Abstract
Hippo–YAP signaling is crucial to organ development and tumorigenesis. VGLL4, which occupies TEAD to prevent YAP binding, is the main transcriptional repressor of Hippo–YAP activity. Here we identified the nuclear E3 ligase ubiquitin protein ligase E3 component n-recognin 5 (UBR5) poly-ubiquitinated VGLL4 at Lys61 for its degradation, which permits Hippo–YAP signaling for the development of the liver biliary system in mice and multiple cancers in humans. In mouse liver development, Ubr5 and Vgll4 exhibited reciprocal expression patterns spatiotemporally. Ubr5 deletion impaired cholangiocyte development and hepatocyte reprogramming, which could be efficiently rescued by restoring Hippo–YAP through ablating Vgll4. We also found that the UBR5–VGLL4–YAP axis is associated with the progression of human pan-cancers. Targeting nuclear E3 ligases in multiple types of patient-derived tumor organoids suppressed their expansion. Our identification of UBR5 as the bona fide E3 ligase of VGLL4 offers a molecular framework of nuclear Hippo–YAP regulation and suggests nuclear ubiquitination as a potential therapeutic target for YAP-dependent malignancies.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Bulk RNA-seq and CUT&Tag data that support the findings of this article have been deposited in the Sequence Read Archive under accession PRJNA1106624. MS data were deposited in iProx with the accession IPX0008733000 or URL (https://www.iprox.cn/page/PSV023.html;?url=1739411243211OtXu, code: Zd70). Source data are provided with this paper.
Change history
10 June 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41589-025-01958-5
References
Yu, F. X., Zhao, B. & Guan, K. L. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell 163, 811–828 (2015).
Pan, D. The Hippo signaling pathway in development and cancer. Dev. Cell 19, 491–505 (2010).
Zhao, B. et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 21, 2747–2761 (2007).
Moya, I. M. & Halder, G. Hippo–YAP/TAZ signalling in organ regeneration and regenerative medicine. Nat. Rev. Mol. Cell Biol. 20, 211–226 (2019).
Totaro, A., Panciera, T. & Piccolo, S. YAP/TAZ upstream signals and downstream responses. Nat. Cell Biol. 20, 888–899 (2018).
Zhang, W. et al. VGLL4 functions as a new tumor suppressor in lung cancer by negatively regulating the YAP–TEAD transcriptional complex. Cell Res. 24, 331–343 (2014).
Koontz, L. M. et al. The Hippo effector Yorkie controls normal tissue growth by antagonizing scalloped-mediated default repression. Dev. Cell 25, 388–401 (2013).
Cai, J. et al. YAP–VGLL4 antagonism defines the major physiological function of the Hippo signaling effector YAP. Genes Dev. 36, 1119–1128 (2022).
Russell, J. O. & Camargo, F. D. Hippo signalling in the liver: role in development, regeneration and disease. Nat. Rev. Gastroenterol. Hepatol. 19, 297–312 (2022).
Samant, R. S., Livingston, C. M., Sontag, E. M. & Frydman, J. Distinct proteostasis circuits cooperate in nuclear and cytoplasmic protein quality control. Nature 563, 407–411 (2018).
Callaghan, M. J. et al. Identification of a human HECT family protein with homology to the Drosophila tumor suppressor gene hyperplastic discs. Oncogene 17, 3479–3491 (1998).
Mansfield, E., Hersperger, E., Biggs, J. & Shearn, A. Genetic and molecular analysis of hyperplastic discs, a gene whose product is required for regulation of cell proliferation in Drosophila melanogaster imaginal discs and germ cells. Dev. Biol. 165, 507–526 (1994).
Saunders, D. N. et al. Edd, the murine hyperplastic disc gene, is essential for yolk sac vascularization and chorioallantoic fusion. Mol. Cell. Biol. 24, 7225–7234 (2004).
Rutz, S. et al. Deubiquitinase DUBA is a post-translational brake on interleukin-17 production in T cells. Nature 518, 417–421 (2015).
