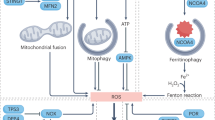
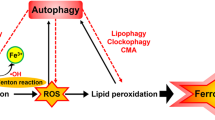
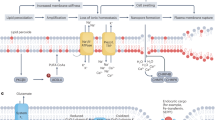
Abstract
Ferroptosis is a necrotic, non-apoptotic cell death modality triggered by unrestrained iron-dependent lipid peroxidation. By unveiling the regulatory mechanisms of ferroptosis and its relevance to various diseases, research over the past decade has positioned ferroptosis as a promising therapeutic target. The rapid growth of this research field presents challenges, associated with potentially inadequate experimental approaches that may lead to misinterpretations in the assessment of ferroptosis. Typical examples include assessing whether an observed phenotype is indeed linked to ferroptosis, and selecting appropriate animal models and small-molecule modulators of ferroptotic cell death. This Expert Recommendation outlines state-of-the-art methods and tools to reliably study ferroptosis and increase the reproducibility and robustness of experimental results. We present highly validated compounds and animal models, and discuss their advantages and limitations. Furthermore, we provide an overview of the regulatory mechanisms and the best-studied players in ferroptosis regulation, such as GPX4, FSP1, SLC7A11 and ACSL4, discussing frequent pitfalls in experimental design and relevant guidance. These recommendations are intended for researchers at all levels, including those entering the expanding and exciting field of ferroptosis research.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Dixon, S. J. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072 (2012). This seminal study coined the term ferroptosis, galvanizing various lines of research at the intersection of redox biology, iron and lipid homeostasis and cell death.
Dixon, S. J. & Olzmann, J. A. The cell biology of ferroptosis. Nat. Rev. Mol. Cell Biol. 25, 424–442 (2024).
Jiang, X., Stockwell, B. R. & Conrad, M. Ferroptosis: mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 22, 266–282 (2021).
Ingold, I. et al. Selenium utilization by GPX4 is required to prevent hydroperoxide-induced ferroptosis. Cell 172, 409–422.e421 (2018).
Badgley, M. A. et al. Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science 368, 85–89 (2020).
Zhao, J. et al. Human hematopoietic stem cell vulnerability to ferroptosis. Cell 186, 732–747.e716 (2023).
Friedmann Angeli, J. P. et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 16, 1180–1191 (2014). This study bdemonstrates that genetic deletion of Gpx4 induces ferroptosis, leading to acute renal failure in mice, and identifies Lip-1 as a potent ferroptosis inhibitor.
Viswanathan, V. S. et al. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature 547, 453–457 (2017).
Hangauer, M. J. et al. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature 551, 247–250 (2017). This seminal work demonstrates that drug-tolerant persister cancer cells depend on GPX4 for survival and that inhibiting GPX4 induces ferroptotic cell death in these cells, thereby preventing tumour relapse in vivo.
Yang, W. S. et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 156, 317–331 (2014).
Wang, W. et al. CD8+ T cells regulate tumour ferroptosis during cancer immunotherapy. Nature 569, 270–274 (2019).
Wu, J. et al. Intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signalling. Nature 572, 402–406 (2019).
Ubellacker, J. M. et al. Lymph protects metastasizing melanoma cells from ferroptosis. Nature 585, 113–118 (2020).
Kim, R. et al. Ferroptosis of tumour neutrophils causes immune suppression in cancer. Nature 612, 338–346 (2022).
Kalkavan, H. et al. Sublethal cytochrome c release generates drug-tolerant persister cells. Cell 185, 3356–3374.e3322 (2022).
Hambright, W. S., Fonseca, R. S., Chen, L., Na, R. & Ran, Q. Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration. Redox Biol. 12, 8–17 (2017).
Linkermann, A. et al. Synchronized renal tubular cell death involves ferroptosis. Proc. Natl Acad. Sci. USA 111, 16836–16841 (2014).
Berndt, C. et al. Ferroptosis in health and disease. Redox Biol. 75, 103211 (2024).
Nakamura, T. & Conrad, M. Exploiting ferroptosis vulnerabilities in cancer. Nat. Cell Biol. 26, 1407–1419 (2024).
Schwab, A. et al. Zeb1 mediates EMT/plasticity-associated ferroptosis sensitivity in cancer cells by regulating lipogenic enzyme expression and phospholipid composition. Nat. Cell Biol. 26, 1470–1481 (2024).
Hirata, Y. et al. Lipid peroxidation increases membrane tension, Piezo1 gating, and cation permeability to execute ferroptosis. Curr. Biol. 33, 1282–1294.e1285 (2023).
Wiernicki, B. et al. Excessive phospholipid peroxidation distinguishes ferroptosis from other cell death modes including pyroptosis. Cell Death Dis. 11, 922 (2020).
Seiler, A. et al. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab. 8, 237–248 (2008). The authors describe a lethal phenotype after deletion of Gpx4, iconically describing the observed cell death as ‘a-yet-unrecognized cell death’.
Thomas, J. P., Maiorino, M., Ursini, F. & Girotti, A. W. Protective action of phospholipid hydroperoxide glutathione peroxidase against membrane-damaging lipid peroxidation. In situ reduction of phospholipid and cholesterol hydroperoxides. J. Biol. Chem. 265, 454–461 (1990).
