Abstract
Patients with treatment-refractory pancreatic cancer often succumb to systemic metastases1,2,3; however, the transcriptomic heterogeneity that underlies therapeutic recalcitrance remains understudied, particularly in a spatial context. Here we construct high-resolution maps of lineage states, clonal architecture and the tumour microenvironment (TME) using spatially resolved transcriptomics from 55 samples of primary tumour and metastases (liver, lung and peritoneum) collected from rapid autopsies of 13 people. We observe discernible transcriptomic shifts in cancer-cell lineage states as tumours transition from primary sites to organ-specific metastases, with the most pronounced intra-patient distinctions between liver and lung. Phylogenetic trees constructed from inferred copy number variations in primary and metastatic loci in each patient highlight diverse patient-specific evolutionary trajectories and clonal dissemination. We show that multiple tumour lineage states co-exist in each tissue, including concurrent metastatic foci in the same organ. Agnostic to tissue site, lineage states correlate with distinct TME features, such as the spatial proximity of TGFB1-expressing myofibroblastic cancer-associated fibroblasts (myCAFs) to aggressive ‘basal-like’ cancer cells, but not to cells in the ‘classical’ or ‘intermediate’ states. These findings were validated through orthogonal and cross-species analyses using mouse tissues and patient-derived organoids. Notably, basal-like cancer cells aligned with myCAFs correlate with plasma-cell exclusion from the tumour milieu, and neighbouring cell analyses suggest that CXCR4–CXCL12 signalling is the underlying basis for observed immune exclusion. Collectively, our findings underscore the profound transcriptomic heterogeneity and microenvironmental dynamics that characterize treatment-refractory pancreatic cancer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The raw and processed spatial transcriptome data and CosMx data generated in this study have been deposited at the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO) with accession numbers GSE274557 and GSE277782. The combined datasets can be accessed at GEO with accession number GSE277783.
Code availability
All R and Python scripts supporting the findings of this study are available in the GitHub repository at https://github.com/Coolgenome/PDAC.
References
Halbrook, C. J., Lyssiotis, C. A., Pasca di Magliano, M. & Maitra, A. Pancreatic cancer: advances and challenges. Cell 186, 1729–1754 (2023).
Hidalgo, M. et al. Addressing the challenges of pancreatic cancer: future directions for improving outcomes. Pancreatology 15, 8–18 (2015).
Park, W., Chawla, A. & O’Reilly, E. M. Pancreatic cancer: a review. JAMA 326, 851–862 (2021).
Groot, V. P. et al. Implications of the pattern of disease recurrence on survival following pancreatectomy for pancreatic ductal adenocarcinoma. Ann. Surg. Oncol. 25, 2475–2483 (2018).
Liu, K. H. et al. Lung metastases in patients with stage IV pancreatic cancer: prevalence, risk factors, and survival impact. J. Clin. Med. 8, 1402 (2019).
Sahin, I. H., Elias, H., Chou, J. F., Capanu, M. & O’Reilly, E. M. Pancreatic adenocarcinoma: insights into patterns of recurrence and disease behavior. BMC Cancer 18, 769 (2018).
Yachida, S. et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 467, 1114–1117 (2010).
Yachida, S. et al. Clinical significance of the genetic landscape of pancreatic cancer and implications for identification of potential long-term survivors. Clin. Cancer Res. 18, 6339–6347 (2012).
Makohon-Moore, A. P. et al. Limited heterogeneity of known driver gene mutations among the metastases of individual patients with pancreatic cancer. Nat. Genet. 49, 358–366 (2017).
Reiter, J. G. et al. Minimal functional driver gene heterogeneity among untreated metastases. Science 361, 1033–1037 (2018).
Notta, F. et al. A renewed model of pancreatic cancer evolution based on genomic rearrangement patterns. Nature 538, 378–382 (2016).
McDonald, O. G. et al. Epigenomic reprogramming during pancreatic cancer progression links anabolic glucose metabolism to distant metastasis. Nat. Genet. 49, 367–376 (2017).
