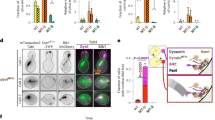
Abstract
Cytoplasmic dynein 1 (dynein) is the primary motor responsible for the retrograde transport of intracellular cargoes along microtubules. Activation of dynein requires the opening its autoinhibited Phi conformation, a process driven by Lis1 and Nde1/Ndel1. Using biochemical reconstitution and cryo-electron microscopy, we demonstrate that Nde1 enhances Lis1 binding to autoinhibited dynein and facilitates Phi opening. We identify a key intermediate in this activation pathway where a single Lis1 dimer binds between Phi-like (PhiL) motor rings. In this ‘PhiL–Lis1’ complex, Lis1 interacts with one motor domain through canonical sites at the AAA+ (adenosine triphosphatases associated with diverse cellular activities) ring and stalk, and with AAA5, AAA6 and linker regions of the other motor domain. Mutagenesis and motility assays confirm the critical role of the PhiL–Lis1 interface in dynein activation. This intermediate forms rapidly in the presence of Nde1, although Nde1 is not part of PhiL–Lis1. These findings provide key insights into how Nde1 promotes Lis1-mediated Phi opening.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Cryo-EM density maps and models were deposited to the EM Data Bank and PDB under the following accession numbers: dynein and Lis1 condition, PDB 9E12 and EMD-47381 for full-length Phi, PDB 9E10 and EMD-47379 for the motor domains of Phi, PDB 9E13 and EMD-47382 for full-length PhiL–Lis1 and PDB 9E11 and EMD-47380 for motor domains of PhiL–Lis1; in the dynein, Lis1 and Nde1 condition, PDB 9E14 and EMD-47383 for full-length PhiL–Lis1 and PDB 9E0Z and EMD-47378 for motor domains of PhiL–Lis1. Source data are provided with this paper.
References
Reck-Peterson, S. L., Redwine, W. B., Vale, R. D. & Carter, A. P. The cytoplasmic dynein transport machinery and its many cargoes. Nat. Rev. Mol. Cell Biol. 19, 382–398 (2018).
McNally, F. J. Mechanisms of spindle positioning. J. Cell Biol. 200, 131–140 (2013).
Markus, S. M., Marzo, M. G. & McKenney, R. J. New insights into the mechanism of dynein motor regulation by lissencephaly-1. eLife 9, e59737 (2020).
Willemsen, M. H. et al. Mutations in DYNC1H1 cause severe intellectual disability with neuronal migration defects. J. Med Genet. 49, 179–183 (2012).
Scoto, M. et al. Novel mutations expand the clinical spectrum of DYNC1H1-associated spinal muscular atrophy. Neurology 84, 668–679 (2015).
Poirier, K. et al. Mutations in TUBG1, DYNC1H1, KIF5C and KIF2A cause malformations of cortical development and microcephaly. Nat. Genet. 45, 639–647 (2013).
Guven, A., Gunduz, A., Bozoglu, T. M., Yalcinkaya, C. & Tolun, A. Novel NDE1 homozygous mutation resulting in microhydranencephaly and not microlyssencephaly. Neurogenetics 13, 189–194 (2012).
Lipka, J., Kuijpers, M., Jaworski, J. & Hoogenraad, C. C. Mutations in cytoplasmic dynein and its regulators cause malformations of cortical development and neurodegenerative diseases. Biochem. Soc. Trans. 41, 1605–1612 (2013).
Eschbach, J. & Dupuis, L. Cytoplasmic dynein in neurodegeneration. Pharmacol. Ther. 130, 348–363 (2011).
Urnavicius, L. et al. Cryo-EM shows how dynactin recruits two dyneins for faster movement. Nature 554, 202–206 (2018).
Singh, K. et al. Molecular mechanism of dynein–dynactin complex assembly by LIS1. Science 383, eadk8544 (2024).
Okada, K. et al. Conserved roles for the dynein intermediate chain and Ndel1 in assembly and activation of dynein. Nat. Commun. 14, 5833 (2023).
Chaaban, S. & Carter, A. P. Structure of dynein–dynactin on microtubules shows tandem adaptor binding. Nature 610, 212–216 (2022).
Zhang, K. et al. Cryo-EM reveals how human cytoplasmic dynein is auto-inhibited and activated. Cell 169, 1303–1314(2017).
