Abstract
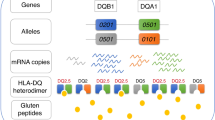
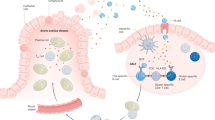
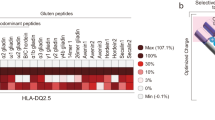
In vitro models of autoimmunity are constrained by an inability to culture affected epithelium alongside the complex tissue-resident immune microenvironment. Coeliac disease (CeD) is an autoimmune disease in which dietary gluten-derived peptides bind to the major histocompatibility complex (MHC) class II human leukocyte antigen molecules (HLA)-DQ2 or HLA-DQ8 to initiate immune-mediated duodenal mucosal injury1,2,3,4. Here, we generated air–liquid interface (ALI) duodenal organoids from intact fragments of endoscopic biopsies that preserve epithelium alongside native mesenchyme and tissue-resident immune cells as a unit without requiring reconstitution. The immune diversity of ALI organoids spanned T cells, B and plasma cells, natural killer (NK) cells and myeloid cells, with extensive T-cell and B-cell receptor repertoires. HLA-DQ2.5-restricted gluten peptides selectively instigated epithelial destruction in HLA-DQ2.5-expressing organoids derived from CeD patients, and this was antagonized by blocking MHC-II or NKG2C/D. Gluten epitopes stimulated a CeD organoid immune network response in lymphoid and myeloid subsets alongside anti-transglutaminase 2 (TG2) autoantibody production. Functional studies in CeD organoids revealed that interleukin-7 (IL-7) is a gluten-inducible pathogenic modulator that regulates CD8+ T-cell NKG2C/D expression and is necessary and sufficient for epithelial destruction. Furthermore, endogenous IL-7 was markedly upregulated in patient biopsies from active CeD compared with remission disease from gluten-free diets, predominantly in lamina propria mesenchyme. By preserving the epithelium alongside diverse immune populations, this human in vitro CeD model recapitulates gluten-dependent pathology, enables mechanistic investigation and establishes a proof of principle for the organoid modelling of autoimmunity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Data sets for scRNA-seq have been deposited in Gene Expression Omnibus with the accession code GSE200075. Source data are provided with this paper.
References
Catassi, C., Verdu, E. F., Bai, J. C. & Lionetti, E. Coeliac disease. Lancet 399, 2413–2426 (2022).
Levescot, A., Malamut, G. & Cerf-Bensussan, N. Immunopathogenesis and environmental triggers in coeliac disease. Gut 71, 2337–2349 (2022).
Iversen, R. & Sollid, L. M. The immunobiology and pathogenesis of celiac disease. Annu. Rev. Pathol. 18, 47–70 (2023).
Marsh, M. N. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘celiac sprue’). Gastroenterology 102, 330–354 (1992).
Jabri, B. et al. Selective expansion of intraepithelial lymphocytes expressing the HLA-E-specific natural killer receptor CD94 in celiac disease. Gastroenterology 118, 867–879 (2000).
Hüe, S. et al. A direct role for NKG2D/MICA interaction in villous atrophy during celiac disease. Immunity 21, 367–377 (2004).
Meresse, B. et al. Coordinated induction by IL15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity 21, 357–366 (2004).
Pinto-Sanchez, M. I. et al. Society for the Study of Celiac Disease position statement on gaps and opportunities in coeliac disease. Nat. Rev. Gastroenterol. Hepatol. 18, 875–884 (2021).
Barone, M. V. et al. Gliadin-mediated proliferation and innate immune activation in celiac disease are due to alterations in vesicular trafficking. PLoS ONE 6, e17039 (2011).
Castellanos-Rubio, A. et al. Long-term and acute effects of gliadin on small intestine of patients on potentially pathogenic networks in celiac disease. Autoimmunity 43, 131–139 (2010).
Palova-Jelinkova, L. et al. Gliadin fragments induce phenotypic and functional maturation of human dendritic cells. J. Immunol. 175, 7038–7045 (2005).
Freire, R. et al. Human gut derived-organoids provide model to study gluten response and effects of microbiota-derived molecules in celiac disease. Sci. Rep. 9, 7029 (2019).
de Kauwe, A. L. et al. Resistance to celiac disease in humanized HLA-DR3-DQ2-transgenic mice expressing specific anti-gliadin CD4+ T cells. J. Immunol. 182, 7440–7450 (2009).