Swenson, S. A. et al. UBR5 HECT domain mutations identified in mantle cell lymphoma control maturation of B cells. Blood 136, 299–312 (2020).
Cojocaru, M. et al. Transcription factor IIS cooperates with the E3 ligase UBR5 to ubiquitinate the CDK9 subunit of the positive transcription elongation factor B. J. Biol. Chem. 286, 5012–5022 (2011).
Jiang, W. et al. Acetylation regulates gluconeogenesis by promoting PEPCK1 degradation via recruiting the UBR5 ubiquitin ligase. Mol. Cell 43, 33–44 (2011).
Gudjonsson, T. et al. TRIP12 and UBR5 suppress spreading of chromatin ubiquitylation at damaged chromosomes. Cell 150, 697–709 (2012).
Zhang, T., Cronshaw, J., Kanu, N., Snijders, A. P. & Behrens, A. UBR5-mediated ubiquitination of ATMIN is required for ionizing radiation-induced ATM signaling and function. Proc. Natl Acad. Sci. USA 111, 12091–12096 (2014).
Sanchez, A. et al. BMI1–UBR5 axis regulates transcriptional repression at damaged chromatin. Proc. Natl Acad. Sci. USA 113, 11243–11248 (2016).
Hyer, M. L. et al. A small-molecule inhibitor of the ubiquitin activating enzyme for cancer treatment. Nat. Med. 24, 186–193 (2018).
Buckley, S. M. et al. Regulation of pluripotency and cellular reprogramming by the ubiquitin–proteasome system. Cell Stem Cell 11, 783–798 (2012).
Zhou, Y. et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 10, 1523 (2019).
de Vivo, A. et al. The OTUD5–UBR5 complex regulates FACT-mediated transcription at damaged chromatin. Nucleic Acids Res. 47, 729–746 (2019).
Jiao, S. et al. VGLL4 targets a TCF4–TEAD4 complex to coregulate Wnt and Hippo signalling in colorectal cancer. Nat. Commun. 8, 14058 (2017).
Jiao, S. et al. A peptide mimicking VGLL4 function acts as a YAP antagonist therapy against gastric cancer. Cancer Cell 25, 166–180 (2014).
Werner, A., Manford, A. G. & Rape, M. Ubiquitin-dependent regulation of stem cell biology. Trends Cell Biol. 27, 568–579 (2017).
Huang, T. P., Newby, G. A. & Liu, D. R. Precision genome editing using cytosine and adenine base editors in mammalian cells. Nat. Protoc. 16, 1089–1128 (2021).
Driskill, J. H. & Pan, D. The Hippo pathway in liver homeostasis and pathophysiology. Annu Rev. Pathol. 16, 299–322 (2021).
Cai, S. Y. et al. Bile acids initiate cholestatic liver injury by triggering a hepatocyte-specific inflammatory response. JCI Insight 2, e90780 (2017).
Verboven, E. et al. Regeneration defects in Yap and Taz mutant mouse livers are caused by bile duct disruption and cholestasis. Gastroenterology 160, 847–862 (2021).
Schaub, J. R. et al. De novo formation of the biliary system by TGFβ-mediated hepatocyte transdifferentiation. Nature 557, 247–251 (2018).
Katsuda, T. et al. Conversion of terminally committed hepatocytes to culturable bipotent progenitor cells with regenerative capacity. Cell Stem Cell 20, 41–55 (2017).
Bou Saleh, M. et al. Loss of hepatocyte identity following aberrant YAP activation: a key mechanism in alcoholic hepatitis. J. Hepatol. 75, 912–923 (2021).
Liu, Y. et al. Yap–Sox9 signaling determines hepatocyte plasticity and lineage-specific hepatocarcinogenesis. J. Hepatol. 76, 652–664 (2022).
Michalopoulos, G. K., Barua, L. & Bowen, W. C. Transdifferentiation of rat hepatocytes into biliary cells after bile duct ligation and toxic biliary injury. Hepatology 41, 535–544 (2005).
