., Lauffenburger, D. A. & Horwitz, A. F. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature 385, 537–540 (1997).
DiMilla, P. A., Barbee, K. & Lauffenburger, D. A. Mathematical model for the effects of adhesion and mechanics on cell migration speed. Biophys. J. 60, 15–37 (1991).
Tanimoto, H. & Sano, M. A simple force-motion relation for migrating cells revealed by multipole analysis of traction stress. Biophys. J. 106, 16–25 (2014).
Hennig, K. et al. Stick-slip dynamics of cell adhesion triggers spontaneous symmetry breaking and directional migration of mesenchymal cells on one-dimensional lines. Sci. Adv. 6, eaau5670 (2020).
Rossetti, L. et al. Optogenetic generation of leader cells reveals a force–velocity relation for collective cell migration. Nat. Phys. 20, 1659–1669 (2024).
Sens, P. Stick–slip model for actin-driven cell protrusions, cell polarization, and crawling. Proc. Natl Acad. Sci. USA 117, 24670–24678 (2020).
Brückner, D. B. et al. Stochastic nonlinear dynamics of confined cell migration in two-state systems. Nat. Phys. 15, 595–601 (2019).
Brückner, D. B. & Broedersz, C. P. Learning dynamical models of single and collective cell migration: a review. Rep. Prog. Phys. 87, 056601 (2024).
Skoge, M. et al. Cellular memory in eukaryotic chemotaxis. Proc. Natl Acad. Sci. USA 111, 14448–14453 (2014).
Hamza, B. et al. Retrotaxis of human neutrophils during mechanical confinement inside microfluidic channels. Integr. Biol. 6, 175–183 (2014).
Isomursu, A. et al. Directed cell migration towards softer environments. Nat. Mater. 21, 1081–1090 (2022).
Shellard, A. et al. Frictiotaxis underlies focal adhesion-independent durotaxis. Nat. Commun. 16, 3811 (2025).
Raab, M. et al. Crawling from soft to stiff matrix polarizes the cytoskeleton and phosphoregulates myosin-II heavy chain. J. Cell Biol. 199, 669–683 (2012).
Mahdisoltani, S., Zinati, R. B. A., Duclut, C., Gambassi, A. & Golestanian, R. Nonequilibrium polarity-induced chemotaxis: emergent Galilean symmetry and exact scaling exponents. Phys. Rev. Res. 3, 013100 (2021).
Elgeti, J., Winkler, R. G. & Gompper, G. Physics of microswimmers—single particle motion and collective behavior: a review. Rep. Prog. Phys. 78, 056601 (2015).
Lee, G., Hynes, R. & Kirschner, M. Temporal and spatial regulation of fibronectin in early Xenopus development. Cell 36, 729–740 (1984).
Davidson, L. A., Hoffstrom, B. G., Keller, R. & DeSimone, D. W. Mesendoderm extension and mantle closure in Xenopus laevis gastrulation: combined roles for integrin α5β1, fibronectin, and tissue geometry. Dev. Biol. 242, 109–129 (2002).
Zhu, M. et al. A fibronectin gradient remodels mixed-phase mesoderm. Sci. Adv. 10, eadl6366 (2024).
Nia, H. T., Munn, L. L. & Jain, R. K. Physical traits of cancer. Science 370, eaaz0868 (2020).
Acerbi, I. et al. Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integr. Biol. 7, 1120–1134 (2015).
Plodinec, M. et al. The nanomechanical signature of breast cancer. Nat. Nanotechnol. 7, 757–765 (2012).
Wynn, T. A. & Ramalingam, T. R. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat. Med. 18, 1028–1040 (2012).
Ortiz, C. et al. Extracellular matrix remodeling in chronic liver disease. Curr. Tissue Microenviron. Rep. 2, 41–52 (2021).
Mongera, A. et al. Mechanics of the cellular microenvironment as probed by cells in vivo during zebrafish presomitic mesoderm differentiation. Nat. Mater. 22, 135–143 (2023).
Ilina, O. et al. Cell–cell adhesion and 3D matrix confinement determine jamming transitions in breast cancer invasion. Nat. Cell Biol. 22, 1103–1115 (2020).
