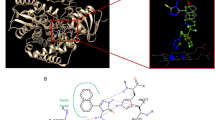
Abstract
Negamycin, a hydrazine-containing dipeptide-like antibiotic, was first isolated in 1970 from three strains of Streptomyces purpeofuscus. Its pronounced antibacterial properties render it an appealing candidate for combating multi-drug-resistant Gram-negative bacteria. Additionally, the unique readthrough-promoting activity makes it a subject for research as a potential therapeutic agent for Duchenne muscular dystrophy and other hereditary diseases. Here we use the unusual (R)-β-lysine found in negamycin as a guide to identify the biosynthetic pathway of negamycin and then carry out gene deletion and chemical complementation, stable isotope feeding and enzyme assays to elucidate the key precursors for negamycin assembly. Our work identified NegB as a lysine-2,3-aminomutase that converts lysine into (R)-β-lysine and NegJ as a heme-dependent, N–N bond-forming enzyme. We show that NegJ, together with a ferredoxin encoded outside of the negamycin gene cluster, directly forms hydrazinoacetic acid from glycine and nitrite. NegJ is a novel biocatalyst for N–N bond formation, and our work highlights its potential for genome mining of N–N bond-containing natural products.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The sequence of the negamycin biosynthetic gene cluster (neg gene cluster) in this study has been deposited to NCBI (GenBank accession no. PQ860822). The accession numbers of all ferredoxins, Spb and CreD/E homologs from strain S. purpeofuscus ATCC 21470 are provided in Supplementary Data 1. The remaining data that support the findings of this study are available within the main text and the Supplementary Information file. Source data are provided with this paper.
References
De Oliveira, D. M. et al. Antimicrobial resistance in ESKAPE pathogens. Clin. Microbiol. Rev. 33, e00181 (2020).
Mckinney, D. C. et al. Structural insights lead to a negamycin analogue with improved antimicrobial activity against Gram-negative pathogens. ACS Med. Chem. Lett. 6, 930–935 (2015).
Arakawa, M. et al. Negamycin restores dystrophin expression in skeletal and cardiac muscles of mdx mice. J. Biochem. 134, 751–758 (2003).
Hamada, K. et al. New negamycin-based potent readthrough derivative effective against TGA-type nonsense mutations. ACS Med. Chem. Lett. 10, 1450–1456 (2019).
Omura, N. et al. Development of conformationally restricted negamycin derivatives for potent readthrough activity. ACS Med. Chem. Lett. 14, 1807–1814 (2023).
Hamada, K. et al. Structure-activity relationship studies of 3-epi-deoxynegamycin derivatives as potent readthrough drug candidates. ACS Med. Chem. Lett. 6, 689–694 (2015).
Taguchi, A. et al. Discovery of natural products possessing selective eukaryotic readthrough activity: 3-epi-deoxynegamycin and its leucine adduct. Chem. Med. Chem. 9, 2233–2237 (2014).
Kondo, S., Yamamoto, H., Maeda, K. & Umezawa, H. Leucylnegamycin, an antibiotic from negamycin-producing Streptomyces. J. Antibiot. 24, 732–734 (1971).
Hamada, M. et al. New antibiotic, negamycin. J. Antibiot. 23, 170–171 (1970).
Umezawa, H., Kondo, S., Hamada, M., Maeda, K. & Takeuchi, T. Antibiotic, negamycin, and processes for the preparation thereof. US patent 3743580 (1973).
Matsuda, K. et al. Discovery of unprecedented hydrazine-forming machinery in bacteria. J. Am. Chem. Soc. 140, 9083–9086 (2018).
Ikeda, Y. et al. d-β-Lysylmethanediamine, a new biogenetic amine produced by a Streptomyces. J. Antibiot. 39, 476–478 (1986).
Maruyama, C. et al. A stand-alone adenylation domain forms amide bonds in streptothricin biosynthesis. Nat. Chem. Biol. 8, 791–797 (2012).
Barkei, J. J., Kevany, B. M., Felnagle, E. A. & Thomas, M. G. Investigations into viomycin biosynthesis by using heterologous production in Streptomyces lividans. ChemBioChem 10, 366–376 (2009).