Sato, H., Tamba, M., Ishii, T. & Bannai, S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J. Biol. Chem. 274, 11455–11458 (1999). In this milestone work, the authors clone and characterize the plasma membrane cystine/glutamate exchange transporter, system xc−.
Doll, S. et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature 575, 693–698 (2019). This work identifies FSP1 as a novel, glutathione-independent suppressor of ferroptosis, demonstrating that FSP1 reduces extramitochondrial coenzyme Q10 to prevent lipid peroxidation and cell death.
Bersuker, K. et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 575, 688–692 (2019).
Mishima, E. et al. A non-canonical vitamin K cycle is a potent ferroptosis suppressor. Nature 608, 778–783 (2022).
Kraft, V. A. N. et al. GTP cyclohydrolase 1/tetrahydrobiopterin counteract ferroptosis through lipid remodeling. ACS Cent. Sci. 6, 41–53 (2020).
Soula, M. et al. Metabolic determinants of cancer cell sensitivity to canonical ferroptosis inducers. Nat. Chem. Biol. 16, 1351–1360 (2020).
Ingold, K. U. & Pratt, D. A. Advances in radical-trapping antioxidant chemistry in the 21st century: a kinetics and mechanisms perspective. Chem. Rev. 114, 9022–9046 (2014).
Li, Y. et al. 7-Dehydrocholesterol dictates ferroptosis sensitivity. Nature 626, 411–418 (2024).
Freitas, F. P. et al. 7-Dehydrocholesterol is an endogenous suppressor of ferroptosis. Nature 626, 401–410 (2024).
Garcia-Bermudez, J. et al. Squalene accumulation in cholesterol auxotrophic lymphomas prevents oxidative cell death. Nature 567, 118–122 (2019).
Warner, G. J. et al. Inhibition of selenoprotein synthesis by selenocysteine tRNA[Ser]Sec lacking isopentenyladenosine. J. Biol. Chem. 275, 28110–28119 (2000).
Aldrovandi, M., Fedorova, M. & Conrad, M. Juggling with lipids, a game of Russian roulette. Trends Endocrinol. Metab. 32, 463–473 (2021).
Li, Z., Lange, M., Dixon, S. J. & Olzmann, J. A. Lipid quality control and ferroptosis: from concept to mechanism. Annu. Rev. Biochem. 93, 499–528 (2024).
Hirata, Y. & Mishima, E. Membrane dynamics and cation handling in ferroptosis. Physiology 39, 73–87 (2024).
Doll, S. et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 13, 91–98 (2017). Using a genome-wide CRISPR-based genetic screen, this study identified ACSL4 as a key determinant of ferroptosis sensitivity.
Kagan, V. E. et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat. Chem. Biol. 13, 81–90 (2017).
Xu, L., Davis, T. A. & Porter, N. A. Rate constants for peroxidation of polyunsaturated fatty acids and sterols in solution and in liposomes. J. Am. Chem. Soc. 131, 13037–13044 (2009).
Morgan, P. K. et al. A lipid atlas of human and mouse immune cells provides insights into ferroptosis susceptibility. Nat. Cell Biol. 26, 645–659 (2024).
Qiu, B. et al. Phospholipids with two polyunsaturated fatty acyl tails promote ferroptosis. Cell 187, 1177–1190.e1118 (2024).
Magtanong, L. et al. Exogenous monounsaturated fatty acids promote a ferroptosis-resistant cell state. Cell Chem. Biol. 26, 420–432.e429 (2019).
Zhang, H. L. et al. PKCβII phosphorylates ACSL4 to amplify lipid peroxidation to induce ferroptosis. Nat. Cell Biol. 24, 88–98 (2022).
Beatty, A. et al. Ferroptotic cell death triggered by conjugated linolenic acids is mediated by ACSL1. Nat. Commun. 12, 2244 (2021).
Tesfay, L. et al. Stearoyl-CoA desaturase 1 protects ovarian cancer cells from ferroptotic cell death. Cancer Res. 79, 5355–5366 (2019).
Rodencal, J. et al. Sensitization of cancer cells to ferroptosis coincident with cell cycle arrest. Cell Chem. Biol. 31, 234–248.e213 (2024).
Liang, D. et al. Ferroptosis surveillance independent of GPX4 and differentially regulated by sex hormones. Cell 186, 2748–2764.e2722 (2023).
Conrad, M. & Pratt, D. A. The chemical basis of ferroptosis. Nat. Chem. Biol. 15, 1137–1147 (2019).
Galy, B., Conrad, M. & Muckenthaler, M. Mechanisms controlling cellular and systemic iron homeostasis. Nat. Rev. Mol. Cell Biol. 25, 133–155 (2024).
Aron, A. T., Loehr, M. O., Bogena, J. & Chang, C. J. An endoperoxide reactivity-based FRET probe for ratiometric fluorescence imaging of labile iron pools in living cells. J. Am. Chem. Soc. 138, 14338–14346 (2016).
Hirayama, T., Miki, A. & Nagasawa, H. Organelle-specific analysis of labile Fe(II) during ferroptosis by using a cocktail of various colour organelle-targeted fluorescent probes. Metallomics 11, 111–117 (2019).
Dixon, S. J. & Lee, M. J. Quick tips for interpreting cell death experiments. Nat. Cell Biol. 25, 1720–1723 (2023).