Saraswathibhatla, A., Indana, D. & Chaudhuri, O. Cell–extracellular matrix mechanotransduction in 3D. Nat. Rev. Mol. Cell Biol. 24, 495–516 (2023).
Shatkin, G., Yeoman, B., Birmingham, K., Katira, P. & Engler, A. J. Computational models of migration modes improve our understanding of metastasis. APL Bioeng. 4, 041505 (2020).
Larsen, A. M. H. et al. Collagen density modulates the immunosuppressive functions of macrophages. J. Immunol. 205, 1461–1472 (2020).
Zhu, P. et al. Targeted mechanical forces enhance the effects of tumor immunotherapy by regulating immune cells in the tumor microenvironment. Cancer Biol. Med. 20, 44–55 (2023).
Latorre, E. et al. Active superelasticity in three-dimensional epithelia of controlled shape. Nature 563, 203–208 (2018).
Strale, P. et al. Multiprotein printing by light‐induced molecular adsorption. Adv. Mater. 28, 2024–2029 (2016).
Hornung, A. et al. A bistable mechanism mediated by integrins controls mechanotaxis of leukocytes. Biophys. J. 118, 565–577 (2020).
Ershov, D. et al. TrackMate 7: integrating state-of-the-art segmentation algorithms into tracking pipelines. Nat. Methods 19, 829–832 (2022).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Butler, J. P., Tolić-Nørrelykke, I. M., Fabry, B. & Fredberg, J. J. Traction fields, moments, and strain energy that cells exert on their surroundings. Am. J. Physiol. Cell Physiol. 282, C595–C605 (2002).
Trepat, X. et al. Physical forces during collective cell migration. Nat. Phys. 5, 426–430 (2009).
Serra-Picamal, X., Conte, V., Sunyer, R., Muñoz, J. J. & Trepat, X. Mapping forces and kinematics during collective cell migration. Methods Cell. Biol. 125, 309–330 (2015).
Acknowledgements
We thank all the members of our groups for discussions and support. We thank A. Menéndez, S. Usieto, M. Purciolas and E. Coderch for technical assistance. We thank G. Charras (London Centre for Nanotechnology, UK) and M. Sheetz (Columbia University, USA) for sharing cells used in this work. We thank J. Ivaska (University of Turku, Finland) for sharing integrin α5-GFP DNA plasmid. We thank P. Guillamat for technical advice and A. Labernardie for providing the microfluidic channels. We thank M. Gómez-González for sharing the 2D traction microscopy algorithm. Finally, we thank P. Guillamat, J. Abenza, G. Ceada, L. Faure, E. Dalaka, M. Matejčić, A. Beedle, I. Granero, O. Baguer, A. Albajar and N. Chahare for discussions. This paper was funded by the Generalitat de Catalunya (Grant Nos. AGAUR SGR-2017-01602 to X.T. and 2021 SGR 00523 to R.S. and the CERCA Programme and ICREA Academia awards to P.R.-C.), the Spanish Ministry for Science and Innovation MICCINN/FEDER (Grant Nos. PID2021-128635NB-I00, MCIN/AEI/10.13039/501100011033 and ERDF-EU A way of making Europe to X.T., PID2021-128674OB-I00 and CNS2022-135533 to R.S. and PID2019-110298GB-I00 to P.R.-C.), the European Research Council (Grant Nos. 101097753 to P.R.-C. and Adv-883739 to X.T.), Fundació la Marató de TV3 (Project Award 201903-30-31-32 to X.T.), the European Commission (Grant No. H2020-FETPROACT-01-2016-731957 to P.R.-C. and X.T.) and La Caixa Foundation (Grant No. LCF/PR/HR20/52400004 to P.R.-C. and X.T.). R.S. is a Serra-Hunter fellow. D.B.B. was supported by the NOMIS foundation as a NOMIS fellow, by the European Molecular Biology Organization (Postdoctoral Fellowship ALTF 343-2022) and by the Austrian Academy of Sciences through an APART-MINT Fellowship. I.C.F. acknowledges support from the European Foundation for the Study of Chronic Liver Failure. IBEC is recipient of a Severo Ochoa Award of Excellence from MINECO.