Yamanaka, K. et al. Discovery of a polyamino acid antibiotic solely comprising l-β-lysine by potential producer prioritization-guided genome mining. ACS Chem. Biol. 17, 171–180 (2022).
Kudo, F., Miyanaga, A. & Eguchi, T. Biosynthesis of natural products containing β-amino acids. Nat. Prod. Rep. 31, 1056–1073 (2014).
Behshad, E. et al. Enantiomeric free radicals and enzymatic control of stereochemistry in a radical mechanism: the case of lysine 2,3-aminomutases. Biochemistry 45, 12639–12646 (2006).
Vey, J. L. & Drennan, C. L. Structural insights into radical generation by the radical SAM superfamily. Chem. Rev. 111, 2487–2506 (2011).
Roy, H. et al. The tRNA synthetase paralog PoxA modifies elongation factor-P with (R)-β-lysine. Nat. Chem. Biol. 7, 667–669 (2011).
Peil, L. et al. Lys34 of translation elongation factor EF-P is hydroxylated by YfcM. Nat. Chem. Biol. 8, 695–697 (2012).
Sugai, Y., Katsuyama, Y. & Ohnishi, Y. A nitrous acid biosynthetic pathway for diazo group formation in bacteria. Nat. Chem. Biol. 12, 73–75 (2015).
Velasco, A., Leguina, J. & Lazcano, A. Molecular evolution of the lysine biosynthetic pathways. J. Mol. Evol. 55, 445–449 (2002).
Cosper, N. J., Booker, S. J., Ruzicka, F., Frey, P. A. & Scott, R. A. Direct FeS cluster involvement in generation of a radical in lysine 2, 3-aminomutase. Biochemistry 39, 15668–15673 (2000).
Fujii, K., Ikai, Y., Oka, H., Suzuki, M. & Harada, K.-i A nonempirical method using LC/MS for determination of the absolute configuration of constituent amino acids in a peptide: combination of Marfey’s method with mass spectrometry and its practical application. Anal. Chem. 69, 5146–5151 (1997).
Bianchi, V. et al. Escherichia coli ferredoxin NADP+ reductase: activation of E. coli anaerobic ribonucleotide reduction, cloning of the gene (fpr), and overexpression of the protein. J. Bacteriol. 175, 1590–1595 (1993).
Brazeau, B. J., Gort, S. J., Jessen, H. J., Andrew, A. J. & Liao, H. H. Enzymatic activation of lysine 2,3-aminomutase from Porphyromonas gingivalis. Appl. Environ. Microbiol. 72, 6402–6404 (2006).
Ma, L. et al. Reconstitution of the in vitro activity of the cyclosporine-specific P450 hydroxylase from Sebekia benihana and development of a heterologous whole-cell biotransformation system. Appl. Environ. Microbiol. 81, 6268–6275 (2015).
Cordoza, J. L. et al. Mechanistic and structural insights into a divergent PLP-dependent l-enduracididine cyclase from a toxic cyanobacterium. ACS Catal. 13, 9817–9828 (2023).
Huang, Z., Wang, K.-K. A. & van der Donk, W. A. New insights into the biosynthesis of fosfazinomycin. Chem. Sci. 7, 5219–5223 (2016).
He, H.-Y., Niikura, H., Du, Y.-L. & Ryan, K. S. Synthetic and biosynthetic routes to nitrogen–nitrogen bonds. Chem. Soc. Rev. 51, 2991–3046 (2022).
Janso, J. E. et al. Discovery of the lomaiviticin biosynthetic gene cluster in Salinispora pacifica. Tetrahedron 70, 4156–4164 (2014).
Gao, J. et al. Use of a phosphonate methyltransferase in the identification of the fosfazinomycin biosynthetic gene cluster. Angew. Chem. 126, 1358–1361 (2014).
Wang, K.-K. A. et al. Glutamic acid is a carrier for hydrazine during the biosyntheses of fosfazinomycin and kinamycin. Nat. Commun. 9, 3687 (2018).
Falk, J. E. Porphyrins and Metalloporphyrins (Elsevier, 1964).
Barr, I. & Guo, F. Pyridine hemochromagen assay for determining the concentration of heme in purified protein solutions. Bio Protoc. 5, e1594–e1594 (2015).
Poulos, T. L. Heme enzyme structure and function. Chem. Rev. 114, 3919–3962 (2014).