Canty, J. T. & Yildiz, A. Activation and regulation of cytoplasmic dynein. Trends Biochem. Sci. 45, 440–453 (2020).
Rao, L. & Gennerich, A. Structure and function of dynein’s non-catalytic Subunits. Cells 13, 330 (2024).
Garrott, S. R. et al. Ndel1 disfavors dynein–dynactin–adaptor complex formation in two distinct ways. J. Biol. Chem. 299, 104735 (2023).
Nyarko, A., Song, Y. J. & Barbar, E. Intrinsic disorder in dynein intermediate chain modulates its interactions with NudE and dynactin. J. Biol. Chem. 287, 24884–24893 (2012).
McKenney, R. J., Weil, S. J., Scherer, J. & Vallee, R. B. Mutually exclusive cytoplasmic dynein regulation by NudE–Lis1 and dynactin. J. Biol. Chem. 286, 39615–39622 (2011).
Neuwald, A. F., Aravind, L., Spouge, J. L. & Koonin, E. V. AAA+: a class of chaperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Res. 9, 27–43 (1999).
Carter, A. P., Cho, C., Jin, L. & Vale, R. D. Crystal structure of the dynein motor domain. Science 331, 1159–1165 (2011).
Canty, J. T., Tan, R., Kusakci, E., Fernandes, J. & Yildiz, A. Structure and mechanics of dynein motors. Annu. Rev. Biophys. 50, 549–574 (2021).
Chai, P. et al. The mechanochemical cycle of reactive full-length human dynein 1. Nat. Struct. Mol. Biol. https://doi.org/10.1038/s41594-025-01543-3 (2025).
Cianfrocco, M. A., DeSantis, M. E., Leschziner, A. E. & Reck-Peterson, S. L. Mechanism and regulation of cytoplasmic dynein. Annu. Rev. Cell Dev. Biol. 31, 83–108 (2015).
Amos, L. A. Brain dynein crossbridges microtubules into bundles. J. Cell Sci. 93, 19–28 (1989).
Torisawa, T. et al. Autoinhibition and cooperative activation mechanisms of cytoplasmic dynein. Nat. Cell Biol. 16, 1118–1124 (2014).
Garrott, S. R., Gillies, J. P. & DeSantis, M. E. Nde1 and Ndel1: outstanding mysteries in dynein-mediated transport. Front. Cell Dev. Biol. 10, 871935 (2022).
Bradshaw, N. J., Hennah, W. & Soares, D. C. Nde1 and Ndel1: twin neurodevelopmental proteins with similar ‘nature’ but different ‘nurture’. Biomol. Concepts 4, 447–464 (2013).
Monda, J. K. & Cheeseman, I. M. Nde1 promotes diverse dynein functions through differential interactions and exhibits an isoform-specific proteasome association. Mol. Biol. Cell 29, 2336–2345 (2018).
Zhao, Y., Oten, S. & Yildiz, A. Nde1 promotes Lis1-mediated activation of dynein. Nat. Commun. 14, 7221 (2023).
Shu, T. Z. et al. Ndel1 operates in a common pathway with Lis1 and cytoplasmic dynein to regulate cortical neuronal positioning. Neuron 44, 263–277 (2004).
Stehman, S. A., Chen, Y., McKenney, R. J. & Vallee, R. B. NudE and NudEL are required for mitotic progression and are involved in dynein recruitment to kinetochores. J. Cell Biol. 178, 583–594 (2007).
Wang, S. S. et al. Nudel/NudE and Lis1 promote dynein and dynactin interaction in the context of spindle morphogenesis. Mol. Biol. Cell 24, 3522–3533 (2013).
McKenney, R. J., Vershinin, M., Kunwar, A., Vallee, R. B. & Gross, S. P. Lis1 and NudE induce a persistent dynein force-producing state. Cell 141, 304–314 (2010).
Reiner, O. & Sapir, T. Lis1 functions in normal development and disease. Curr. Opin. Neurobiol. 23, 951–956 (2013).
Huang, J., Roberts, A. J., Leschziner, A. E. & Reck-Peterson, S. L. Lis1 acts as a ‘clutch’ between the ATPase and microtubule-binding domains of the dynein motor. Cell 150, 975–986 (2012).