Abadie, V. et al. IL-15, gluten and HLA-DQ8 drive tissue destruction in coeliac disease. Nature 578, 600–604 (2020).
Goel, G. et al. Serum cytokines elevated during gluten-mediated cytokine release in coeliac disease. Clin. Exp. Immunol. 199, 68–78 (2020).
Tye-Din, J. A. et al. Patient factors influencing acute gluten reactions and cytokine release in treated coeliac disease. BMC Med. 18, 362 (2020).
Jabri, B. & Abadie, V. IL-15 functions as a danger signal to regulate tissue-resident T cells and tissue destruction. Nat. Rev. Immunol. 15, 771–783 (2015).
Lähdeaho, M.-L. et al. Safety and efficacy of AMG 714 in adults with coeliac disease exposed to gluten challenge: a phase 2a, randomised, double-blind, placebo-controlled study. Lancet Gastroenterol. Hepatol. 4, 948–959 (2019).
Rochman, Y., Spolski, R. & Leonard, W. J. New insights into the regulation of T cells by γc family cytokines. Nat. Rev. Immunol. 9, 480–490 (2009).
Lei, X. et al. Down-regulation of interleukin 7 receptor (IL-7R) contributes to central nervous system demyelination. Oncotarget 8, 28395–28407 (2017).
Churchman, S. M. et al. Modulation of peripheral T-cell function by interleukin-7 in rheumatoid arthritis. Arthritis Res. Ther. 16, 511 (2014).
Penaranda, C. et al. IL-7 receptor blockade reverses autoimmune diabetes by promoting inhibition of effector/memory T cells. Proc. Natl Acad. Sci. USA 109, 12668–12673 (2012).
Lee, L. -F. et al. Anti-IL-7 receptor-α reverses established type 1 diabetes in nonobese diabetic mice by modulating effector T-cell function. Proc. Natl Acad. Sci. USA 109, 12674–12679 (2012).
Belarif, L. et al. IL-7 receptor influences anti-TNF responsiveness and T cell gut homing in inflammatory bowel disease. J. Clin. Invest. 129, 1910–1925 (2019).
Watanabe, M. et al. Interleukin 7 transgenic mice develop chronic colitis with decreased interleukin 7 protein accumulation in the colonic mucosa. J. Exp. Med. 187, 389–402 (1998).
Saligrama, N. et al. Opposing T cell responses in experimental autoimmune encephalomyelitis. Nature 572, 481–487 (2019).
Li, J. et al. KIR+CD8+ T cells suppress pathogenic T cells and are active in autoimmune diseases and COVID-19. Science 376, eabi9591 (2022).
Sollid, L. M. The roles of MHC class II genes and post-translational modification in celiac disease. Immunogenetics 69, 605–616 (2017).
Anderson, R. P., Degano, P., Godkin, A. J., Jewell, D. P. & Hill, A. V. S. In vivo antigen challenge in celiac disease identifies a single transglutaminase-modified peptide as the dominant A-gliadin T-cell epitope. Nat. Med. 6, 337–342 (2000).
Petersen, J. et al. T-cell receptor recognition of HLA-DQ2–gliadin complexes associated with celiac disease. Nat. Struct. Mol. Biol. 21, 480–488 (2014).
Sollid, L. M. et al. Update 2020: nomenclature and listing of celiac disease-relevant gluten epitopes recognized by CD4+ T cells. Immunogenetics 72, 85–88 (2020).
Sette, A., Southwood, S., Miller, J. & Appella, E. Binding of major histocompatibility complex class II to the invariant chain-derived peptide, CLIP, is regulated by allelic polymorphism in class II. J. Exp. Med. 181, 677–683 (1995).
Korneychuk, N. et al. Interleukin 15 and CD4+ T cells cooperate to promote small intestinal enteropathy in response to dietary antigen. Gastroenterology 146, 1017–1027 (2014).
Glanville, J. et al. Identifying specificity groups in the T cell receptor repertoire. Nature 547, 94–98 (2017).