Kim, M. S. et al. Heterogeneity of pancreatic cancer metastases in a single patient revealed by quantitative proteomics. Mol. Cell. Proteomics 13, 2803–2811 (2014).
Erickson, A. et al. Spatially resolved clonal copy number alterations in benign and malignant tissue. Nature 608, 360–367 (2022).
O’Kane, G. M. et al. GATA6 expression distinguishes classical and basal-like subtypes in advanced pancreatic cancer. Clin. Cancer Res. 26, 4901–4910 (2020).
Raghavan, S. et al. Microenvironment drives cell state, plasticity, and drug response in pancreatic cancer. Cell 184, 6119–6137 (2021).
Wormann, S. M. et al. APOBEC3A drives deaminase domain-independent chromosomal instability to promote pancreatic cancer metastasis. Nat. Cancer 2, 1338–1356 (2021).
Collisson, E. A. et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat. Med. 17, 500–503 (2011).
Bailey, P. et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 531, 47–52 (2016).
Gavish, A. et al. Hallmarks of transcriptional intratumour heterogeneity across a thousand tumours. Nature 618, 598–606 (2023).
Gabitova-Cornell, L. et al. Cholesterol pathway inhibition induces TGF-β signaling to promote basal differentiation in pancreatic cancer. Cancer Cell 38, 567–583 (2020).
Zhang, D. et al. Inferring super-resolution tissue architecture by integrating spatial transcriptomics with histology. Nat. Biotechnol. 42, 1372–1377 (2024).
Elyada, E. et al. Cross-species single-cell analysis of pancreatic ductal adenocarcinoma reveals antigen-presenting cancer-associated fibroblasts. Cancer Discov. 9, 1102–1123 (2019).
Sahai, E. et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 20, 174–186 (2020).
Dilly, J. et al. Mechanisms of resistance to oncogenic KRAS inhibition in pancreatic cancer. Cancer Discov. 14, 2135–2161 (2024).
Zhang, Y. et al. Macropinocytosis in cancer-associated fibroblasts is dependent on CaMKK2/ARHGEF2 signaling and functions to support tumor and stromal cell fitness. Cancer Discov. 11, 1808–1825 (2021).
Ma, R. Y., Black, A. & Qian, B. Z. Macrophage diversity in cancer revisited in the era of single-cell omics. Trends Immunol. 43, 546–563 (2022).
Blando, J. et al. Comparison of immune infiltrates in melanoma and pancreatic cancer highlights VISTA as a potential target in pancreatic cancer. Proc. Natl Acad. Sci. USA 116, 1692–1697 (2019).
Carstens, J. L. et al. Spatial computation of intratumoral T cells correlates with survival of patients with pancreatic cancer. Nat. Commun. 8, 15095 (2017).
Falcomata, C. et al. Context-specific determinants of the immunosuppressive tumor microenvironment in pancreatic cancer. Cancer Discov. 13, 278–297 (2023).
Biffi, G. et al. IL1-induced JAK/STAT signaling is antagonized by TGFβ to shape CAF heterogeneity in pancreatic ductal adenocarcinoma. Cancer Discov. 9, 282–301 (2019).
Feig, C. et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc. Natl Acad. Sci. USA 110, 20212–20217 (2013).
Jiang, H. et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat. Med. 22, 851–860 (2016).
He, S. et al. High-plex imaging of RNA and proteins at subcellular resolution in fixed tissue by spatial molecular imaging. Nat. Biotechnol. 40, 1794–1806 (2022).
Connor, A. A. et al. Integration of genomic and transcriptional features in pancreatic cancer reveals increased cell cycle progression in metastases. Cancer Cell 35, 267–282 (2019).
Chan-Seng-Yue, M. et al. Transcription phenotypes of pancreatic cancer are driven by genomic events during tumor evolution. Nat. Genet. 52, 231–240 (2020).
Moffitt, R. A. et al. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat. Genet. 47, 1168–1178 (2015).
Puleo, F. et al. Stratification of pancreatic ductal adenocarcinomas based on tumor and microenvironment features. Gastroenterology 155, 1999–2013 (2018).