Author information
Authors and Affiliations
Contributions
R.S., X.T. and I.C.F. conceived of the project. R.S. and X.T. supervised the project. I.C.F. and R.S. developed the haptotactic system. I.C.F., R.S. and X.T. designed the experiments. I.C.F. performed all the experiments, except those involving MEFs and soft PDMS without beads. R.N. performed the experiments with MEFs and soft PDMS without beads. R.S., I.C.F., S.G., L.R. and M.B.-P. developed the computational analysis tools. I.C.F. processed the experimental data. I.C.F., S.G., R.S. and D.B.B. analysed the data. D.B.B. and E.H. developed the theoretical model. D.B.B. implemented and performed the simulations. J.T. and P.R.-C. contributed technical expertise and materials and to the discussion. X.T., I.C.F., R.S., D.B.B. and E.H. wrote the paper. All authors participated in revising the completed paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Physics thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Experimental setup.
a, Illustration of micropatterning soft elastomeric substrates by light-induced molecular adsorption of proteins (LIMAP) using PRIMO system. b, Median fibronectin density of 1D gradients. Error bars are 25 and 75 percentiles. N = 173. c, Fibronectin density profile of low (N = 71), mid (N = 58) and high (N = 44) 1D gradients. d, Maximum fibronectin density of cells migrating on low (N = 71), mid (N = 58) and high (N = 44) 1D gradients. Error bars are s.d. P values obtained using two-sided Mann-Whitney test. **** indicates P < 0.0001. For panels c-d, low, mid and high magnitudes have mean fibronectin density of 24.293 ± 10.244 ng/cm2, 46.784 ± 12.82 ng/cm2 and 67.579 ± 20.613 ng/cm2, respectively. Data were obtained from four independent experiments.
Extended Data Fig. 2 Examples of full cell trajectories.
25 full trajectories representative of N = 173 cells migrating on 1D gradients.
Extended Data Fig. 3 Cell deposition during migration.
a, Example of protein deposition using MCF10A cells overexpressing α5 integrin-GFP. White arrow indicates the deposited tail. b, Example of membrane deposition using MCF10A cells labelled with Flipper-TR probe (Tebu-bio, SC020). White arrow indicates the deposited tail. c, Mean velocity in x for the 1st, 2nd and 3rd runs of cells migrating up and down 1D gradients. Error bars are s.d. Significant difference between groups was identified using the Kruskal-Wallis test. To determine specific pairwise differences, Dunn’s multiple comparisons test was applied. The reported P values correspond to Dunn’s test. **** indicates P < 0.0001. Immunofluorescence data were obtained from one independent experiment. Velocity data were obtained from four independent experiments. Scale bars, 10 μm (a), 5 μm (b).
Extended Data Fig. 4 Haptotactic response remains consistent on glass substrates and substrates without beads.
a, First-run trajectories of cells migrating on 1D gradients patterned on glass substrates (N = 79). Colour coding represents haptotactic (blue, N = 61) and anti-haptotactic (yellow, N = 18) cells. b, First-run trajectories of cells migrating on 1D gradients patterned on soft PDMS substrates that did not contain fluorescence beads for traction microscopy (N = 81). Colour coding represents haptotactic (blue, N = 62) and anti-haptotactic (yellow, N = 19) cells. Data on glass substrates were obtained from one independent experiment. Data on substrates without beads were obtained from two independent experiments.
Extended Data Fig. 5 Migration and traction forces on homogenous patterns.