Broughton, S. E. et al. Biased T cell receptor usage directed against human leukocyte antigen DQ8-restricted gliadin peptides is associated with celiac disease. Immunity 37, 611–621 (2012).
Huang, H., Wang, C., Rubelt, F., Scriba, T. J. & Davis, M. M. Analyzing the Mycobacterium tuberculosis immune response by T-cell receptor clustering with GLIPH2 and genome-wide antigen screening. Nat. Biotechnol. 38, 1194–1202 (2020).
Dahal-Koirala, S. et al. Comprehensive analysis of CDR3 sequences in gluten-specific T-cell receptors reveals a dominant R-motif and several new minor motifs. Front. Immunol. 12, 639672 (2021).
Galeano Niño, J. L. et al. Cytotoxic T cells swarm by homotypic chemokine signalling. eLife 9, e56554 (2020).
Christophersen, A., Risnes, L. F., Dahal-Koirala, S. & Sollid, L. M. Therapeutic and diagnostic implications of T cell scarring in celiac disease and beyond. Trends Mol. Med. 25, 836–852 (2019).
Hamilton, J. A. GM-CSF-dependent inflammatory pathways. Front. Immunol. 10, 2055 (2019).
Olaussen, R. W. et al. Interferon-γ-secreting T cells localize to the epithelium in coeliac disease. Scand. J. Immunol. 56, 652–664 (2002).
Husby, S. et al. European Society Paediatric Gastroenterology, Hepatology and Nutrition guidelines for diagnosing coeliac disease 2020. J. Pediatr. Gastroenterol. Nutr. 70, 141–156 (2020).
Di Niro, R. et al. High abundance of plasma cells secreting transglutaminase 2-specific IgA autoantibodies with limited somatic hypermutation in celiac disease intestinal lesions. Nat. Med. 18, 441–445 (2012).
Kivelä, L. et al. Current and emerging therapies for coeliac disease. Nat. Rev. Gastroenterol. Hepatol. 18, 181–195 (2021).
Dooms, H. Interleukin-7: fuel for the autoimmune attack. J. Autoimmun. 45, 40–48 (2013).
Meresse, B. et al. Reprogramming of CTLs into natural killer-like cells in celiac disease. J. Exp. Med. 203, 1343–1355 (2006).
Lundin, K. E. et al. Gliadin-specific, HLA-DQ(α1*0501,β1*0201) restricted T cells isolated from the small intestinal mucosa of celiac disease patients. J. Exp. Med. 178, 187–196 (1993).
Ootani, A. et al. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat. Med. 15, 701–706 (2009).
Neal, J. T. et al. Organoid modeling of the tumor immune microenvironment. Cell 175, 1972–1988 (2018).
Maiuri, L. et al. Association between innate response to gliadin and activation of pathogenic T cells in coeliac disease. Lancet 362, 30–37 (2003).
Yang, H., Spencer, A. U. & Teitelbaum, D. H. Interleukin-7 administration alters intestinal intraepithelial lymphocyte phenotype and function in vivo. Cytokine 31, 419–428 (2005).
Porter, B. O. & Malek, T. R. Thymic and intestinal intraepithelial T lymphocyte development are each regulated by the γc-dependent cytokines IL-2, IL-7, and IL-15. Semin. Immunol. 12, 465–474 (2000).
Dong, L.-H., Lv, P. & Han, M. Roles of SM22α in cellular plasticity and vascular diseases. Cardiovasc. Hematol. Disord. Drug Targets 12, 119–125 (2012).
Watanabe, M. et al. Interleukin 7 is produced by human intestinal epithelial cells and regulates the proliferation of intestinal mucosal lymphocytes. J. Clin. Invest. 95, 2945–2953 (1995).
Christophersen, A. et al. Distinct phenotype of CD4+ T cells driving celiac disease identified in multiple autoimmune conditions. Nat. Med. 25, 734–737 (2019).
Gandini, A., Gededzha, M. P., De Maayer, T., Barrow, P. & Mayne, E. Diagnosing coeliac disease: a literature review. Hum. Immunol. 82, 930–936 (2021).
Berg, S. et al. ilastik: interactive machine learning for (bio)image analysis. Nat. Methods 16, 1226–1232 (2019).