Lee, J. J. et al. Elucidation of tumor-stromal heterogeneity and the ligand-receptor interactome by single-cell transcriptomics in real-world pancreatic cancer biopsies. Clin. Cancer Res. 27, 5912–5921 (2021).
Topham, J. T. et al. Subtype-discordant pancreatic ductal adenocarcinoma tumors show intermediate clinical and molecular characteristics. Clin. Cancer Res. 27, 150–157 (2021).
Williams, H. L. et al. Spatially resolved single-cell assessment of pancreatic cancer expression subtypes reveals co-expressor phenotypes and extensive intratumoral heterogeneity. Cancer Res. 83, 441–455 (2023).
Wang, Z. et al. Carcinomas assemble a filamentous CXCL12-keratin-19 coating that suppresses T cell-mediated immune attack. Proc. Natl Acad. Sci. USA 119, e2119463119 (2022).
Yan, R., Moresco, P., Gegenhuber, B. & Fearon, D. T. T cell-mediated development of stromal fibroblasts with an immune-enhancing chemokine profile. Cancer Immunol. Res. 11, 1044–1054 (2023).
Meylan, M. et al. Tertiary lymphoid structures generate and propagate anti-tumor antibody-producing plasma cells in renal cell cancer. Immunity 55, 527–541 (2022).
Law, H. C. et al. The proteomic landscape of pancreatic ductal adenocarcinoma liver metastases identifies molecular subtypes and associations with clinical response. Clin. Cancer Res. 26, 1065–1076 (2020).
Stuart, T. et al. Comprehensive integration of single-cell data. Cell 177, 1888–1902 (2019).
McInnes, L., Healy, J., Saul, N. & Grossberger, L. UMAP: uniform manifold approximation and projection. J. Open Source Softw. 3, 861 (2018).
Chu, Y. et al. Pan-cancer T cell atlas links a cellular stress response state to immunotherapy resistance. Nat. Med. 29, 1550–1562 (2023).
Wang, R. et al. Evolution of immune and stromal cell states and ecotypes during gastric adenocarcinoma progression. Cancer Cell 41, 1407–1426 (2023).
Wang, R. et al. Single-cell dissection of intratumoral heterogeneity and lineage diversity in metastatic gastric adenocarcinoma. Nat. Med. 27, 141–151 (2021).
Sans, M. et al. Spatial transcriptomics of intraductal papillary mucinous neoplasms of the pancreas identifies NKX6-2 as a driver of gastric differentiation and indolent biological potential. Cancer Discov. 13, 1844–1861 (2023).
Yu, G., Smith, D. K., Zhu, H., Guan, Y. & Lam, T. T. Y. ggtree: an R package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods Ecol. Evol. 8, 28–36 (2017).
Gao, R. et al. Delineating copy number and clonal substructure in human tumors from single-cell transcriptomes. Nat. Biotechnol. 39, 599–608 (2021).
De Falco, A., Caruso, F., Su, X.-D., Iavarone, A. & Ceccarelli, M. A variational algorithm to detect the clonal copy number substructure of tumors from scRNA-seq data. Nat. Commun. 14, 1074 (2023).
Schliep, K. phangorn: phylogenetic analysis in R. Bioinformatics 27, 592–593 (2011).
Liberzon, A. et al. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 1, 417–425 (2015).
Hao, D. et al. The single-cell immunogenomic landscape of B and plasma cells in early-stage lung adenocarcinoma. Cancer Discov. 12, 2626–2645 (2022).
Liao, Y., Wang, J., Jaehnig, E. J., Shi, Z. & Zhang, B. WebGestalt 2019: gene set analysis toolkit with revamped UIs and APIs. Nucleic Acids Res. 47, W199–W205 (2019).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300 (1995).
Ohlund, D. et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J. Exp. Med. 214, 579–596 (2017).
Cable, D. M. et al. Robust decomposition of cell type mixtures in spatial transcriptomics. Nat. Biotechnol. 40, 517–526 (2022).
Wei, R. et al. Spatial charting of single-cell transcriptomes in tissues. Nat. Biotechnol. 40, 1190–1199 (2022).