Toyokuni, S. et al. Iron as spirit of life to share under monopoly. J. Clin. Biochem. Nutr. 71, 78–88 (2022).
Cheng, Z. et al. Ferroptosis resistance determines high susceptibility of murine A/J strain to iron-induced renal carcinogenesis. Cancer Sci. 113, 65–78 (2022).
Zheng, H., Jiang, L., Tsuduki, T., Conrad, M. & Toyokuni, S. Embryonal erythropoiesis and aging exploit ferroptosis. Redox Biol. 48, 102175 (2021).
Tonnus, W. et al. The pathological features of regulated necrosis. J. Pathol. 247, 697–707 (2019).
Devisscher, L. et al. Discovery of novel, drug-like ferroptosis inhibitors with in vivo efficacy. J. Med. Chem. 61, 10126–10140 (2018).
Van Coillie, S. et al. Targeting ferroptosis protects against experimental (multi)organ dysfunction and death. Nat. Commun. 13, 1046 (2022).
Proneth, B. & Conrad, M. Ferroptosis and necroinflammation, a yet poorly explored link. Cell Death Differ. 26, 14–24 (2019).
Drummen, G. P., van Liebergen, L. C., Op den Kamp, J. A. & Post, J. A. C11-BODIPY(581/591), an oxidation-sensitive fluorescent lipid peroxidation probe: (micro)spectroscopic characterization and validation of methodology. Free Radic. Biol. Med. 33, 473–490 (2002).
Shah, R., Farmer, L. A., Zilka, O., Van Kessel, A. T. M. & Pratt, D. A. Beyond DPPH: use of fluorescence-enabled inhibited autoxidation to predict oxidative cell death rescue. Cell Chem. Biol. 26, 1594–1607.e1597 (2019). In this study, the authors establish the FENIX assay designed to predict potential RTA activity of anti-ferroptotic compounds.
Poon, J. F., Zilka, O. & Pratt, D. A. Potent ferroptosis inhibitors can catalyze the cross-dismutation of phospholipid-derived peroxyl radicals and hydroperoxyl radicals. J. Am. Chem. Soc. 142, 14331–14342 (2020).
Zilka, O., Poon, J. F. & Pratt, D. A. Radical-trapping antioxidant activity of copper and nickel bis(thiosemicarbazone) complexes underlies their potency as inhibitors of ferroptotic cell death. J. Am. Chem. Soc. 143, 19043–19057 (2021).
Long, E. K. & Picklo, M. J. Sr Trans-4-hydroxy-2-hexenal, a product of n-3 fatty acid peroxidation: make some room HNE. Free Radic. Biol. Med. 49, 1–8 (2010).
Criscuolo, A. et al. Analytical and computational workflow for in-depth analysis of oxidized complex lipids in blood plasma. Nat. Commun. 13, 6547 (2022). This study provides a workflow based on the combination of bioinformatics and LC–MS/MS technologies to support identification and relative quantification of oxidized complex lipids.
Wolk, M., Prabutzki, P. & Fedorova, M. Analytical toolbox to unlock the diversity of oxidized lipids. Acc. Chem. Res. 56, 835–845 (2023).
Ni, Z. et al. Guiding the choice of informatics software and tools for lipidomics research applications. Nat. Methods 20, 193–204 (2023).
Tyurina, Y. Y. et al. Redox phospholipidomics discovers pro-ferroptotic death signals in A375 melanoma cells in vitro and in vivo. Redox Biol. 61, 102650 (2023).
Zou, Y. et al. Plasticity of ether lipids promotes ferroptosis susceptibility and evasion. Nature 585, 603–608 (2020).
Cui, W., Liu, D., Gu, W. & Chu, B. Peroxisome-driven ether-linked phospholipids biosynthesis is essential for ferroptosis. Cell Death Differ. 28, 2536–2551 (2021).
Perez, M. A. et al. Ether lipid deficiency disrupts lipid homeostasis leading to ferroptosis sensitivity. PLoS Genet. 18, e1010436 (2022).
Breitzig, M., Bhimineni, C., Lockey, R. & Kolliputi, N. 4-Hydroxy-2-nonenal: a critical target in oxidative stress? Am. J. Physiol. Cell Physiol 311, C537–C543 (2016).
Mallais, M., Hanson, C. S., Giray, M. & Pratt, D. A. General approach to identify, assess, and characterize inhibitors of lipid peroxidation and associated cell death. ACS Chem. Biol. 18, 561–571 (2023).
Vinik, Y. et al. Programming a ferroptosis-to-apoptosis transition landscape revealed ferroptosis biomarkers and repressors for cancer therapy. Adv. Sci. 11, e2307263 (2024).
Feng, H. et al. Transferrin receptor is a specific ferroptosis marker. Cell Rep. 30, 3411–3423.e3417 (2020).
Dixon, S. J. et al. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. eLife 3, e02523 (2014).
Kang, Y. J., Mbonye, U. R., DeLong, C. J., Wada, M. & Smith, W. L. Regulation of intracellular cyclooxygenase levels by gene transcription and protein degradation. Prog. Lipid Res. 46, 108–125 (2007).
Meinert, M. et al. Thiol starvation triggers melanoma state switching in an ATF4 and NRF2-dependent manner. Redox Biol. 70, 103011 (2024).