Stirling, D. R. et al. CellProfiler 4: improvements in speed, utility and usability. BMC Bioinformatics 22, 433 (2021).
Ráki, M. et al. Tetramer visualization of gut-homing gluten-specific T cells in the peripheral blood of celiac disease patients. Proc. Natl Acad. Sci. USA 104, 2831–2836 (2007).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587 (2021).
Efremova, M., Vento-Tormo, M., Teichmann, S. A. & Vento-Tormo, R. CellPhoneDB: inferring cell–cell communication from combined expression of multi-subunit ligand–receptor complexes. Nat. Protoc. 15, 1484–1506 (2020).
Acknowledgements
We thank members of the Kuo, Davis, Mellins and Sollid groups for discussions; the Stanford FACS, Functional Genomics, Human Histology (P. Chu), Cell Sciences Imaging and Human Immune Monitoring cores for technical expertise; the Stanford Tissue Bank for providing surgical samples; E. Sanjines, A. Adiao, D. Souki, G. Tan and G. Masarweh for collection and delivery of endoscopy samples; B. Simonsen for the HLA-DQ molecules used for tetramer assembly; and J. and R. Triebsch for support from the Stanford Celiac Translational Research Program. This work was also supported by funding from the Stanford Medicine Children’s Health Center for IBD and Celiac Disease. V.v.U. was supported by a Netherlands Organization for Scientific Research Rubicon grant (452181214). We acknowledge funding from the South-Eastern Norway Regional Health Authority (projects 2016113 and 2020027 to L.M.S.), a Stanford Maternal Child Health Research Institute seed grant (C.J.K.), NIH RM1-HG007735 (H.Y.C.), NIH U19AI057229 (M.M.D.), NIH U01DK085527, U19AI116484, R01CA251514, R01DK130414, R01DK115728 (C.J.K.), the NIDDK Intestinal Stem Cell Consortium and the NIAID Biomimetic U19 Consortium. We dedicate this work to the memory of Elizabeth D. Mellins, whose experimental design and guidance were crucial for this study.
Author information
Authors and Affiliations
Contributions
A.J.M.S. conceived, designed and performed experiments, analysed data and wrote the manuscript. V.v.U. conceived experiments and analysed scRNA-seq data. Z.L. designed experiments, did organoid cultures, confocal imaging and analysis, analysed scRNA-seq data and did RT–qPCR. S.M.C. collected tissue FFPE blocks and coordinated sectioning for haplotyping and IL7 in situ hybridization and analysed data. N.H. did organoid cultures and RT–qPCR. A. Batish did RT–qPCR. J.E.C. measured and analysed organoid sizes. J.C. did organoid cultures, cryopreservation and recovery, imaging and analysis. E.T.Z. sectioned frozen tissue blocks and helped with their staining and imaging. Q.M. did organoid cultures. A.G.-S. did confocal imaging and analysis. M.T. did RT–qPCR and data analysis. D.C. sectioned FFPE tissue blocks and did IL7 in situ hybridization. S.V. did IL7 in situ hybridization. S.S.C. did confocal imaging and schematic design. A.C. produced HLA-DQ monomers for tetramer assembly. A. Baghdasaryan provided resources. K.E.Y. prepared libraries for scRNA-seq. K.K. used Cell Ranger for scRNA-seq. A.H. assisted with 10x Genomics cell capture. J.L. did HLA genotyping. H.D. provided resources and supervision. Z.M.S. provided samples and guidance. H.Y.C. provided resources and supervision. J.C.Y.D. provided surgical samples. B.M.Z. did HLA genotyping and analysis. E.D.M. conceived and designed experiments and provided resources and supervision. L.M.S. provided resources, supervision and guidance. N.Q.F.-B. identified CeD patients and controls, coordinated and collected endoscopy samples and provided guidance. M.M.D. provided resources, supervision and guidance. C.J.K. conceived and designed experiments, analysed data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
C.J.K. and A.J.M.S. are inventors on patent WO 2020/247528 describing methods and uses of patient-derived celiac intestinal organoids. C.J.K. and M.M.D. are founders of Mozart Therapeutics and NextVivo, Inc. L.M.S has been a consultant during the last 3 years for BMS, GSK, Mozart Therapeutics, Ono Pharmaceutical, Precigen ActoBio, Sanofi-Aventis, SQZ Biotech, Takeda and Topas Therapeutics. All other authors declare no competing interests.