Vahid, M. R. et al. High-resolution alignment of single-cell and spatial transcriptomes with CytoSPACE. Nat. Biotechnol. 41, 1543–1548 (2023).
Pei, G. et al. deCS: a tool for systematic cell type annotations of single-cell RNA sequencing data among human tissues. Genomics Proteomics Bioinformatics 21, 370–384 (2023).
Belle, J. I. & DeNardo, D. G. A single-cell window into pancreas cancer fibroblast heterogeneity. Cancer Discov. 9, 1001–1002 (2019).
Boyd, L. N. C., Andini, K. D., Peters, G. J., Kazemier, G. & Giovannetti, E. Heterogeneity and plasticity of cancer-associated fibroblasts in the pancreatic tumor microenvironment. Semin. Cancer Biol. 82, 184–196 (2022).
Hanzelmann, S., Castelo, R. & Guinney, J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinformatics 14, 7 (2013).
Acknowledgements
A.M. was supported by the MD Anderson Pancreatic Cancer Moon Shot Program; the Sheikh Khalifa Bin Zayed Al-Nahyan Foundation; Break Through Cancer; and NIH grants (U54CA274371, U01CA200468 and U24CA274274). L.W. was supported by NIH–NCI grants (R01CA266280, U24CA274274, U01CA294518 and U01CA264583); research funding provided by the James P. Allison Institute; the Institute for Data Science in Oncology; the University of Texas MD Anderson Cancer Center; and Break Through Cancer. L.W. is an Associate Member of the James P. Allison Institute and an Andrew Sabin Family Foundation Fellow at the MD Anderson Cancer Center. G.P. acknowledges support from NIH–NCI grant U01CA294518 and the Program for T Cell-based Therapy at the MD Anderson Cancer Center. A.S. was supported by the the ACCENT (B-487.0012) and BONFOR program (O-112.0070). A.M.L. was supported by a NIH grant (U01CA274295). A.J.A. was funded by the Hale Center for Pancreatic Cancer Research; Break Through Cancer; the Lustgarten Foundation; the Pancreatic Cancer Action Network; NIH–NCI grants (P50CA127003, U01CA274276 and R01CA276268); and the Dana-Farber Cancer Institute Hale Center for Pancreatic Cancer Research. P.M.G. and M.A.H. were supported by the Pancreatic Cancer Detection Consortium (U01CA210240); a NCI Cancer Center Support grant (P30CA36727); and a NCI Research Specialist award (R50CA211462). The cyclic IF staining was performed in the Flow Cytometry and Cellular Imaging Core Facility, which is supported in part by the NIH through MD Anderson’s Cancer Center Support grant (P30CA016672), the NCI’s Research Specialist 1 (R50CA243707-01A1) and a Shared Instrumentation award from the Cancer Prevention Research Institution of Texas (CPRIT). We thank A. S. Multani for the FISH experiments and S. P. So, E. E. Rodriguez, A. T. Reckard, Y. A. Zuberi, A. V. Basi and J. A. Gomez for technical assistance.