a, Representative fluorescence image of 1D homogeneous patterns with 20%, 40%, 60%, 80% and 100% fibronectin. b, Mean velocity in x as a function of fibronectin density. The continuous line connects the mean data for 10 fibronectin density bins. Bin1 = [6.905; 10] (N = 24), bin2 = [10; 11.5] (N = 21), bin3 = [11.5; 15.99] (N = 43), bin4 = [15.99; 29.35] (N = 86), bin5 = [29.35; 43.35] (N = 90), bin6 = [43.35; 58.97] (N = 91), bin7 = [58.97; 78] (N = 94), bin8 = [78; 110] (N = 56), bin9 = [110; 135] (N = 15) and bin10 = [135; 185.05] (N = 13). c, Representative fluorescence images of cells migrating on 1D patterns with homogeneous fibronectin. d, Median fibronectin density of 1D patterns with homogeneous fibronectin. Error bars are 25 and 75 percentiles. N = 202. e, First-run trajectories of cells migrating on 1D patterns with homogeneous fibronectin. N = 202. f, Mean traction magnitude for cells migrating on homogeneous patterns (N = 11) and gradient patterns (N = 23). Error bars are s.e.m. g, Time lapse of the normalized traction map of a representative cell migrating on a homogeneous pattern. h, Mean normalized traction magnitude for cells migrating on homogeneous patterns (N = 11). Error bars are s.e.m. i, Mean profiles of x component of the normalized traction forces for cells migrating on homogeneous patterns (N = 11). Error bars are s.e.m. j, Mean normalized traction quadrupole of cells migrating on homogeneous patterns (N = 11). Error bars are s.e.m. For panels a-b, data of 1D homogeneous patterns with 100% fibronectin were obtained from nine independent experiments and with 20%, 40%, 60% and 80% fibronectin were obtained from four independent experiments. For panels c-e, data were obtained from four independent experiments. For panels f-j, data were obtained from three independent experiments. FN, fibronectin. Scale bars, 50 μm (a), 50 μm (c).
Extended Data Fig. 6 Mouse embryonic fibroblasts (MEFs) follow fibronectin gradients.
a, Representative time lapse of one MEF migrating on a 1D gradient. b, First-run trajectories of MEFs migrating on 1D gradients (N = 18). Colour coding represents haptotactic (blue) and anti-haptotactic (yellow) cells. c, Percentage of MEFs migrating towards the centre and against the centre on homogeneous (N = 15) and gradient (N = 18) fibronectin 1D patterns. Data were obtained from two independent experiments. FN, fibronectin. Scale bar: 50 μm.
Extended Data Fig. 7 Cell length and velocity is affected upon inhibition of myosin or ROCK in 1D patterns with homogeneous fibronectin.
a, Mean length of cells migrating on 1D patterns with homogeneous fibronectin when incubated with blebbistatin (N = 32) or Y-27632 (N = 26), compared to the control group (N = 151). Error bars are s.d. b, Mean velocity in the x-axis of cells migrating on 1D patterns with homogeneous fibronectin with treatments of blebbistatin (N = 32) and Y-27632 (N = 26), compared to the control group (N = 151). Error bars are s.d. For panels a-b, a significant difference between groups was identified using the Kruskal-Wallis test. To determine specific pairwise differences, Dunn’s multiple comparisons test was applied. The reported P values correspond to Dunn’s test. **** indicates P < 0.0001. c, First-run trajectories of cells migrating on 1D patterns with homogeneous fibronectin treated with blebbistatin (N = 32). d, First-run trajectories of cells migrating on 1D patterns with homogeneous fibronectin treated with Y-27632 (N = 26). Control data were obtained from four independent experiments. Y-27632 and blebbistatin data were obtained from three independent experiments.
Extended Data Fig. 8 Inverted gradients and sawtooth patterns.
a, Representative time lapse of one cell migrating on a 1D inverted gradient. b, Representative trajectories of three cells migrating on 1D inverted gradients. c, First-run trajectories of cells migrating on 1D inverted gradients (N = 16). Colour coding represents haptotactic (blue) and anti-haptotactic (yellow) cells. d, First-run mean distance migrated by cells moving on 1D homogeneous (N = 202), gradient (N = 173) and inverted gradient (N = 16) patterns. Error bars are s.d. e, Experimental (N = 16) and simulated (N = 500) percentage of haptotactic and anti-haptotactic cells migrating on 1D inverted gradients. f, Representative simulated trajectories of five cells migrating on 1D inverted gradients. g, Simulated first-run trajectories on 1D inverted gradients with the same duration as in experiments. h, Simulated mean distance migrated by cells moving on 1D homogeneous (N = 501), gradient (N = 501) and inverted gradient (N = 501) patterns. Error bars are s.d. In panel d and h, significant difference between groups was identified using the Kruskal-Wallis test. **** indicates P < 0.0001. To determine specific pairwise differences, Dunn’s multiple comparisons test was applied. The reported P values correspond to Dunn’s test. i, Representative time lapse of one cell migrating on a sawtooth pattern. j, Representative examples of sawtooth patterns with different gradient magnitudes. k, Percentage of cells crossing at least one ratchet-like transition in low (N = 28), mid (N = 47), and high (N = 48) gradient magnitudes. l, Comparison of model predictions and experimental data showing the percentage of cells crossing as a function of different normalized gradient levels. The gradient in experiment and model was normalized by the gradient of the “Low” condition. m, Simulated percentage of cells crossing at least one sawtooth in low, mid and high sawtooth pattern magnitudes. Data were obtained from one independent experiment. Model parameters are τ = 2 h, D = 800 μm2 h−3 and χ = 7×109 μm4/ng (Supplementary Information). FN, fibronectin. Scale bars, 50 μm (a), 50 μm (i), 20 μm (j).