Peer review
Peer review information
Nature thanks Nadine Cerf-Bensussan, Toshiro Sato and Detlef Schuppan for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Small intestine ALI organoids possess different mesenchymal and epithelial cell types.
a, IF whole-mount staining of small intestine organoids at day 14 showing SMA+ or PDGFRA+ fibroblasts, CD31+ endothelial cells and PGP9.5+ neurons (green), ECAD+ epithelium (white) and DAPI (blue) (representative images from n = 3 biological replicates). b, IF whole-mount staining of small intestine organoids at day 14 showing MUC2+ goblet cells, CHGA+ enteroendocrine cells, LYZ1+ Paneth cells (green), ECAD+ epithelium (white) and DAPI (blue) (representative images from n = 3 biological replicates). c, Enlargement of organoid cup-shaped MUC2+ goblet cells (representative image from n = 3 biological replicates). d, Violin plots of CD14 and CD68 mRNA expression from CeD organoid scRNA-seq and a scatter plot of CD14 and CD68 mRNA co-expression in the myeloid compartment. e, Whole-mount IF staining of small intestine ALI organoid CD4+ (green) and CD8+ (red) T cells, showing enrichment of CD8+ T cells within the EPCAM+ epithelial compartment (white). DAPI (blue). In contrast, CD4+ T cells localize to non-epithelial lamina propria-like areas (representative image from n = 3 biological replicates). All scale bars are 100 µm, except (c) in which the scale bar is 50 µm.
Extended Data Fig. 2 Duodenal ALI organoids contain diverse immune populations, related to Fig. 1.
a, Integrated UMAP plot of CD45+-sorted cells from scRNA-seq, revealing diverse immune populations in small intestine ALI organoids at day 14, n = 6 CeD patients. b, Violin plots showing expression of genes used to identify the immune populations shown in (a). c, UMAP plots of overlap between tissue and organoid CD45+ immune populations as in (a, b). d, scRNA-seq Jaccard index of TCR overlap between fresh small intestine tissue (n = 1 CeD patient) and ALI organoids (n = 4 CeD patients). e, Integrated UMAP from scRNA-seq of active CeD organoid T cells (n = 6 patients). Cells expressing KIR3DL1 or KIR2DL3 are rendered in red. f, Plot of CD8+ T cells from (e). g, Integrated UMAP from scRNA-seq of active CeD organoid T cells (top left) (n = 6 patients). Cells in red exhibit expression of KIR3DL1 or KIR2DL3 (top right), NKG2C (bottom left) and NKG2D (bottom right). h, Pie bar graph showing organoid-derived TCR counts in which each segment represents a unique clonotype, n = 5 patients. Expanded clonotypes (TCR counts ≥ 2) are indicated in red.
Extended Data Fig. 3 Cytokine supplementation and cryopreservation of intestinal ALI organoids.
a, FACS-based tSNE plots depicting time course abundance of EPCAM+ and CD45+ cells (top) and CD4+ and CD8+ T cells (bottom) as a percentage of total live single ileal organoid cells with or without addition of IL-2 and IL-7, representative experiment of n = 3 biological replicates. b, Organoids grown for 14 days (control) have similar percentages of epithelium and immune components as organoids grown for 5 days, frozen in-gel at −80 °C, cryorecovered, and replated for the indicated durations. c-d, ALI organoids demonstrate persistent growth after being frozen in-gel at −80 °C, cryorecovered and replated (c, arrows), with maintenance of epithelial protrusions by H&E (d). Numerous air bubbles in the collagen are present on initial plating post-cryorecovery and progressively disappear with culture. (b-d) depict representative experiments from n = 4 biological replicates. Scale bar is 5 mm for (c) and 100 µm for (d).