Qiu, R. D., Zhang, J. & Xiang, X. Lis1 regulates cargo-adapter-mediated activation of dynein by overcoming its autoinhibition in vivo. J. Cell Biol. 218, 3630–3646 (2019).
Elshenawy, M. M. et al. Lis1 activates dynein motility by modulating its pairing with dynactin. Nat. Cell Biol. 22, 570–578 (2020).
Htet, Z. M. et al. Lis1 promotes the formation of activated cytoplasmic dynein-1 complexes. Nat. Cell Biol. 22, 518–525 (2020).
McKenney, R. J. Lis1 cracks open dynein. Nat. Cell Biol. 22, 515–517 (2020).
Karasmanis, E. P. et al. Lis1 relieves cytoplasmic dynein-1 autoinhibition by acting as a molecular wedge. Nat. Struct. Mol. Biol. 30, 1357–1364 (2023).
Ton, W. D. et al. Microtubule-binding-induced allostery triggers Lis1 dissociation from dynein prior to cargo transport. Nat. Struct. Mol. Biol. 30, 1365–1379 (2023).
Kusakci, E. et al. Lis1 slows force-induced detachment of cytoplasmic dynein from microtubules. Nat. Chem. Biol. 20, 521–529 (2024).
Neer, E. J., Schmidt, C. J., Nambudripad, R. & Smith, T. F. The ancient regulatory-protein family of WD-repeat proteins. Nature 371, 297–300 (1994).
Emes, R. D. & Ponting, C. P. A new sequence motif linking lissencephaly, Treacher Collins and oral-facial-digital type 1 syndromes, microtubule dynamics and cell migration. Hum. Mol. Genet 10, 2813–2820 (2001).
Mateja, A., Cierpicki, T., Paduch, M., Derewenda, Z. S. & Otlewski, J. The dimerization mechanism of Lis1 and its implication for proteins containing the LisH motif. J. Mol. Biol. 357, 621–631 (2006).
Reimer, J. M., DeSantis, M. E., Reck-Peterson, S. L. & Leschziner, A. E. Structures of human dynein in complex with the lissencephaly 1 protein, Lis1. eLife 12, e84302 (2023).
Marzo, M. G., Griswold, J. M. & Markus, S. M. Pac1/Lis1 stabilizes an uninhibited conformation of dynein to coordinate its localization and activity. Nat. Cell Biol. 22, 559–569 (2020).
Geohring, I. C. et al. A nucleotide code governs Lis1’s ability to relieve dynein autoinhibition. Preprint at bioRxiv https://doi.org/10.1101/2024.12.30.630615 (2024).
Wynne, C. L. & Vallee, R. B. CDK1 phosphorylation of the dynein adapter Nde1 controls cargo binding from G2 to anaphase. J. Cell Biol. 217, 3019–3029 (2018).
Lam, C., Vergnolle, M. A., Thorpe, L., Woodman, P. G. & Allan, V. J. Functional interplay between Lis1, Nde1 and Ndel1 in dynein-dependent organelle positioning. J. Cell Sci. 123, 202–212 (2010).
Zhang, Y. F. et al. Nde1 is a Rab9 effector for loading late endosomes to cytoplasmic dynein motor complex. Structure 30, 386–395(2022).
Doobin, D. J., Helmer, P., Carabalona, A., Bertipaglia, C. & Vallee, R. B. The role of Nde1 phosphorylation in interkinetic nuclear migration and neural migration during cortical development. Mol. Biol. Cell 35, ar129 (2024).
Pei, Z. et al. The expression and roles of Nde1 and Ndel1 in the adult mammalian central nervous system. Neuroscience 271, 119–136 (2014).
Derewenda, U. et al. The structure of the coiled-coil domain of Ndel1 and the basis of its interaction with Lis1, the causal protein of Miller–Dieker lissencephaly. Structure 15, 1467–1481 (2007).
Ye, F. et al. DISC1 regulates neurogenesis via modulating kinetochore attachment of Ndel1/Nde1 during mitosis. Neuron 96, 1204 (2017).
Moon, H. M. et al. Lis1 controls mitosis and mitotic spindle organization via the Lis1–Ndel1–dynein complex. Hum. Mol. Genet 23, 449–466 (2014).