Yi, J. et al. Large tumor suppressor homologs 1 and 2 regulate mouse liver progenitor cell proliferation and maturation through antagonism of the coactivators YAP and TAZ. Hepatology 64, 1757–1772 (2016).
Lee, D. H. et al. LATS-YAP/TAZ controls lineage specification by regulating TGFβ signaling and Hnf4α expression during liver development. Nat. Commun. 7, 11961 (2016).
Shearer, R. F., Iconomou, M., Watts, C. K. & Saunders, D. N. Functional roles of the E3 ubiquitin ligase UBR5 in cancer. Mol. Cancer Res. 13, 1523–1532 (2015).
Gao, Q. et al. Integrated proteogenomic characterization of HBV-related hepatocellular carcinoma. Cell 179, 561–577 (2019).
Li, H. et al. YAP/TAZ drives cell proliferation and tumour growth via a polyamine-eIF5A hypusination-LSD1 axis. Nat. Cell Biol. 24, 373–383 (2022).
Tarcic, O. et al. RNF20 and histone H2B ubiquitylation exert opposing effects in basal-like versus luminal breast cancer. Cell Death Differ. 24, 694–704 (2017).
Zhao, J. et al. Olaparib and enzalutamide synergistically suppress HCC progression via the AR-mediated miR-146a-5p/BRCA1 signaling. FASEB J. 34, 5877–5891 (2020).
Li, X. et al. Ubiquitylation of phosphatidylinositol 4-phosphate 5-kinase type I γ by HECTD1 regulates focal adhesion dynamics and cell migration. J. Cell Sci. 126, 2617–2628 (2013).
Tsai, J. M. et al. UBR5 forms ligand-dependent complexes on chromatin to regulate nuclear hormone receptor stability. Mol. Cell 83, 2753–2767 (2023).
Lampersberger, L. et al. Loss of the E3 ubiquitin ligases UBR-5 or HECD-1 restores Caenorhabditis elegans development in the absence of SWI/SNF function. Proc. Natl Acad. Sci. USA 120, e2217992120 (2023).
Kaisari, S. et al. Role of ubiquitin–protein ligase UBR5 in the disassembly of mitotic checkpoint complexes. Proc. Natl Acad. Sci. USA 119, e2121478119 (2022).
Cipolla, L. et al. UBR5 interacts with the replication fork and protects DNA replication from DNA polymerase eta toxicity. Nucleic Acids Res. 47, 11268–11283 (2019).
Wang, F. et al. Structure of the human UBR5 E3 ubiquitin ligase. Structure 31, 541–552 (2023).
Hodáková, Z. et al. Cryo-EM structure of the chain-elongating E3 ubiquitin ligase UBR5. EMBO J. 42, e113348 (2023).
Hehl, L. A. et al. Structural snapshots along K48-linked ubiquitin chain formation by the HECT E3 UBR5. Nat. Chem. Biol. 20, 190–200 (2023).
Pepe-Mooney, B. J. et al. Single-cell analysis of the liver epithelium reveals dynamic heterogeneity and an essential role for YAP in homeostasis and regeneration. Cell Stem Cell 25, 23–38 (2019).
Molina, L. M. et al. Compensatory hepatic adaptation accompanies permanent absence of intrahepatic biliary network due to YAP1 loss in liver progenitors. Cell Rep. 36, 109310 (2021).
Guofeng, L. et al. Near-Infrared-Responded High Sensitivity Nanoprobe for Steady and Visualized Detection of Albumin in Hepatic Organoids and Mouse Liver. Adv. Sci. 9, 2202505 (2022).
Zhao, B. et al. PICK1 promotes caveolin-dependent degradation of TGF-β type I receptor. Cell Res. 22, 1467–1478 (2012).
Charni-Natan, M. & Goldstein, I. Protocol for primary mouse hepatocyte isolation. STAR Protoc. 1, 100086 (2020).
Wei, J. et al. Gene manipulation in liver ductal organoids by optimized recombinant adeno-associated virus vectors. J. Biol. Chem. 294, 14096–14104 (2019).