Extended Data Fig. 4 Gliadin induces loss of villus-like structures in CeD organoids.
a, Duodenal ALI organoids from celiac (CeD) or non-celiac control donors were established for 9–12 days followed by gliadin or CLIP treatment for 2 days before analysis, unless stated otherwise. The gliadin peptides were a 1:1 mixture of deamidated immunodominant, HLA-DQ2.5-restricted, glia-α1 (LQPFPQPELPYPGS) and glia-α2 (APQPELPYPQPGS) gluten epitopes. b-d, Confirmatory IF staining of sections of human duodenum tissue showing IL-15 (red) in (a), SI (red) in (b) and APOA4 (red) in (c); DAPI (blue) (representative images from n = 3 biological replicates). e, Quantification of SI mRNA in FACS-sorted organoid EPCAM+ cells from 2-day gliadin-treated control or active CeD organoids. RT-qPCR, expressed as a ratio of gliadin:CLIP treatment, from control (n = 4) or CeD (n = 5) biological replicates. Box plots show the median as the center line, the interquartile range as the box limits and the whiskers represent the min and max. *, P = 0.0381; two-tailed Mann-Whitney test. f, Representative H&E staining of different sections of control or active CeD organoids after 2-day gliadin or CLIP treatment. Arrows denote regions where epithelial protrusions are absent. g, Quantification of epithelial protrusions per organoid circumference from (f); control (N = 6 biological replicates), CeD (N = 7 biological replicates), each data point is from an individual organoid. Scatter plots show the median as the center line and the whiskers represent the min and max. ***=P < 0.0001; two-tailed Mann-Whitney test. All scale bars are 100 µm. All CeD organoids were DQ2.5+.
Extended Data Fig. 5 Gliadin induces epithelial proliferation in CeD organoids.
a, Representative IF staining of sections of active CeD organoids after 2-day gliadin or CLIP treatment in EN media showing proliferative KI67+ cells (green), ECAD (red) and DAPI (blue). Scale bar is 50 µm. b, Quantification of KI67 fluorescence from (a), control (n = 3 biological replicates), CeD (n = 5 biological replicates); each data point is from an individual organoid. ***, P < 0.0001; two-tailed Mann-Whitney test. c, Representative brightfield images of active CeD organoids before and after 2-day treatment with gliadin or CLIP peptides. Scale bar is 5 mm. d, Automated quantification of fold change in CeD organoid area from (c), 2 days after treatment with gliadin or CLIP. n = 10 CeD patients. **, P = 0.002; two-tailed Wilcoxon test. e, LGR5 RT-qPCR from FACS-sorted organoid EPCAM+ cells as ratio of gliadin:CLIP treatment for 2 days in organoids from control (n = 7 biological replicates) or active CeD (n = 8 biological replicates). **, P = 0.0012; two-tailed Mann-Whitney test. f, PCNA RT-qPCR from FACS-sorted organoid EPCAM+ cells as ratio of gliadin:CLIP treatment for 2 days in control or active CeD organoids, (n = 7 biological replicates each). **, P = 0.007; two-tailed Mann-Whitney test. g, CCND1 RT-qPCR from FACS-sorted organoid EPCAM+ cells as ratio of gliadin:CLIP treatment for 2 days in organoids from control (n = 8 biological replicates) or active CeD (n = 7 biological replicates). ***, P = 0.0003; two-tailed Mann-Whitney test. All box plots show the median as the center line, the interquartile range as the box limits and the whiskers represent the min and max. All CeD organoids were DQ2.5+.
Extended Data Fig. 6 TCR sequencing from CeD organoid scRNA-seq reveals known and suspected gliadin-specific TCR motifs.
a, scRNA-seq integrated UMAP plot highlighting TCR-expressing T cells in active CeD organoids at day 14, n = 5 CeD patients. b, GLIPH homology analysis showing conserved CDR3 motifs (red) found between active CeD organoids and gliadin-specific published sequences, n = 5 CeD patients. All CeD organoids in this figure were HLA-DQ2.5+.
Extended Data Fig. 7 Two additional biological replicates of scRNA-seq-derived dot plots.
Dot plots from organoid scRNA-seq from two active CeD patients (a, b); a third patient is shown in Fig. 4a. Depiction of mean expression levels and corresponding percent population expression amongst active CeD organoid CD4+ and CD8+ T cells, Treg, plasma B cells and myeloid cells after 2-day gliadin or CLIP treatment. The patient in (a) is HLA-DQ2.5, as is the patient in Fig. 4a. The patient in (b) is HLA-DQ2.2, which manifests low-affinity binding to the HLA-DQ2.5 gliadin peptides used in the study (Bodd et al, Gastroenterology, 2012 Mar;142(3):552-61).