Zylkiewicz, E. et al. The N-terminal coiled-coil of Ndel1 is a regulated scaffold that recruits Lis1 to dynein. J. Cell Biol. 192, 433–445 (2011).
Wang, S. S. & Zheng, Y. X. Identification of a novel dynein binding domain in nudel essential for spindle pole organization in Xenopus egg extract. J. Biol. Chem. 286, 587–593 (2011).
Efimov, V. P. Roles of NudE and NudF proteins of Aspergillus nidulans: insights from intracellular localization and overexpression effects. Mol. Biol. Cell 14, 871–888 (2003).
Toropova, K. et al. Lis1 regulates dynein by sterically blocking its mechanochemical cycle. eLife 3, e03372 (2014).
Cianfrocco, M. A. et al. Lis1 has two distinct modes of regulating dynein’s mechanochemical cycle. Biophys. J. 112, 43A (2017).
Gillies, J. P. et al. Structural basis for cytoplasmic dynein-1 regulation by Lis1. eLife 11, e71229 (2022).
Jie, J., Lohr, F. & Barbar, E. Dynein binding of competitive regulators dynactin and NudE involves novel interplay between phosphorylation site and disordered spliced linkers. Structure 25, 421–433 (2017).
Schlager, M. A., Hoang, H. T., Urnavicius, L., Bullock, S. L. & Carter, A. P. In vitro reconstitution of a highly processive recombinant human dynein complex. EMBO J. 33, 1855–1868 (2014).
Urnavicius, L. et al. The structure of the dynactin complex and its interaction with dynein. Science 347, 1441–1446 (2015).
Casanal, A., Lohkamp, B. & Emsley, P. Current developments in Coot for macromolecular model building of electron cryo-microscopy and crystallographic data. Protein Sci. 29, 1069–1078 (2020).
Kidmose, R. T. et al. Namdinator—automatic molecular dynamics flexible fitting of structural models into cryo-EM and crystallography experimental maps. IUCrJ 6, 526–531 (2019).
Afonine, P. V. et al. Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr. D 74, 531–544 (2018).
Acknowledgements
We are grateful to members of the K.Z. and A.Y. laboratories for their valuable discussions. This work was funded by the National Institutes of Health (NIH) National Institute of General Medical Sciences (GM136414 to A.Y. and GM142959 to K.Z.) and in part by a Collaboration Development Award Program (to K.Z.) from the Pittsburgh Center for Human Immunodeficiency Virus Protein Interactions (U54AI170791). The cryo-EM data were collected at the Yale ScienceHill cryo-EM facility. We thank J. Lin and K. Zhou for assistance with the data collection. The Yale Cryo-EM Resource is funded in part by the NIH grant S10OD023603 awarded to F. J. Sigworth.
Author information
Authors and Affiliations
Contributions
K.Z. and A.Y. designed the study. J.Y. expressed and purified the dynein, Lis1 and Nde1 proteins for EM. J.Y. and P.C. prepared the cryo-EM samples, collected and processed the data and built the PDB models. P.C. and J.Y. processed the negative-stain EM data and quantified the particle numbers. Y.Z. performed the Lis1 mutagenesis, protein preparation, TIRF imaging and MP assays. J.Y., P.C., Y.Z., K.Z. and A.Y. analyzed the data and prepared the figures. J.Y., Y.Z., P.C., K.Z. and A.Y. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Biology thanks Richard McKenney and the other, anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 MP analysis of nucleotide conditions on Nde1 and Lis1 binding to WT dynein.
MP shows that under apo buffer (a), 0.1 mM ATP (b), ADP (c), ATP.vi (d), and AMPPNP (e) conditions, Nde1 promotes Lis1 binding to dynein, forming a 1:1 dynein-Lis1 (DL) complex. The nucleotide condition does not affect Nde1’s ability to tether Lis1 to dynein. Importantly, the formation of dynein-Nde1, dynein-Lis1-Nde1, and 1:2 dynein-Lis1 complexes was not observed. In the Lis1-alone condition, no significant DL complex was formed immediately.