Tanimizu, N. et al. Intrahepatic bile ducts are developed through formation of homogeneous continuous luminal network and its dynamic rearrangement in mice. Hepatology 64, 175–188 (2016).
Tag, C. G. et al. Bile duct ligation in mice: induction of inflammatory liver injury and fibrosis by obstructive cholestasis. J. Vis. Exp. 10, 52438 (2015).
Kluesner, M. G. et al. EditR: a method to quantify base editing from sanger sequencing. CRISPR J. 1, 239–250 (2018).
Acknowledgements
We thank S. Huppert (Division of Gastroenterology, Hepatology and Nutrition at Cincinnati Children’s Hospital Medical Center), L. Zhang (School of Life Science, Zhejiang University), L. Hui (Center for Excellence in Molecular Cell Science, CAS) and L. An (Shanghai Tenth People’s Hospital, Tongji University School of Medicine) for insightful discussion.
This work was supported by grants from the National Key Research and Development Program of China (2024YFA1307400), the National Natural Science Foundation of China (82372663 to B.Z. and 32400573 to J.W.), the Key Research and Development Program of Jiangxi Province (20232BBG70024 to B.Z.), the Key Research and Development Program of Yunnan Province (202302AA310024 to B.Z.) and the Natural Science Foundation of Shandong Province (ZR2023LSW008 to B.Z.).
Author information
Authors and Affiliations
Contributions
J.W. performed the AP–MS experiments and identified VGLL4 as the potential substrate of UBR5. Z.C. and R.Z. performed the majority of the biochemistry experiments to demonstrate that UBR5 is the E3 ligase of VGLL4 and identified the ubiquitination site. J.W., Q.L. and Xiaoyu Li performed the functional assay in mice and ductal organoids and discovered the Ubr5–Vgll4–Yap axis in liver cell fate regulation. Q.W. performed the functional assays in PDOs and analyzed the data. Y.Z. and Xinyang Li analyzed the RNA-seq, scRNA-seq and CUT&Tag data. X.W. and Q.D. performed the immunostaining and RNA scope and analyzed the data. Z.Z. and F.S. contributed to the discussion of the results. S.J. and B.Z. supervised the study. J.W. and B.Z. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declared no conflict of interest.
Peer review
Peer review information
Nature Chemical Biology thanks Shuguo Sun and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
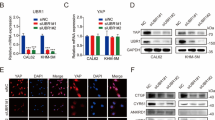
Extended Data Fig. 1 Nuclear ubiquitination permits Hippo–Yap signaling.
a, Evaluating the effect of global ubiquitination on TGF-β and Wnt/β-catenin signaling pathways. Data were presented as mean ± s.d. (n = 3 biological replicates), and P values were calculated by two-tailed unpaired Student’s t test. b, Screening of nuclear ubiquitin E3 ligases that regulate Hippo–Yap signaling. Data were presented as mean ± s.d. (n = 3 biological replicates), and p values were calculated by one-way ANOVA with Dunnett’s post hoc analysis, compared with shControl. ***P ˂ 0.0001. c, HEK293T cells transfected with HOP flash and UBR5 shRNAs were treated with 10 μM TRULI for 12 h and collected for luciferase measurement. Data were presented as mean ± s.d. (n = 3 biological replicates). The P values were calculated by one-way ANOVA with Dunnett’s post hoc analysis, compared with shControl. d, Evaluation of UBR5 knockdown efficiency in HepG2 cells. Images are representative of three independent experiments. e, HepG2 cells that stably integrated control and UBR5 shRNAs were stained for YAP (red) and DAPI (blue), and then subjected to confocal imaging. Images are representative of three independent experiments. Scale bar, 50 μm. f, YAP localization frequencies were quantified according to e. N > C, YAP is enriched in nucleus; N = C, YAP is evenly distributed in cytoplasm and nucleus; N < C, YAP is enriched in cytoplasm. Data were represented as mean ± s.d. (n = 3 biological replicates). The P values were calculated by one-way ANOVA with Dunnett’s post hoc analysis, compared with shControl. g, HepG2 cells that stably integrated with UBR5 shRNAs were transfected with HOP flash and collected for luciferase measurement. Data were represented as mean ± s.d. (n = 3 biological replicates). The P values were calculated by one-way ANOVA with Dunnett’s post hoc analysis, compared with control.