Oh-Hashi, K. et al. Transcriptional and post-translational regulation of mouse cation transport regulator homolog 1. Mol. Cell Biochem. 380, 97–106 (2013).
Cui, S. et al. Identification of hyperoxidized PRDX3 as a ferroptosis marker reveals ferroptotic damage in chronic liver diseases. Mol. Cell 83, 3931–3939.e3935 (2023).
Hattori, K. et al. Cold stress-induced ferroptosis involves the ASK1–p38 pathway. EMBO Rep. 18, 2067–2078 (2017).
Brown, K. K., Eriksson, S. E., Arner, E. S. & Hampton, M. B. Mitochondrial peroxiredoxin 3 is rapidly oxidized in cells treated with isothiocyanates. Free Radic. Biol. Med. 45, 494–502 (2008).
Takeda, K., Noguchi, T., Naguro, I. & Ichijo, H. Apoptosis signal-regulating kinase 1 in stress and immune response. Annu. Rev. Pharmacol. Toxicol. 48, 199–225 (2008).
Chorley, B. N. et al. Identification of novel NRF2-regulated genes by ChIP-Seq: influence on retinoid X receptor alpha. Nucleic Acids Res. 40, 7416–7429 (2012).
Koppula, P. et al. A targetable CoQ-FSP1 axis drives ferroptosis- and radiation-resistance in KEAP1 inactive lung cancers. Nat. Commun. 13, 2206 (2022).
Mishima, E. et al. DHODH inhibitors sensitize to ferroptosis by FSP1 inhibition. Nature 619, E9–E18 (2023).
Yant, L. J. et al. The selenoprotein GPX4 is essential for mouse development and protects from radiation and oxidative damage insults. Free Radic. Biol. Med. 34, 496–502 (2003).
Liang, H. et al. Short form glutathione peroxidase 4 is the essential isoform required for survival and somatic mitochondrial functions. J. Biol. Chem. 284, 30836–30844 (2009).
Azuma, K. et al. Mitochondrial glutathione peroxidase 4 is indispensable for photoreceptor development and survival in mice. J. Biol. Chem. 298, 101824 (2022).
Imai, H. et al. Depletion of selenoprotein GPx4 in spermatocytes causes male infertility in mice. J. Biol. Chem. 284, 32522–32532 (2009).
Schneider, M. et al. Mitochondrial glutathione peroxidase 4 disruption causes male infertility. FASEB J. 23, 3233–3242 (2009).
Moreno, S. G., Laux, G., Brielmeier, M., Bornkamm, G. W. & Conrad, M. Testis-specific expression of the nuclear form of phospholipid hydroperoxide glutathione peroxidase (PHGPx). Biol. Chem. 384, 635–643 (2003).
Conrad, M. et al. The nuclear form of phospholipid hydroperoxide glutathione peroxidase is a protein thiol peroxidase contributing to sperm chromatin stability. Mol. Cell Biol. 25, 7637–7644 (2005).
Labunskyy, V. M., Hatfield, D. L. & Gladyshev, V. N. Selenoproteins: molecular pathways and physiological roles. Physiol. Rev. 94, 739–777 (2014).
Li, Z. et al. Ribosome stalling during selenoprotein translation exposes a ferroptosis vulnerability. Nat. Chem. Biol. 18, 751–761 (2022).
Alborzinia, H. et al. LRP8-mediated selenocysteine uptake is a targetable vulnerability in MYCN-amplified neuroblastoma. EMBO Mol. Med. 15, e18014 (2023).
Nakamura, T. et al. A tangible method to assess native ferroptosis suppressor activity. Cell Rep. Methods 4, 100710 (2024).
Hurst, R. et al. Hyperresistance to cholesterol hydroperoxide-induced peroxidative injury and apoptotic death in a tumor cell line that overexpresses glutathione peroxidase isotype-4. Free Radic. Biol. Med. 31, 1051–1065 (2001).
Vuckovic, A. M. et al. Inactivation of the glutathione peroxidase GPx4 by the ferroptosis-inducing molecule RSL3 requires the adaptor protein 14-3-3epsilon. FEBS Lett. 594, 611–624 (2020).
Sato, H., Tamba, M., Kuriyama-Matsumura, K., Okuno, S. & Bannai, S. Molecular cloning and expression of human xCT, the light chain of amino acid transport system xc. Antioxid. Redox Signal. 2, 665–671 (2000).
Sato, H. et al. Redox imbalance in cystine/glutamate transporter-deficient mice. J. Biol. Chem. 280, 37423–37429 (2005).
Armenta, D. A. et al. Ferroptosis inhibition by lysosome-dependent catabolism of extracellular protein. Cell Chem. Biol. 29, 1588–1600.e1587 (2022).
Zheng, J. et al. Sorafenib fails to trigger ferroptosis across a wide range of cancer cell lines. Cell Death Dis. 12, 698 (2021).
Poltorack, C. D. & Dixon, S. J. Understanding the role of cysteine in ferroptosis: progress & paradoxes. FEBS J. 289, 374–385 (2022).
Kobayashi, S. et al. Carnosine dipeptidase II (CNDP2) protects cells under cysteine insufficiency by hydrolyzing glutathione-related peptides. Free Radic. Biol. Med. 174, 12–27 (2021).