Extended Data Fig. 9 Initial migration trajectories on gradients are independent of confinement width.
a, First-run trajectories of cells migrating on gradients with widths of 20 µm (N = 27), 40 µm (N = 27), 60 µm (N = 30), 80 µm (N = 22) and 250 µm (N = 25). b, Haptotaxis probability of cells migrating on gradients with widths of 20 µm (N = 27), 40 µm (N = 27), 60 µm (N = 30), 80 µm (N = 22) and 250 µm (N = 25). 20, 40, 60 and 80 µm widths data were obtained from six replicates. 250 µm width data were obtained from five replicates.
Extended Data Fig. 10 Effect of migrating cells on neighbouring gradients.
a, Representative time lapse of cells migrating on a 1D gradient without and with neighbouring cells. b, Percentage of haptotatic and anti-haptotatic cells on patterns with (N = 27) and without (N = 34) neighbours. Colour coding represents haptotactic (blue) and anti-haptotactic (yellow) cells. Data were obtained from three independent experiments. Scale bar: 50 μm.
Supplementary information
Supplementary Information
Supplementary theory notes for the model.
Supplementary Video 1
Representative time-lapse video of MCF10A cells migrating on confined fibronectin density gradient patterns. Cells initially move along the fibronectin gradient but continue to migrate persistently against it, resulting in oscillatory trajectories. The fibronectin pattern is labelled with Alexa-647. Video rate is 15 frames per second.
Supplementary Video 2
Representative time-lapse video of MCF10A-H2B-GFP cells migrating on confined homogeneous fibronectin density patterns. Cells randomly initiate migration and oscillate between the pattern extremes. The fibronectin pattern is labelled with Alexa-647. Video rate is 15 frames per second.
Supplementary Video 3
Representative time-lapse video of actin flows of MCF10A-LifeAct-GFP cells migrating up (left-hand side) and down (right-hand side) confined fibronectin density gradient patterns. The leading edge of cells migrating down the gradient displays more dynamic behaviour compared with those migrating up the gradient, with faster retrograde actin flow, increased actin polymerization and a higher lamellipodial collapse rate. Video rate is 15 frames per second.
Supplementary Video 4
Representative time-lapse video of MCF10A-H2B-GFP cells migrating on fibronectin density gradient patterns with various widths. Upon adhesion, cells move towards the maximum protein region, irrespective of the confinement level. However, the long-term migration dynamics is strongly affected by the confinement. On confined gradients (20 µm and 40 µm widths), cells migrate down the gradient once they reach the fibronectin peak. For intermediate lane widths of 60 µm and 80 µm, cells exhibited non-trivial patterns, including circular rotations around the midline where concentration is maximal. In near 2D patterns (250 µm), cells do not migrate down the gradient but rather made a 90° turn, migrating along the ridge defined by the maximal fibronectin density. The fibronectin pattern is labelled with Alexa-647. Video rate is 15 frames per second.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fortunato, I.C., Brückner, D.B., Grosser, S. et al. Single-cell migration along and against confined haptotactic gradients. Nat. Phys. 21, 1638–1647 (2025). https://doi.org/10.1038/s41567-025-03015-3
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41567-025-03015-3
This article is cited by
-
Simple cell motility on adhesive gradients
Nature Physics (2025)