Extended Data Fig. 2 UBR5 mediated the ubiquitination of endogenous VGLL4 via its K61 site.
a, 293A cells that stably integrated with control shRNA and UBR5 shRNA were subjected to VGLL4 immunoprecipitation (VGLL4 IP) to detect the endogenous ubiquitination VGLL4 (ubiquitin immunoblotting, middle), or subjected to ubiquitin immunoprecipitation (ubiquitin IP) to detect endogenous ubiquitination VGLL4 (VGLL4 immunoblotting, right). The input was detected with ubiquitin, VGLL4, UBR5 and β-actin as indicated (left). b, 293A cells were transfected with vectors expressing Flag-VGLL4WT and Flag-VGLL4K61R and then treated with CHX (50 μM) for indicated time course. Whole cell lysates were subjected to western blotting analysis to detect the Flag-tagged VGLL4WT and VGLL4K61R. β-Actin served as loading control. c, Quantification of the relative VGLL4 protein level in b. Data were presented as mean ± s.d. (n = 3 biological replicates); P values were calculated by multiple two-tailed unpaired Student’s t test at each time point. In a and b, images are representative of three independent experiments.
Extended Data Fig. 3 Ubr5 knockout in liver leads to cholestasis.
a, H&E staining of liver sections from control and Ubr5-LKO mice at P21, P35 and P56, respectively. Arrows indicate the necrosis foci. Scale bar, 200 μm. b,c, ALT activity (b) and total bile acid content (c) in serum from control (n = 6 mice) and Ubr5-LKO (n = 5 mice) mice at P21. Data are presented as mean ± s.d., and P values were calculated by two-tailed unpaired Student’s t test. d, Mdr1 IHC staining images of liver sections from control and Ubr5-LKO mice at P21, P35 and P56, respectively. Scale bar, 100 μm. Magnification = ×2.5. e, Quantification of the Mdr1 width (bile canaliculus width) in control and Ubr5-LKO livers at P21, P35 and P56, respectively. Data are presented as mean ± s.d. (for control/Ubr5-LKO mice at each time point, about 40 random bile canaliculi from 3 different mice were counted), and P values were calculated by comparing two-tailed unpaired Student’s t test at each time point. f, Schematic of AAV-Ttr-Cre mediated hepatocyte specific deletion of Ubr5. g, In situ fluorescence imaging and H&E staining of the liver frozen sections from AAV-Ttr-Cre or PBS-injected mice. Scale bar, 200 μm. In a, d and g, images are representative of three independent experiments.
Extended Data Fig. 4 Ubr5 depletion attenuates Yap signaling in liver.
a, Western blotting analysis of Ubr5 and Vgll4 level in control and Ubr5-LKO livers at 3 weeks. Vinculin served as a loading control. Images are representative of three independent experiments. b, Immunofluorescent staining for Ubr5 (red) and Vgll4 (green) in control and Ubr5-LKO liver tissue. Images are representative of three independent experiments. Scale bar, 100 μm. c, Quantification of Vgll4 fluorescence intensity in b was shown as mean ± s.d. (n = 3 biological replicates), and P value was calculated by two-tailed unpaired Student’s t test. d, Quantitative real-time PCR (qRT–PCR) analysis for the Ctgf, Cyr61, Ankrd1, Myc, Jag1 and Ubr5 expression in liver tissues from control and Ubr5-LKO mice at P7, P14 and P21, respectively. Histone H3 was used as an internal control. Data were represented as mean ± s.d. (n = 3 biological replicates). The P values were calculated by unpaired two-tailed Student’s t test.