Author information
Authors and Affiliations
Contributions
L.W. and A.M. conceived and jointly supervised the study. V. Branchi, J.L.G., T.C.C., P.M.G. and M.A.H. contributed to sample and patient information collection. V. Branchi and B.C.S. processed tissues and prepared libraries for SRT. A.M., V. Branchi and D.S. contributed to pathology review. L.W. supervised the processing, analysis and interpretation of bioinformatics data. D.Z. and M.L. contributed to tool development. Y.L., K.S.C., Y.C., E.D., V. Bernard, A.S. and G.H. assisted with data analysis. V. Branchi, G.P. and K.I.R. contributed to the processing of sequencing data and integrative analyses. F.T., K.T., B.G., H.T., A.M.L., and A.J.A. provided mouse tissues, human organoid lines, human CAF lines and relevant resources. J.K.B. provided support and resources for cyclic IF. J.M. performed in vitro experiments and data analysis. G.P. and J.M. analysed data and generated figures and tables for the manuscript. G.P., V. Branchi, J.M., K.I.R., C.Y., P.A.G., L.W. and A.M. contributed to data interpretation. J.M., G.P., L.W. and A.M. wrote and revised the manuscript, and all co-authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
A.M. is listed as an inventor on a patent that has been licensed by Johns Hopkins University to Thrive Earlier Detection and serves as a consultant for Tezcat Biosciences. A.J.A. has consulted for Anji Pharmaceuticals, Affini-T Therapeutics, Arrakis Therapeutics, AstraZeneca, Boehringer Ingelheim, Kestrel Therapeutics, Merck, Mirati Therapeutics, Nimbus Therapeutics, Oncorus, Plexium, Quanta Therapeutics, Revolution Medicines, Reactive Biosciences, Riva Therapeutics, Servier Pharmaceuticals, Syros Pharmaceuticals, T-knife Therapeutics, Third Rock Ventures and Ventus Therapeutics; holds equity in Riva Therapeutics and Kestrel Therapeutics; and has research funding from Boehringer Ingelheim, Bristol Myers Squibb, Deerfield, Eli Lilly, Mirati Therapeutics, Novartis, Novo Ventures, Revolution Medicines and Syros Pharmaceuticals. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature thanks Jen Jen Yeh, Nancy Zhang and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 UMAP plots of 134,776 SRT spots from 13 patients.
a,b, Each dot represents a single spot coloured by (a) patient and (b) treatment history. c,d, Venn diagrams showing the overlap of significantly upregulated genes in the treated group (c) or untreated group (d) across four different tissue sites.
Extended Data Fig. 2 Workflow for the construction of phylogenetic clone trees based on SRT data.
The workflow comprises four key steps: (1) annotation of tumour spots, (2) inference of CNVs at spot level, (3) construction of phylogenetic tree, and (4) spatial mapping of inferred subclones.
Extended Data Fig. 3 Phylogenetic clone trees reveal diverse evolutionary patterns in PDAC.
a, Pt-3. b, Pt-6. c, Pt-12.
Extended Data Fig. 4 FISH validation of MYC (8q24.2).
a,e, Spatial visualization of subclones from Fig. 2, along with fluorescence images for MYC (red) and control (Ctrl, green) probes targeting the centromere of chromosome 8 in Pt-10 (a) and Pt-1 (e). Dotted boxes and numbers indicate the enlarged areas. b,f, MYC CNV scores from the inferCNV analysis are shown in b (Pt-10) and f (Pt-1). Each dot represents each subclone. Data are presented as mean ± standard deviation (SD). For Pt-10 (b), clone A (n = 2), clone B (n = 3), clone C (n = 5) and clone D (n = 1). For Pt-1 (f), clone A (n = 2), clone B (n = 3) and clone C (n = 5). c,g, Per-cell copy number (CN) ratio for MYC in Pt-10 (c) and Pt-1 (g). d,h, Percentages of cells with MYC amplification (CN ratio over 1) in Pt-10 (d) and Pt-1 (h).
Extended Data Fig. 5 Characterization of lineage-state heterogeneity and plasticity.
a, UMAP plot displaying 67,990 “neoplastic” spots cross lineage states (left), patients (middle) or tissue origin sites (right). b, Bar plots displaying the relative fraction of tumour spots in classical, intermediate, and basal lineage states in each ST sample. The samples were ordered by tissue sites and the fraction of basal lineage. The top pie charts represent the global lineage composition in all Pri, LiM, PerM and LuM sites. c, Co-immunostaining of PanCK (green), S100A2 (red), and GATA6 (blue) in matched liver and lung metastases of three KPCY mice. Dotted boxes indicate the enlarged areas. d, Mean intensity of S100A2 and GATA6 across individual cells in tumour ROIs of liver and lung metastases. Each dot with connected lines represents data from the same mouse (n = 3). Student’s paired t-test. P values are indicated above the plot.
Extended Data Fig. 6 Subtyping of mesenchymal and squamous components in the basal-lineage-enriched tumour spots.
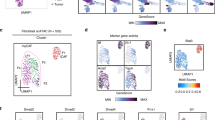
a, Pearson correlation analysis among classical, basal lineage, mesenchymal, squamous, and 8 core basal gene signature scores across all tumour spots. b, Bar plots comparing the relative fraction of tumour spots with mesenchymal and squamous lineages in each ST sample. c, Overview of 41 MPs among classical, intermediate, mesenchymal, and squamous lineage tumour enriched spots.