Zhang, W. et al. Mechanistic insights into interactions between bacterial class I P450 enzymes and redox partners. ACS Catal. 8, 9992–10003 (2018).
Freibert, S.-A. et al. Biochemical reconstitution and spectroscopic analysis of iron-sulfur proteins. Methods Enzymol. 599, 197–226 (2018).
Abramson, J. et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 630, 493–500 (2024).
Williams, P. A. et al. Haem-ligand switching during catalysis in crystals of a nitrogen-cycle enzyme. Nature 389, 406–412 (1997).
Guengerich, F. P. Common and uncommon cytochrome P450 reactions related to metabolism and chemical toxicity. Chem. Res. Toxicol. 14, 611–650 (2001).
Goeptar, A. R., Scheerens, H. & Vermeulen, N. P. Oxygen and xenobiotic reductase activities of cytochrome P450. Crit. Rev. Toxicol. 25, 25–65 (1995).
Einsle, O., Messerschmidt, A., Huber, R., Kroneck, P. M. & Neese, F. Mechanism of the six-electron reduction of nitrite to ammonia by cytochrome c nitrite reductase. J. Am. Chem. Soc. 124, 11737–11745 (2002).
Higgins, M. A. et al. Structure and mechanism of haem-dependent nitrogen−nitrogen bond formation in piperazate synthase. Nat. Catal. 8, 207–217 (2025).
Gao, S. et al. Enzymatic nitrogen incorporation using hydroxylamine. J. Am. Chem. Soc. 145, 20196–20201 (2023).
Athavale, S. V. et al. Enzymatic nitrogen insertion into unactivated C–H bonds. J. Am. Chem. Soc. 144, 19097–19105 (2022).
Katsuyama, Y. & Matsuda, K. Recent advance in the biosynthesis of nitrogen-nitrogen bond-containing natural products. Curr. Opin. Chem. Biol. 59, 62–68 (2020).
Matsuda, K. & Wakimoto, T. Bacterial hydrazine biosynthetic pathways featuring cupin/methionyl tRNA synthetase‐like enzymes. Chem. Bio. Chem. 25, e202300874 (2024).
Zhao, G. et al. Molecular basis of enzymatic nitrogen-nitrogen formation by a family of zinc-binding cupin enzymes. Nat. Commun. 12, 7205 (2021).
Rea, S. et al. Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature 406, 593–599 (2000).
Moutiez, M., Belin, P. & Gondry, M. Aminoacyl-tRNA-utilizing enzymes in natural product biosynthesis. Chem. Rev. 117, 5578–5618 (2017).
Kieser, T., Bibb, M. J., Buttner, M. J., Chater, K. F. & Hopwood, D. A. Practical Streptomyces Genetics (John Innes Foundation, 2000).
Gust, B., Kieser, T. & Chater, K. Redirect Technology: PCR-Targeting System in Streptomyces coelicolor (John Innes Foundation, 2002).
Heinzelmann, E. et al. The phosphinomethylmalate isomerase gene pmi, encoding an aconitase-like enzyme, is involved in the synthesis of phosphinothricin tripeptide in Streptomyces viridochromogenes. Appl. Environ. Microbiol. 67, 3603–3609 (2001).
Gust, B., Challis, G. L., Fowler, K., Kieser, T. & Chater, K. F. PCR-targeted Streptomyces gene replacement identifies a protein domain needed for biosynthesis of the sesquiterpene soil odor geosmin. Proc. Natl Acad. Sci. USA 100, 1541–1546 (2003).
Gerlt, J. A. et al. Enzyme Function Initiative-Enzyme Similarity Tool (EFI-EST): a web tool for generating protein sequence similarity networks. Biochim. Biophys. Acta 1854, 1019–1037 (2015).
Zallot, R., Oberg, N. & Gerlt, J. A. The EFI web resource for genomic enzymology tools: leveraging protein, genome, and metagenome databases to discover novel enzymes and metabolic pathways. Biochemistry 58, 4169–4182 (2019).
Acknowledgements
Funding for this work was provided by the Canadian Institutes for Health Research (AWD-024206 CIHR 2022 and ARB-196016 CIHR 2024 to K.S.R.) and the Michael Smith Foundation for Health Research (RT-2020-0591 to M.W.) We are grateful to L. Eltis and J. Grigg for helpful discussions and assistance with the anaerobic assays.