Extended Data Fig. 5 Ubr5 is required for hepatocyte plasticity.
a, Schematic of the induction of liver progenitor cells from isolated primary hepatocytes. The primary hepatocytes are isolated by liver perfusion and subjected to YAC medium (Y-27632, A8301 and CHIR99021) for CLiP (chemically induced liver progenitor) formation. Cells are collected for immunostaining at day 0 and day 5. b, Freshly isolated hepatocytes (day 0) and YAC-induced primary hepatocytes (day 5) from control and Ubr5-LKO mice were stained for Sox9 (red) and Hnf4α (green) and then subjected to confocal imaging. A representative result of three independent experiments is shown. Scale bar, 100 μm. c, Quantification of the percentage of Sox9/Hnf4α double-positive cells in control and Ubr5-LKO groups at day 0 and day 5 after YAC medium treatment. Data are presented as mean ± s.d. (n = 3); P value was calculated by two-tailed Student’s t test.
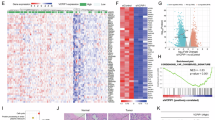
Extended Data Fig. 6 Ubr5 and Vgll4 exhibit reciprocal expression pattern spatiotemporally to orchestrate liver development.
a, The liver samples from E13.5, E18, P0 and adult mice were stained for Ubr5 (red), Vgll4 (green) and DAPI (blue) and then subjected to confocal imaging. A representative result of six independent experiments is shown. Scale bar, 100 μm. b, The fluorescence intensity of Ubr5 and Vgll4 at different developmental stages shown in a was quantified. Data were represented as mean ± s.d. (n = 6 biological replicates). c, Representative immunofluorescent imaging of P0 liver stained with Ubr5 (red) and Vgll4 (green). Scale bar, 100 μm. d, Percentage quantification of Vgll4 single-positive, Ubr5 single-positive and Vgll4/Ubr5 double-positive cells in P0 liver as shown in c. Data were represented as mean ± s.d. (n = 3 biological replicates); P values were calculated by one-way ANOVA with Dunnett’s post hoc analysis, compared with column 2 (Vgll4/Ubr5 double positive).
Extended Data Fig. 7 Characterization of Ubr5 and Vgll4 expression pattern in cholangiocytes during liver development.
a, The liver samples from E13.5, E18, P0 and P21 mice were stained for Ubr5 (red), Sox9 (green) and DAPI (blue) and then subjected to confocal imaging. A representative result of three independent experiments is shown. Scale bar, 100 μm. b, Percentage quantification of Ubr5-positive ductal cells during liver development as shown in a. Data were represented as mean ± s.d. (n = 3 biological replicates). c, Scheme of the Ubr5/Vgll4 expression pattern during liver development. d, The liver samples from E13.5, E18, P0 and P21 mice were stained for Vgll4 (green), Sox9 (red) and DAPI (blue) and then subjected to confocal imaging. A representative result of four independent experiments is shown. Scale bar, 100 μm. Magnification = ×2. e, Percentage quantification of Vgll4 positive ductal cells during liver development as shown in d. Data were represented as mean ± s.d. (n = 4 biological replicates).
Extended Data Fig. 8 Vgll4 knockout restores the Yap signaling in Ubr5-LKO liver.
a, Simultaneous interfering Ubr5 and Vgll4 rescued the organoid expansion. Ubr5fl/fl ductal organoids infected with indicated AAV vectors were cultured for 5 days. Representative images were shown for each group (n = 3 biological replicates). Scale bar, 200 μm. b, Quantification of the average diameter of organoids in a. Data were presented as mean ± s.d. (n = 3). The P values were calculated by one-way ANOVA with Dunnett’s post hoc analysis, compared with AAV-Cre-shControl. c, Representative RNA scope image of Ctgf in liver sections from control, Ubr5-LKO (SKO) and Ubr5 and Vgll4 LKO (DKO) mice at P21. A representative result of three independent experiments is shown. Scale bar, 100 μm. d, Quantification of Ctgf signals in c, which is shown by mean ± s.d. (n = 3 biological replicates). The P values were calculated by one-way ANOVA with Dunnett’s post hoc analysis, compared with SKO. e, qRT–PCR analysis of Yap target genes’ expression level in control, SKO and DKO livers at P21. Histone H3 was used as an internal control. Data were represented as mean ± s.d. (n = 3 biological replicates). The P values were calculated by one-way ANOVA with Dunnett’s post hoc analysis, compared with SKO. f, Fresh hepatocytes isolated from SKO and DKO mice were subjected to CUT&Tag against Yap, and the representative genomic view of Cdk1 and Cebpb was shown by IGV. g, Total bile acid content in the serum of control, Ubr5-LKO (SKO) and Ubr5 and Vgll4 LKO (DKO) mice. Data were represented as mean ± s.d. (n = 9 mice). The P values were calculated by one-way ANOVA with Dunnett’s post hoc analysis, compared with SKO.