Extended Data Fig. 2 Workflow for negative-stain EM data processing.
a, Representative micrographs for dynein alone (42 micrographs), dynein-Lis1 at 1:1 (41 micrographs), and dynein-Lis1 at 1:2 (47 micrographs) molar ratios from batch #1. b, Particle picking from representative micrographs in each dataset using a template matching approach based on Phi and open dynein models (particle diameter: 750 Å, distance cutoff: 400 Å). c, Three rounds of 2D classification were performed after extracting all particles (box size: 960 Å × 960 Å), yielding class averages of Phi dynein, open dynein motors, single motors, and junk particles. d, Final classified 2D averages showing Phi dynein (43.7%), two-motor open dynein (43.3%), and single-motor open dynein (13.0%). The particle numbers for each group were counted, and single motors were considered as open dynein by dividing the total number of particles by two.
Extended Data Fig. 3 Quantification of Phi-dynein percentage in the presence or absence of Lis1 and Nde1.
Dynein (D) was incubated with Lis1 (L), Nde1 (N), or both proteins for 90 minutes at the indicated molar ratios of protein dimers. The percentage of Phi-dynein was quantified under each condition, D + L (a), D + N (b), D + L + N (c). Colors represent independent biological replicates. n = 6 biological replicates for D:L = 1:1 and D:L = 1:2; n = 3 biological replicates for D:N = 1:1 and n = 4 biological replicates for D:N = 1:2; n = 4 biological replicates for D:L:N = 1:1:1 and D:L:N = 1:2:2. The control (Ctrl) corresponds to the Phi-dynein percentage measured in the dynein-alone condition.
Extended Data Fig. 4 Cryo-EM data processing for the dynein-Lis1 dataset.
a, A representative cryo-EM micrograph and the flowchart of cryo-EM data processing. b, Fourier Shell Correlation (FSC) curves showing the final resolution estimates for the motor domains of the Phi (2.71 Å) and PhiL-Lis1(2.86 Å) datasets. Orientation distribution of Phi (c) and PhiL-Lis1(d).
Extended Data Fig. 5 Cryo-EM data processing for the dynein-Lis1-Nde1 dataset.
a, A representative cryo-EM micrograph and the flowchart of cryo-EM data processing. b, FSC curve showing the final resolution estimate for the motor domains of the PhiL-Lis1 (2.88 Å) dataset. c, Comparison of Nde1’s effect on Lis1 binding to the open dynein motor. Cryo-EM particle numbers for open dynein-Lis1 and open dynein alone were quantified from the dynein-Lis1 and dynein-Lis1-Nde1 datasets. In the dynein-Lis1 dataset, 493,173 particles correspond to single dynein motors unbound to Lis1, and 624,475 particles correspond to dynein motors bound to Lis1 (including Lis1 dimers and monomers). In the dynein-Lis1-Nde1 dataset, 162,229 particles correspond to dynein motors unbound to Lis1, and 170,995 particles correspond to dynein motors bound to Lis1 (including Lis1 dimers and monomers), indicating that Nde1 does not promote Lis1 binding to the open dynein motor.
Extended Data Fig. 6 Comparison of local resolution, nucleotide binding in AAA1, AAA3, AAA4, and sensor-I loop conformation in MD-A of the Phi and PhiL-Lis1.
Local resolution, and nucleotide binding states in MD-A at AAA1, AAA3, and AAA4 of the Phi (a) and PhiL-Lis1 (b). MD-A and -B share the same nucleotide binding in AAA1, AAA3, and AAA4 across both the Phi and PhiL-Lis1. The sensor-I loop adopts almost the same conformation in MD-A (or -B) of both Phi (c) and PhiL-Lis1 (d), indicating that Lis1 binding does not affect phosphate release. The color scheme is the same as Fig. 4.
Extended Data Fig. 7 Density quality at the dynein MD-A and Lis1 interface in PhiL-Lis1.
a, Well-defined density at the AAA5-Lis1ring and AAA6-Lis1ring regions, showing tight and stable interactions. The color scheme for the motor domains is consistent with Fig. 4. b, Flexible density at the linker-Lis1ring interface, indicating dynamic interactions in this region.