Author information
Authors and Affiliations
Contributions
M.W. and K.S.R. conceptualized and designed the project. M.W. carried out genome extraction, gene deletion/chemical complementation, plasmid construction, in vivo enzymatic assays, LC–MS and MS–MS analysis of isotope feeding studies, screening for the physiological electron partner of NegJ and protein structure prediction and molecular docking. Z.-W.W. performed protein purification, pyridine hemochromagen assays, in vitro enzymatic assays and site-directed mutagenesis. M.W., Z.-W.W. and K.S.R. collaboratively wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Biology thanks Makoto Nishiyama, Changming Zhao and the other, anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Natural products containing β-lysine.
a) The structural motif of β-lysine is highlighted in blue, with the stereochemistry of the β-NH2 being specifically labeled. b) The identified gene clusters for the biosynthesis of viomycin, streptothricin, and poly-β-lysine. The enzymatic activity of those proposed LAMs, including VioP, Orf15, and PblB, has not been characterized.
Extended Data Fig. 2 Production of negamycin in neg cluster containing bacterial strains.
Strain Streptomyces sp. NRRL S-495 contains a complete neg cluster homolog with encoded protein identities between 90% to 95% to Neg proteins in Streptomyces purpeofuscus ATCC 21470. Strains Streptomyces sp. NRRL S-623 and Dactylosporangium aurantiacum sp. NRRL B-8018 lack negA, negG, and negI homologs in their neg homologous gene cluster. Only S. purpeofuscus ATCC 21470 and Streptomyces sp. NRRL S-495 produce negamycin under experimental conditions in this study.
Extended Data Fig. 3 Stable isotope feeding study with 15N-Leu.
a) Detection of negamycin and leucylnegamycin from 5-day culture of strain S. Purpeofuscus. b) HR-MS of negamycin produced from unlabeled and 15N-labeled Leu (2 mM) culture media. c) HR-MS of leucylnegamycin produced from unlabeled and 15N-labeled Leu (2 mM) culture media.
Extended Data Fig. 4 LC-MS profile of NegB in vivo assay.
a) Co-expression of NegB with seFdR (S. elongatus ferredoxin NADP+ reductase), seFd (S. elongatus ferredoxin), or Fld (E. coli flavodoxin) leads to the production of β-lysine. All the assay groups were supplemented with l-Lys (5 mM) and Fe(NH4)2(SO4)2 (200 μM). The in vivo products were derivatized with Fmoc-Cl before LC-MS analysis. b) Production of 5’-deoxyadenosine (5’-dAdoH) in NegB in vitro assay. The full components group contains NegB, SAM, PLP, L-lysine, and sodium dithionite.
Extended Data Fig. 5 SDS-PAGE profile, color, and UV-vis profiles of purified and reconstituted proteins.
a) SDS-PAGE (12%) analysis of NegJ. b) SDS-PAGE (18%) analysis of spFd6. SDS-PAGE analysis of NegJ and spFd6 was performed three times with similar results, and representative results are shown. c) Color differences of NegJ before and after reconstitution with hemin. d) Color differences of spFd6 before and after reconstitution of iron–sulfur cluster. e) α-band and β-band absorption peaks of purified NegJ in the reduced pyridine hemochromogen spectrum. f) The Soret peak of NegJ shifted from 414 nm to 417 nm when incubated with Gly and nitrite.
Extended Data Fig. 6 Screen of ferredoxin reductase and ferredoxin combination for NegJ activity.
a) LC-MS profiles of NegJ in vivo assay with S. purpeofuscus Ferredoxin reductase-1 and all the ferredoxins. b) LC-MS profiles of NegJ in vivo assay with S. purpeofuscus Ferredoxin reductase-2 and all the ferredoxins. c) LC-MS profile of NegJ in vivo assay. Co-expression of NegJ with spFd6 (ferredoxin-6 from S. purpeofuscus ATCC 21470) leads to the production of HAA. All the assay groups were supplemented with Gly (5 mM), sodium nitrite (2 mM), and Fe(NH4)2(SO4)2 (100 μM). The in vivo products were derivatized with DNFB before LC-MS analysis.