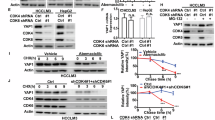
Extended Data Fig. 9 Targeting UBR5 restricts patient-derived tumor organoids (PDOs) proliferation and inhibits YAP signaling.
a, Analysis of UBR5 expression level among different tumor organoids. GAPDH was used as an internal control. Data were represented as mean ± s.d. (n = 3 biological replicates). n.a., not applicable. The P values were calculated by unpaired two-tailed Student’s t test. b–d. PDOs, including HCC organoids (b), LAC organoids (c) and GC organoids (d) were infected with lentiviral UBR5/control shRNA and subjected to paraffin sectioning. The sections were stained with UBR5 (red), cleaved caspase 3 or Ki67 (green) and DAPI (blue) and then subjected to confocal imaging. A representative result of three independent experiments is shown. Scale bar = 50 μm. e,f, HCC organoids (e) and GC organoids (f) infected with lentiviral UBR5/control shRNA were subjected to whole-mount staining. The fixed organoids were stained with UBR5 (red), VGLL4 (green) and DAPI (blue) and then subjected to confocal imaging. A representative result of three independent experiments is shown. Scale bar = 50 μm. g, HCC organoids infected with lentiviral UBR5/control shRNA were subjected to bulk RNA-seq and analyzed with GSEA using the YAP signature genes set. h, qRT–PCR analysis of the mRNA level of UBR5, AMOTL2, ODC1 and NNMT in the HCC organoids infected with lentiviral UBR5/control shRNA. HISTONE H3 was used as an internal control. Data were represented as mean ± s.d. (n = 3 biological replicates). The P values were calculated by unpaired two-tailed Student’s t test. i, Scheme of UBR5–VGLL4 balance in regulating YAP activity and tumor progression.
Extended Data Fig. 10 Targeting nuclear E3 ligases restricts HCC organoid proliferation.
a, Cell viability tracking of the HCC organoids after lentiviral infection of indicated shRNA-targeting nuclear E3 ligases. Data were represented as mean ± s.e.m. (n = 3 biological replicates). b, The representative bright-field images showing the HCC organoids at day 7 post lentiviral infection. A representative result of three independent experiments is shown. Scale bar, 1 mm.
Supplementary information
Supplementary Information
Supplementary Figs. 1–7.
Supplementary Data 1–6.
Supplementary Data 1: AP–MS data of UTI mice livers. Supplementary Data 2: Functional annotation of UBR5 interacting nuclear proteins. Supplementary Data 3: MS data of VGLL4 ubiquitination. Supplementary Data 4: qPCR primers. Supplementary Data 5: shRNA sequences. Supplementary Data 6: Genesets.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Unprocessed western blots.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 3
Unprocessed western blots.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 1
Unprocessed western blots.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 2
Unprocessed western blots.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 4
Unprocessed western blots.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wei, J., Cao, Z., Li, Q. et al. Nuclear ubiquitination permits Hippo–YAP signal for liver development and tumorigenesis. Nat Chem Biol 21, 1565–1576 (2025). https://doi.org/10.1038/s41589-025-01901-8
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41589-025-01901-8
This article is cited by
-
Emerging nanomedicine for liver diseases treatment
Journal of Nanobiotechnology (2025)