Lee, J. et al. The viability of primary hepatocytes is maintained under a low cysteine-glutathione redox state with a marked elevation in ophthalmic acid production. Exp. Cell Res. 361, 178–191 (2017).
Van Liefferinge, J. et al. Comparative analysis of antibodies to xCT (Slc7a11): forewarned is forearmed. J. Comp. Neurol. 524, 1015–1032 (2016).
Nguyen, H. P. et al. Aifm2, a NADH oxidase, supports robust glycolysis and is required for cold- and diet-induced thermogenesis. Mol. Cell 77, 600–617.e604 (2020).
Mishima, E., Wahida, A., Seibt, T. & Conrad, M. Diverse biological functions of vitamin K: from coagulation to ferroptosis. Nat. Metab. 5, 924–932 (2023).
Tonnus, W. et al. Dysfunction of the key ferroptosis-surveilling systems hypersensitizes mice to tubular necrosis during acute kidney injury. Nat. Commun. 12, 4402 (2021).
Soriano-Castell, D., Liang, Z., Maher, P. & Currais, A. Profiling the chemical nature of anti-oxytotic/ferroptotic compounds with phenotypic screening. Free Radic. Biol. Med. 177, 313–325 (2021).
Nakamura, T. et al. Integrated chemical and genetic screens unveil FSP1 mechanisms of ferroptosis regulation. Nat. Struct. Mol. Biol. 30, 1806–1815 (2023).
Xavier da Silva, T. N., Schulte, C., Alves, A. N., Maric, H. M. & Friedmann Angeli, J. P. Molecular characterization of AIFM2/FSP1 inhibition by iFSP1-like molecules. Cell Death Dis. 14, 281 (2023).
Magtanong, L. et al. Context-dependent regulation of ferroptosis sensitivity. Cell Chem. Biol. 29, 1409–1418.e1406 (2022).
Shimbara-Matsubayashi, S., Kuwata, H., Tanaka, N., Kato, M. & Hara, S. Analysis on the substrate specificity of recombinant human acyl-CoA synthetase ACSL4 variants. Biol. Pharm. Bull. 42, 850–855 (2019).
Cao, Y., Traer, E., Zimmerman, G. A., McIntyre, T. M. & Prescott, S. M. Cloning, expression, and chromosomal localization of human long-chain fatty acid-CoA ligase 4 (FACL4). Genomics 49, 327–330 (1998).
Piccini, M. et al. FACL4, a new gene encoding long-chain acyl-CoA synthetase 4, is deleted in a family with Alport syndrome, elliptocytosis, and mental retardation. Genomics 47, 350–358 (1998).
Klett, E. L., Chen, S., Yechoor, A., Lih, F. B. & Coleman, R. A. Long-chain acyl-CoA synthetase isoforms differ in preferences for eicosanoid species and long-chain fatty acids. J. Lipid Res. 58, 884–894 (2017).
Chen, W. C. et al. Systematic analysis of gene expression alterations and clinical outcomes for long-chain acyl-coenzyme A synthetase family in cancer. PLoS ONE 11, e0155660 (2016).
Zilka, O. et al. On the mechanism of cytoprotection by ferrostatin-1 and liproxstatin-1 and the role of lipid peroxidation in ferroptotic cell death. ACS Cent. Sci. 3, 232–243 (2017).
Helberg, J. & Pratt, D. A. Autoxidation vs. antioxidants — the fight for forever. Chem. Soc. Rev. 50, 7343–7358 (2021).
Mishima, E. et al. Drugs repurposed as antiferroptosis agents suppress organ damage, including AKI, by functioning as lipid peroxyl radical scavengers. J. Am. Soc. Nephrol. 31, 280–296 (2020).
Conlon, M. et al. A compendium of kinetic modulatory profiles identifies ferroptosis regulators. Nat. Chem. Biol. 17, 665–674 (2021).
Shah, R., Shchepinov, M. S. & Pratt, D. A. Resolving the role of lipoxygenases in the initiation and execution of ferroptosis. ACS Cent. Sci. 4, 387–396 (2018).
Sato, H. et al. Transcriptional control of cystine/glutamate transporter gene by amino acid deprivation. Biochem. Biophys. Res. Commun. 325, 109–116 (2004).
Forcina, G. C. et al. Ferroptosis regulation by the NGLY1/NFE2L1 pathway. Proc. Natl Acad. Sci. USA 119, e2118646119 (2022).
Chen, Y. et al. Quantitative profiling of protein carbonylations in ferroptosis by an aniline-derived probe. J. Am. Chem. Soc. 140, 4712–4720 (2018).
Gao, J. et al. Selenium-encoded isotopic signature targeted profiling. ACS Cent. Sci. 4, 960–970 (2018).
Eaton, J. K. et al. Selective covalent targeting of GPX4 using masked nitrile-oxide electrophiles. Nat. Chem. Biol. 16, 497–506 (2020).
Chen, T. et al. Discovery of novel potent covalent glutathione peroxidase 4 inhibitors as highly selective ferroptosis inducers for the treatment of triple-negative breast cancer. J. Med. Chem. 66, 10036–10059 (2023).
Yan, B. et al. Membrane damage during ferroptosis is caused by oxidation of phospholipids catalyzed by the oxidoreductases POR and CYB5R1. Mol. Cell 81, 355–369.e310 (2021).