Extended Data Fig. 8 ScRNA-seq-based interactome analysis of novel gliadin-induced immune interactions in CeD organoids.
a, Overview of unique CellPhoneDB immune interactions found in 2-day CLIP- or gliadin-treated active CeD organoids, stratified by immune cell type (CD4+ T, CD8+ T, myeloid, NK and Treg cells). Columns indicate sending:receiving cell type and rows indicate ligand-receptor pairs. P values are indicated by circle size. The mean (log2) average expression levels of interacting molecule 1 and interacting molecule 2 are indicated by the color gradient. b, Corresponding schematic showing potential interactions between immune cells in CeD. Integrated data from n = 4 CeD patients, 3 DQ2.5+ and 1 DQ2.2+.
Extended Data Fig. 9 Sc-RNAseq-based interactome of B cells and plasma cells, and BCR sequence consensus.
a, B cell- and plasma B cell-specific immune interactomes derived from scRNA-seq CellPhoneDB analysis showing 117 unique B and plasma-cell driven interactions in gliadin-treated organoids and absence of unique interactions in CLIP, integrated data from n = 4 CeD patients. b, BCR sequence consensus analysis from scRNA-seq of matched CDR3 sequences from active CeD organoids and published anti-TG2 CDR3 sequences categorized by length; n = 3 CeD patients.
Extended Data Fig. 10 BCR sequencing from CeD organoid scRNA-seq reveals extensive overlap with public anti-TG2 CeD-specific motifs.
a, scRNA-seq integrated UMAP plot highlighting BCR-expressing B and plasma cells in active CeD organoids, n = 3 CeD patients. b, Homology analysis showing conserved CDR3 sequences (red) found between active CeD organoids and anti-TG2 CeD-specific published sequences, n = 3 CeD patients.
Extended Data Fig. 11 IL-7 is upregulated in active celiac duodenal biopsy tissue.
a, Luminex protein analysis of organoid conditioned media from active CeD (N = 7 biological replicates) or control (N = 4 biological replicates) showing fold-increases of IL-7 as ratio of gliadin:CLIP treatment after 2 days. Box plots show the median as the center line, the interquartile range as the box limits and the whiskers represent the min and max. ns, P = 0.072; two-tailed Mann-Whitney test. b-c, Representative IF staining using a rabbit (Rb) anti-IL-7 antibody (red) in fresh duodenal biopsies from (b) 14 remission CeD patients (previously diagnosed with CeD but on gluten-free diet) versus (c) 14 CeD patients with active disease, showing increased IL-7 levels in the latter. Epithelium (CK19, green); DAPI (blue). Figure 6c shows staining for a 15th patient in remission and a 15th patient with active CeD, and quantitation is presented in Fig. 6d. (GFD, n = 15 donors) or active CeD (n = 15 donors). d, Representative IF staining using a mouse (Ms) anti-IL-7 antibody (red) in fresh duodenal biopsies from (b), 4 remission CeD patients versus 4 patients with active CeD. This confirmed elevated IL-7 expression in active CeD seen with a different antibody than in (b) and (c). Scale bars are 100 µm.
Supplementary information
Supplementary Information
This file contains sequential FACS gating strategy, donor DQ-typing and demographics, and primer sequences used for RT-qPCR.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Santos, A.J.M., van Unen, V., Lin, Z. et al. A human autoimmune organoid model reveals IL-7 function in coeliac disease. Nature 632, 401–410 (2024). https://doi.org/10.1038/s41586-024-07716-2
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41586-024-07716-2
This article is cited by
-
The application of organoids in investigating immune evasion in the microenvironment of gastric cancer and screening novel drug candidates
Molecular Cancer (2025)
-
Adapting systems biology to address the complexity of human disease in the single-cell era
Nature Reviews Genetics (2025)
-
Boosting human immunology: harnessing the potential of immune organoids
EMBO Molecular Medicine (2025)
-
Human liver immunology: from in vitro models to new insights
Cellular & Molecular Immunology (2025)
-
RNAseq of peripheral blood mononucleated cells exposed to platelet-rich fibrin and enamel matrix derivatives
Scientific Reports (2025)