Extended Data Fig. 8 Binding characterization of Lis1 mutants and structural prediction of Lis1Δ300–304.
a, MP profiles show binding of Lis1 mutants to open dynein. Open dynein and Lis1 mutants were mixed at a 1:2 ratio and incubated for 2 min prior to measurements. b, MP profiles show binding of the Lis1AAA6 mutant to dynein with Nde1 at different incubation times. Dynein, Lis1AAA6, and Nde1 were mixed at 1:2:2 ratio (D: dynein only, DL: one dynein and one Lis1, DL2N: one dynein, two Lis1s, and one Nde1) and incubated for 2, 6, and 15 minutes before the measurements. c, The percentages of mass populations detected in b. d, Overlay of AlphaFold3-predicted Lis1Δ300-304 and WT Lis1 bound to PhiL dynein motor domains. Lis1Δ300-304 is shown in violet, while WT Lis1 is colored green and sky blue. Dynein motors domains are displayed in grey and white. The structure overlay indicates that deletion of residues 300–304 in Lis1 surface loop does not induce notable conformational change of the rigid elements of Lis1Δ300-304.
Extended Data Fig. 9 Characterization of additional mutants of Lis1 at the PhiL-Lis1 interface.
a, MP profiles illustrate the interaction of Nde1 (N) with Lis1 mutants. Nde1 interacts with one (NL) or two (NL2) Lis1 dimers. b, MP of the binding of Lis1 mutants to open dynein. Dynein and Lis1 were incubated for 2 minutes at 1:2 ratio (D: dynein only, DL: one dynein and one Lis1). c. MP shows the binding of Lis1 mutants to dynein with or without Nde1. Dynein, Lis1 and Nde1 were incubated for 2 minutes at 1:2:2 ratio (DL2N: one dynein, two Lis1s, and one Nde1). d, Representative kymographs show the motility of WT DDR complexes with or without Nde1 and Lis1. e, Run frequency of WT DDR with or without Nde1 and Lis1 (mean ± s.d.; n = 30 MTs for each condition; statistics from two independent experiments). Results were normalized to the -Lis1, -Nde1 condition. P values are calculated from a two-tailed t-test.
Extended Data Fig. 10 Comparison of yeast Chi-Lis1 and human PhiL-Lis1 motor domains.
a, The structure of yeast Chi-Lis1 (PDB:8DZZ)41, showing two tail-truncated yeast dynein motor domains (grey) bound to two Lis1 dimers (colored, Chi-Lis1 1:2). b, Residues of MD-A that interact with Lis1ring are located in AAA6-Lis1ring and AAA5-Lis1ring regions and highlighted with dashed rectangle. Representative residues of MD-A involved in the canonical Lis1ring binding sites are located in Lis1ring-AAA3, Lis1ring-AAA4, Lis1ring-AAA5 and Lis1ring-stalk region. Interactions between MD-A and MD-B are in stalk-stalk region. Residues are displayed in sphere mode and are colored according to the subdomains in Fig. 4. c, Superimposition of the human PhiL-Lis1 and yeast Chi-Lis1 structures, showing that Chi-Lis1 adopts a more expanded conformation, with larger grooves on both the front and back sides compared to the more compact PhiL-Lis1 structure. Lis1 is hidden for clarity. Vectors represent interatomic distances of pairwise Cα atoms between the PhiL-Lis1 and Chi-Lis1 structures.
Supplementary information
Supplementary Information
Supplementary Fig. 1 and Tables 1–4.
Supplementary Video 1
Full-length human dynein in PhiL conformation, bound to a Lis1 dimer and displaying the newly identified interface with Lis1.
Supplementary Video 2
Single-molecule motility recordings of WT DDR complexes, in the presence or absence of Nde1, WT Lis1 and Lis1 mutants. The fluorescence signal originates from BicDR1–mNeonGreen.
Source data
Source Data Fig. 1
Statistical source data of relative percentage of Phi dynein.
Source Data Fig. 4
Statistical source data of dynein motility under Lis1 mutants.
Source Data Extended Data Fig. 3
Statistical source data of Phi dynein percentage.
Source Data Extended Data Fig. 9
Statistical source data of dynein motility under additional Lis1 mutants.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, J., Zhao, Y., Chai, P. et al. Nde1 promotes Lis1 binding to full-length autoinhibited human dynein 1. Nat Chem Biol 22, 274–283 (2026). https://doi.org/10.1038/s41589-025-01981-6
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41589-025-01981-6
This article is cited by
-
A nucleotide code governs Lis1’s ability to relieve dynein autoinhibition
Nature Chemical Biology (2026)