Oh, M. et al. The lipoprotein-associated phospholipase A2 inhibitor Darapladib sensitises cancer cells to ferroptosis by remodelling lipid metabolism. Nat. Commun. 14, 5728 (2023).
Li, J. et al. Tumor-specific GPX4 degradation enhances ferroptosis-initiated antitumor immune response in mouse models of pancreatic cancer. Sci. Transl. Med. 15, eadg3049 (2023).
Luo, T. et al. Intracellular delivery of glutathione peroxidase degrader induces ferroptosis in vivo. Angew. Chem. Int. Ed. Engl. 61, e202206277 (2022).
Cheff, D. M. et al. The ferroptosis inducing compounds RSL3 and ML162 are not direct inhibitors of GPX4 but of TXNRD1. Redox Biol. 62, 102703 (2023).
Fujii, J., Homma, T. & Kobayashi, S. Ferroptosis caused by cysteine insufficiency and oxidative insult. Free Radic. Res. 54, 969–980 (2020).
Sagara, J. I., Miura, K. & Bannai, S. Maintenance of neuronal glutathione by glial cells. J. Neurochem. 61, 1672–1676 (1993).
Gao, M. et al. Role of mitochondria in ferroptosis. Mol. Cell 73, 354–363.e353 (2019).
Zhang, Y. et al. Imidazole ketone erastin induces ferroptosis and slows tumor growth in a mouse lymphoma model. Cell Chem. Biol. 26, 623–633.e629 (2019).
Gout, P. W., Buckley, A. R., Simms, C. R. & Bruchovsky, N. Sulfasalazine, a potent suppressor of lymphoma growth by inhibition of the xc− cystine transporter: a new action for an old drug. Leukemia 15, 1633–1640 (2001).
Griffith, O. W. & Meister, A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J. Biol. Chem. 254, 7558–7560 (1979).
Harris, I. S. et al. Deubiquitinases maintain protein homeostasis and survival of cancer cells upon glutathione depletion. Cell Metab. 29, 1166–1181.e1166 (2019).
Xia, C. et al. Cysteine and homocysteine can be exploited by GPX4 in ferroptosis inhibition independent of GSH synthesis. Redox Biol. 69, 102999 (2024).
Bebber, C. M. et al. Ferroptosis response segregates small cell lung cancer (SCLC) neuroendocrine subtypes. Nat. Commun. 12, 2048 (2021).
Harris, I. S. et al. Glutathione and thioredoxin antioxidant pathways synergize to drive cancer initiation and progression. Cancer Cell 27, 211–222 (2015).
Fujihara, K. M. et al. Eprenetapopt triggers ferroptosis, inhibits NFS1 cysteine desulfurase, and synergizes with serine and glycine dietary restriction. Sci. Adv. 8, eabm9427 (2022).
Nakamura, T. et al. Phase separation of FSP1 promotes ferroptosis. Nature 619, 371–377 (2023).
Hendricks, J. M. et al. Identification of structurally diverse FSP1 inhibitors that sensitize cancer cells to ferroptosis. Cell Chem. Biol. 30, 1090–1103.e1097 (2023).
Falk, M. H., Hultner, L., Milner, A., Gregory, C. D. & Bornkamm, G. W. Irradiated fibroblasts protect Burkitt lymphoma cells from apoptosis by a mechanism independent of BCL-2. Int. J. Cancer 55, 485–491 (1993).
Takashima, H. et al. Impact of selenium content in fetal bovine serum on ferroptosis susceptibility and selenoprotein expression in cultured cells. J. Toxicol. Sci. 49, 555–563 (2024).
Mishima, E. & Conrad, M. Nutritional and metabolic control of ferroptosis. Annu. Rev. Nutr. 42, 275–309 (2022).
Gao, M., Monian, P., Quadri, N., Ramasamy, R. & Jiang, X. Glutaminolysis and transferrin regulate ferroptosis. Mol. Cell 59, 298–308 (2015).
Lee, H. et al. Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat. Cell Biol. 22, 225–234 (2020).
Vuckovic, A. M. et al. Aerobic pyruvate metabolism sensitizes cells to ferroptosis primed by GSH depletion. Free Radic. Biol. Med. 167, 45–53 (2021).
Song, X. et al. PDK4 dictates metabolic resistance to ferroptosis by suppressing pyruvate oxidation and fatty acid synthesis. Cell Rep. 34, 108767 (2021).
Vera, M., Barahona, M. J., Nova-Lamperti, E., Nualart, F. & Ferrada, L. The phenol red compound: a potential artifact in pharmacological induction of ferroptosis. Free Radic. Biol. Med. 222, 397–402 (2024).
Deshwal, S. et al. Mitochondria regulate intracellular coenzyme Q transport and ferroptotic resistance via STARD7. Nat. Cell Biol. 25, 246–257 (2023).
Imai, H. et al. Early embryonic lethality caused by targeted disruption of the mouse PHGPx gene. Biochem. Biophys. Res. Commun. 305, 278–286 (2003).
Conrad, M., Lorenz, S. M. & Proneth, B. Targeting ferroptosis: new hope for as-yet-incurable diseases. Trends Mol. Med. 27, 113–122 (2021).