Extended Data Fig. 7 Comparison of tumour lineage compositions and tumour locations.
a, Schematic diagram illustrating the redefinition of tumour regions based on their distance from non-tumour areas, categorizing them into tumour edge, intermediate, and core regions. b,c, Total spot number (left) and relative composition (right) of tumour spots from the three main different lineages (b), with further subtyping of the basal lineage into mesenchymal and squamous lineages (c), among tumour-edge, tumour-intermediate, and tumour-core regions. d, Overview of 41 MPs based on tumour regions.
Extended Data Fig. 8 Spatial proximity between basal-like tumour cells and myCAFs.
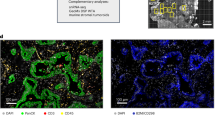
a, Co-immunostaining of PanCK (green), S100A2 (red), and DAPI (blue) in matched liver and lung metastases of three different KPCY mice. White lines denote the tumour bed, and yellow lines denote the juxtalesional areas. b, Mean intensity of α-SMA across individual cells in juxtalesional ROIs between liver (LiM) and lung (LuM) metastases. For mouse 1, n = 86,763 cells (LiM) and n = 4,125 cells (LuM). For mouse 2, n = 21,310 cells (LiM) and n = 5,101 cells (LuM). For mouse 3, n = 194,729 cells (LiM) and n = 11,861 cells (LuM). Each dot with connected lines represents data from the same mouse. Student’s paired t-test. ****P < 0.0001. c, A representative image of E-cadherin (white), α-SMA (green), S100A2 (red), and GATA6 (blue) in a liver metastasis containing both classical-like and basal-like tumour cells. White arrows with numbers denote the enlarged areas. d, Phase contrast, H&E staining, and co-immunostaining for S100A2 (red), α-SMA (green), and DAPI (blue) in classical-like and basal-like PDOs cultured with human CAFs. Full PDO IDs are as follows: 185; PANFR0185_T2, 332; PANFR0332_T1, 172; PANFR0172_T4, and 440; PANFR0440_T1. e, Quantification of the percentage of organoids displaying direct attachments of α-SMA+ CAFs in each PDO line. Each dot represents an individual CAF line. Data are presented as mean ± SD (n = 3, independent experiments). Student’s unpaired t-test. *P < 0.05 (P = 0.014 in 185 vs. 172, P = 0.01 in 185 vs. 440), **P < 0.01 (P = 0.006 in 332 vs. 172, P = 0.005 in 332 vs. 440).
Extended Data Fig. 9 Absence of T cell and B cell infiltration in PDAC SRT samples.
From top to bottom, the images show tumour lineage distribution, iStar-derived T cell signature, iStar-derived B cell signature, and expression levels of CD3D and MS4A1.
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1–12.
Supplementary Tables
This file contains Supplementary Tables 1–10.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pei, G., Min, J., Rajapakshe, K.I. et al. Spatial mapping of transcriptomic plasticity in metastatic pancreatic cancer. Nature 642, 212–221 (2025). https://doi.org/10.1038/s41586-025-08927-x
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41586-025-08927-x
This article is cited by
-
Machine learning-based clinical prediction model and multi-omics integration for assessing pancreatic cancer risk in new-onset diabetes
Journal of Translational Medicine (2026)
-
CRISPR screen of human pancreatic cancer xenografts identifies a KLF5 proliferation vulnerability through epigenetic modifiers NCAPD2 and MTHFD1
Molecular Cancer (2026)
-
Heterocellular crosstalk and architecture of the pancreatic tumour microenvironment
Nature Reviews Cancer (2026)
-
Neoadjuvant modified FOLFIRINOX plus nivolumab in borderline-resectable pancreatic ductal adenocarcinoma: a pilot phase 1 trial
Nature Communications (2026)
-
SPICEiST: subcellular RNA pattern enhances cell clustering of imaging-based spatial transcriptomics
Genomics & Informatics (2025)