Extended Data Fig. 7 The potential residues mediating NegJ catalysis based on AlphaFold3 prediction and molecular docking.
a) Molecular docking of nitrite and glycine with NegJ. The carboxyl group of Gly is predicted to interact via hydrogen bonds with Glu165, Arg498, and Arg523. Nitrite was docked into the same cavity, with its oxygen atom interacting with R444 and N445. The heme cofactor forms the Fe-His bond with His488. b) In vivo assay of NegJ and its variants. The in vivo products were derivatized with DNFB before LC-MS analysis.
Extended Data Fig. 8 In vivo and in vitro assays of NegJ with NH2OH as substrate.
a) In vivo assay of NegJ-spFd6. Group 1 was supplemented with 1 mM nitrite and 5 mM Gly. Groups 2 to 6 were supplemented with 0.5 mM NH2OH and 5 mM Gly. Group 4 and 5 omitted spFd6 and NegJ respectively. Group 6 used pACYCDuet-1 and pET28a instead of NegJ and spFd6. b) In vitro assay of NegJ-spFd6. The substrate concentrations for in vitro assay were 1 mM for Gly, and 2 mM for NH2OH and nitrite. Note: We noticed that the efficiency of NH2OH is lower than that of nitrite in reacting with Gly to form HAA. We assume that nitrite must bind to the heme cofactor of NegJ before being reduced to other intermediates, including hydroxylamine, and that free hydroxylamine might have a weaker binding affinity to heme than nitrite. In addition, we propose that the ferredoxin partner spFd6 is essential for NegJ catalysis not only by facilitating electron transfer but also by stabilizing the heme cofactor, as demonstrated in Supplementary Fig. 22. This explains why the NH2OH + Gly assay still requires spFd6 to produce HAA.
Extended Data Fig. 9 Sequence similarity network (SSN) and genome neighborhood network (GNN) of NegJ.
The networks were visualized and analyzed using Cytoscape 3.10.3. a) SSN of NegJ was constructed using the EFI-EST online tool (https://efi.igb.illinois.edu/efi-est)56. The BLAST query e-value and the alignment score threshold for generating the SSN were set to 5 and 100 respectively. Three NegJ homologs for genome mining (Extended Data Fig. 10) were indicated with arrows. b) GNN was constructed based on SSN of NegJ and created by EFI-GNT web tool (https://efi.igb.illinois.edu/efi-gnt)57. The values for Neighborhood Size and Minimal Co-occurrence Percentage Lower Limit were 10 and 20 respectively. Three GNN clusters (No. 1, 3, and 6) with SSN cluster-hub nodes with Pfam family spoke nodes were randomly selected for genome mining of potential N–N-bond-containing natural products (see Extended Data Fig. 10). The SSN cluster-hub nodes were colored.
Extended Data Fig. 10 Genome mining examples based on GNN of NegJ.
The diagrams were created by EFI-GNDs online tool and edited with Inkscape 1.3.257. Proposed functions of genes neighboring to negJ homologs are highlighted and labeled. a) Partial Genome Neighborhood Diagram for cluster 1. The downstream oxygenase, hydroxylase, and methyltransferase genes indicate diverse structural modification of potential hydrazine based natural products. b) Partial Genome Neighborhood Diagram for cluster 3. The downstream genes encoding ATP-grasp domain containing proteins suggest the formation of amide bonds that connect hydrazine and other amino acids to produce novel N–N-bond containing compounds. c) Partial Genome Neighborhood Diagram for cluster 6. The upstream NRPS elements indicate the biosynthesis of a series of potential N–N-bond containing peptides.
Supplementary information
Supplementary Information
Supplementary Methods, Figs. 1–26 and Tables 1–5.
Supplementary Data 1
The accession numbers of proteins in Supplementary Tables 3, 4 and 5, and in Supplementary Figs. 2 and 3.
Source data
Source Data Extended Data Fig. 5
Unprocessed SDS-PAGE in Extended Data Fig. 5a,b.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, M., Wei, ZW. & Ryan, K.S. A heme-dependent enzyme forms the hydrazine in the antibiotic negamycin. Nat Chem Biol 21, 1012–1020 (2025). https://doi.org/10.1038/s41589-025-01898-0
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41589-025-01898-0