Chen, L., Hambright, W. S., Na, R. & Ran, Q. Ablation of the ferroptosis inhibitor glutathione peroxidase 4 in neurons results in rapid motor neuron degeneration and paralysis. J. Biol. Chem. 290, 28097–28106 (2015).
Wirth, E. K. et al. Neuronal selenoprotein expression is required for interneuron development and prevents seizures and neurodegeneration. FASEB J. 24, 844–852 (2010).
Carlson, B. A. et al. Glutathione peroxidase 4 and vitamin E cooperatively prevent hepatocellular degeneration. Redox Biol. 9, 22–31 (2016).
Wortmann, M. et al. Combined deficiency in glutathione peroxidase 4 and vitamin E causes multiorgan thrombus formation and early death in mice. Circ. Res. 113, 408–417 (2013).
Matsushita, M. et al. T cell lipid peroxidation induces ferroptosis and prevents immunity to infection. J. Exp. Med. 212, 555–568 (2015).
Alim, I. et al. Selenium drives a transcriptional adaptive program to block ferroptosis and treat stroke. Cell 177, 1262–1279.e1225 (2019).
Yamada, N. et al. Ferroptosis driven by radical oxidation of n-6 polyunsaturated fatty acids mediates acetaminophen-induced acute liver failure. Cell Death Dis. 11, 144 (2020).
Martin-Sanchez, D. et al. Ferroptosis, but not necroptosis, is important in nephrotoxic folic acid-induced AKI. J. Am. Soc. Nephrol. 28, 218–229 (2017).
Van San, E. et al. Ferroptosis contributes to multiple sclerosis and its pharmacological targeting suppresses experimental disease progression. Cell Death Differ. 30, 2092–2103 (2023).
Ide, S. et al. Sex differences in resilience to ferroptosis underlie sexual dimorphism in kidney injury and repair. Cell Rep. 41, 111610 (2022).
Ward, N. P., Kang, Y. P., Falzone, A., Boyle, T. A. & DeNicola, G. M. Nicotinamide nucleotide transhydrogenase regulates mitochondrial metabolism in NSCLC through maintenance of Fe-S protein function. J. Exp. Med. 217, e20191689 (2020).
Co, H. K. C., Wu, C. C., Lee, Y. C. & Chen, S. H. Emergence of large-scale cell death through ferroptotic trigger waves. Nature 631, 654–662 (2024).
Davidson, A. J., Heron, R., Das, J., Overholtzer, M. & Wood, W. Ferroptosis-like cell death promotes and prolongs inflammation in Drosophila. Nat. Cell Biol. 26, 1535–1544 (2024).
Ni, Z. et al. Evaluation of air oxidized PAPC: a multi laboratory study by LC–MS/MS. Free Radic. Biol. Med. 144, 156–166 (2019).
Criscuolo, A., Zeller, M. & Fedorova, M. Evaluation of lipid in-source fragmentation on different orbitrap-based mass spectrometers. J. Am. Soc. Mass. Spectrom. 31, 463–466 (2020).
Acknowledgements
We thank all our colleagues, especially K. Ono, J. Ito, W. Zhang, A. Levkina, M. Novikova, J. Zheng, T. Seibt, M. Aldrovandi, S.M. Lorenz, D. Chen, N. Yamada, G. Mardani, C. Xu and B. Henkelmann, for their help in organizing this Expert Recommendation. We thank S. Kobayashi and H. Sato for providing expert views about system xc−. M.C. received funding from the Deutsche Forschungsgemeinschaft (CO 291/7-1, the Priority Program SPP 2306 (grants CO 291/9-1, 461385412; CO 291/10-1, 461507177, CO 291/9-2, CO 291/10-2, CO 291/14-1) and the CRC TRR 353 (CO 291/11-1; 471011418), the German Federal Ministry of Education and Research FERROPATH (grant 01EJ2205B) and the European Research Council under the European Union’s Horizon 2020 research and innovation program (grant GA 884754). D.A.P. thanks the Natural Sciences and Engineering Research Council of Canada for their continued support (RGPIN-2022-05058). Work in the Fedorova lab is supported by ‘Sonderzuweisung zur Unterstützung profilbestimmender Struktureinheiten der TUD’ by the SMWK, TG70 by Sächsische Aufbaubank and SMWK, the measure is cofinanced with tax funds based on the budget passed by the Saxon state parliament (to M.F.), Deutsche Forschungsgemeinschaft (grant FE 1236/5-1 to M.F.) and Bundesministerium für Bildung und Forschung (grant 01EJ2205A, FERROPath to M.F.). S.J.D. is supported by the USA National Institutes of Health (grant R01GM122923). E.M. thanks the Research Grant of Sapporo Bioscience Foundation and Food Science Institute Foundation (Ryoshoku Kenkyuukai).
Author information
Authors and Affiliations
Contributions
E.M., T.N., A.W. and M.C. wrote the article. S.D., B.P., M.F., D.A.P., J.P.F.A. and S.J.D. contributed substantially to the discussion of the content. All authors reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
M.C. and B.P. are cofounders and shareholders of ROSCUE Therapeutics GmbH. D.A.P. is a cofounder and shareholder of Prothegen Inc. S.J.D. holds patents related to ferroptosis. M.C., B.P. and T.N. have filed patents for some of the compounds described herein.
Peer review
Peer review information
Nature Reviews Molecular Cell Biology thanks Andreas Linkermann, Qitao Ran and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
41580_2025_843_MOESM2_ESM.mp4
Supplementary Video 1. Live imaging of cells undergoing ferroptosis. Pfa1 cells with inducible Gpx4 deletion following treatment with 4-hydroxytamoxifen. The video was captured using Nanolive 3D Cell Explorer 42–56 h after the addition of 4-hydroxytamoxifen. E.M. produced the video.
Glossary
- 3′-UTR selenocysteine insertion sequence element
-
A stem loop-like secondary structure located in the 3′-untranslated region (UTR) of selenoprotein mRNAs, which affords decoding of UGA as a selenocysteine during translation.
- 4-hydroxy-2-nonenal
-
(4-HNE). An α,β-unsaturated hydroxyalkenal produced downstream of lipid peroxidation, which can react with specific amino acid residues (that is, histidine, cysteine and lysine) in proteins to generate Michael adducts in cells and tissues.
- C57BL/6J × 129S6/SvEv mixed background
-
F1 mice with mixed background strains derived from two inbred strains of C57BL/6J and 129S6/SvEv mice.
- Epilipidomic studies
-
A method to analyse a subset of natural lipidome formed by lipid modifications (for example oxidation) required to regulate complex biological functions.
- Fenton reaction
-
A reaction in which iron or other transition metals catalyse the disproportionation of hydrogen peroxide into highly reactive hydroxyl radical and a hydroperoxide ion.
- Gpx4 U46C mutant mice
-
Transgenic mice carrying a targeted mutation of the catalytically active site selenocysteine (U46) to Cys of Gpx4.
- Ischaemia-reperfusion injury
-
(IRI). Transient ischaemia, followed by reperfusion, generates oxygen-centred radicals that trigger extensive cell death and inflammatory responses in the affected organs, leading to acute tissue damage.
- Karyolysis
-
Complete dissolution of nuclear components in a dying cell.
- Lipid hydroperoxides
-
The primary products of lipid peroxidation, resulting from propagation by H-atom transfer, can be further reduced or oxidized to yield radicals that initiate additional lipid peroxidation and/or produce secondary reactive lipid aldehydes.
- Lipid peroxidation
-
Generally refers to the autoxidation of lipids, a free radical chain reaction in which oxygen is incorporated into hydrocarbons to form peroxides, resulting in the production of lipid hydroperoxides when the chain reaction is propagated by H-atom transfer from a lipid or lipid peroxides when propagated by addition to a lipid.
- Necroptosis
-
A regulated, necrotic cell death modality mediated by receptor-interacting protein kinase 3 (RIPK3) activity and ensuing pore formation by mixed-lineage kinase domain-like pseudokinase.
- Parvalbumin-expressing GABAergic interneurons
-
The principal inhibitory interneurons in the brain cortex.
- Phospholipids
-
Amphiphilic molecules with a hydrophilic head containing a phosphate group (for example phosphocholine and phosphoethanolamine) and two hydrophobic fatty acid tails esterified to the glycerol moiety, which are key component of cell membranes.
- Plasmalogens
-
A unique class of phospholipids containing a vinyl ether bond at the sn-1 position, with its synthesis initiated in peroxisomes and endoplasmic reticulum.
- Pyknosis
-
Condensation of the nucleus and chromatin, often observed in cells undergoing cell death.
- Radical-trapping antioxidants
-
(RTAs). Compounds that react with radical chain-propagating radicals to form non-propagating radicals31,123.
- Regulated necrosis
-
A type of programmed cell death involving plasma membrane rupture and including various modalities, such as ferroptosis and necroptosis.
- Selenocysteinyl-tRNA
-
A specific tRNA responsible for incorporating selenocysteine into selenoproteins during translation.
- Selenoproteins
-
An exclusive group of selenium-containing proteins in which selenocysteine, the 21st proteinogenic amino acid, is cotranslationally incorporated into the protein and is usually present at the catalytically active site.
- Tetrahydrobiopterin
-
(BH4). A redox-active cofactor for several biosynthetic enzymes, also functioning as an RTA by reacting with peroxyl radicals, yielding oxidation products such as dihydrobiopterin (BH2), which can be reduced back to BH4 by the enzyme dihydrofolate reductase.
- Thiol-containing molecules
-
Organic molecules that contain a sulfhydryl group.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mishima, E., Nakamura, T., Doll, S. et al. Recommendations for robust and reproducible research on ferroptosis. Nat Rev Mol Cell Biol 26, 615–630 (2025). https://doi.org/10.1038/s41580-025-00843-2
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41580-025-00843-2
This article is cited by
-
Ferroptosis in cancer toward molecular insights and clinical translation in pancreatic cancer
Molecular Cancer (2026)
-
Tocotrienols exhibit superior ferroptosis inhibition over tocopherols
Scientific Reports (2026)
-
Copper-Based Targeted Nanocatalytic Therapeutics for Non-Small Cell Lung Cancer
Nano-Micro Letters (2026)
-
ZKSCAN5 transcriptional regulation of APOC1 modulates ferroptosis via PI3K/AKT/SREBP2/SLC1A5 axis
Journal of Translational Medicine (2025)
-
P23 acts as a negative regulator of ferroptosis in NSCLC by blocking GPX4 degradation via chaperone-mediated autophagy
Molecular Cancer (2025)