Abstract
The mechanisms underlying the efficacy of anti-programmed cell death protein 1 (PD-1) and anti-cytotoxic T lymphocyte-associated protein 4 (CTLA-4) therapy are incompletely understood. Here, by immune profiling responding PD-1+CD8+ T (TResp) cell populations from patients with advanced melanoma, we identified differential programming of TResp cells in response to combination therapy, from an exhausted toward a more cytotoxic effector program. This effect does not occur with anti-PD-1 monotherapy. Single-cell transcriptome and T cell receptor repertoire analysis was used to identify altered effector programming of expanding PD-1+CD8+ T cell clones with distinct regulon usage, STAT1 and STAT3 utilization and antitumor specificity connected to interleukin (IL)-21 signaling in combination and anti-CTLA-4 monotherapy. Therapeutic efficacy of CTLA-4 blockade was lost in B16F10 melanoma models with either Il21r− deficiency or anti-IL-21 receptor blockade. Together, these results show how IL-21 signaling to TResp is critical for anti-CTLA-4-based checkpoint therapies and highlight major signaling differences to anti-PD-1 monotherapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
CITE–seq data have been deposited in the Gene Expression Omnibus under accession number GSE268364. ScRNA-seq and TCR-seq data are available via Zenodo at https://doi.org/10.5281/zenodo.13971562 (ref. 43).
Code availability
RDS objects including the regulon analysis are available via Zenodo at https://doi.org/10.5281/zenodo.13971562 (ref. 43). Code availability for CheckMate 238: original code is deposited in Zenodo (https://doi.org/10.5281/zenodo.14224147). Requests for resources or further information should be directed to the lead contact at NYU Grossman School of Medicine for CheckMate 238.
References
Huang, A. C. & Zappasodi, R. A decade of checkpoint blockade immunotherapy in melanoma: understanding the molecular basis for immune sensitivity and resistance. Nat. Immunol. 23, 660–670 (2022).
Weber, J. et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N. Engl. J. Med. 377, 1824–1835 (2017).
Larkin, J. et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 381, 1535–1546 (2019).
Huang, A. C. et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 545, 60–65 (2017).
Huang, A. C. et al. A single dose of neoadjuvant PD-1 blockade predicts clinical outcomes in resectable melanoma. Nat. Med. 25, 454–461 (2019).
Fairfax, B. P. et al. Peripheral CD8+ T cell characteristics associated with durable responses to immune checkpoint blockade in patients with metastatic melanoma. Nat. Med. 26, 193–199 (2020).
Miller, B. C. et al. Author correction: subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade. Nat. Immunol. 20, 1556 (2019).
Sotomayor, E. M., Borrello, I., Tubb, E., Allison, J. P. & Levitsky, H. I. In vivo blockade of CTLA-4 enhances the priming of responsive T cells but fails to prevent the induction of tumor antigen-specific tolerance. Proc. Natl Acad. Sci. USA 96, 11476–11481 (1999).
Wei, S. C. et al. Distinct cellular mechanisms underlie anti-CTLA-4 and anti-PD-1 checkpoint blockade. Cell 170, 1120–1133.e1117 (2017).
Das, R. et al. Combination therapy with anti-CTLA-4 and anti-PD-1 leads to distinct immunologic changes in vivo. J Immunol 194, 950–959 (2015).
Franken, A. et al. CD4+ T cell activation distinguishes response to anti-PD-L1 + anti-CTLA4 therapy from anti-PD-L1 monotherapy. Immunity 57, 541–558.e547 (2024).
Codarri Deak, L. et al. PD-1-cis IL-2R agonism yields better effectors from stem-like CD8+ T cells. Nature 610, 161–172 (2022).
Hashimoto, M. et al. PD-1 combination therapy with IL-2 modifies CD8+ T cell exhaustion program. Nature 610, 173–181 (2022).
Bengsch, B. et al. Epigenomic-guided mass cytometry profiling reveals disease-specific features of exhausted CD8 T cells. Immunity 48, 1029–1045.e1025 (2018).
Chronister, W. D. et al. TCRMatch: predicting T-cell receptor specificity based on sequence similarity to previously characterized receptors. Front. Immunol. 12, 640725 (2021).
Tsui, C. et al. MYB orchestrates T cell exhaustion and response to checkpoint inhibition. Nature 609, 354–360 (2022).
Aibar, S. et al. SCENIC: single-cell regulatory network inference and clustering. Nat. Methods 14, 1083–1086 (2017).
McLane, L. M. et al. Role of nuclear localization in the regulation and function of T-bet and Eomes in exhausted CD8 T cells. Cell Rep. 35, 109120 (2021).
Seo, H. et al. TOX and TOX2 transcription factors cooperate with NR4A transcription factors to impose CD8+ T cell exhaustion. Proc. Natl Acad. Sci. USA 116, 12410–12415 (2019).
Ohl, K. et al. CREM alpha enhances IL-21 production in T cells in vivo and in vitro. Front. Immunol. 7, 618 (2016).
Hermans, D. et al. Lactate dehydrogenase inhibition synergizes with IL-21 to promote CD8+ T cell stemness and antitumor immunity. Proc. Natl Acad. Sci. USA 117, 6047–6055 (2020).
Leonard, W. J. & Wan, C. K. IL-21 Signaling in Immunity. F1000Res 5, F1000 (2016).
Tian, Y. et al. A context-dependent role for IL-21 in modulating the differentiation, distribution, and abundance of effector and memory CD8 T cell subsets. J. Immunol. 196, 2153–2166 (2016).
Cui, W., Liu, Y., Weinstein, J. S., Craft, J. & Kaech, S. M. An interleukin-21-interleukin-10–STAT3 pathway is critical for functional maturation of memory CD8+ T cells. Immunity 35, 792–805 (2011).
Mastroianni, J. et al. miR-146a controls immune response in the melanoma microenvironment. Cancer Res. 79, 183–195 (2019).
Curran, M. A., Montalvo, W., Yagita, H. & Allison, J. P. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc. Natl Acad. Sci. USA 107, 4275–4280 (2010).
Gangaev, A. et al. Differential effects of PD-1 and CTLA-4 blockade on the melanoma-reactive CD8 T cell response. Proc. Natl Acad. Sci. USA 118, e2102849118 (2021).
Gros, A. et al. Prospective identification of neoantigen-specific lymphocytes in the peripheral blood of melanoma patients. Nat. Med. 22, 433–438 (2016).
Yossef, R. et al. Phenotypic signatures of circulating neoantigen-reactive CD8+ T cells in patients with metastatic cancers. Cancer Cell 41, 2154–2165.e2155 (2023).
Spitzer, M. H. et al. Systemic immunity is required for effective cancer immunotherapy. Cell 168, 487–502.e415 (2017).
Zander, R. et al. CD4+ T cell help is required for the formation of a cytolytic CD8+ T cell subset that protects against chronic infection and cancer. Immunity 51, 1028–1042.e1024 (2019).
Choi, Y. S. et al. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity 34, 932–946 (2011).
Bauquet, A. T. et al. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH17 cells. Nat. Immunol. 10, 167–175 (2009).
Wang, C. J. et al. CTLA-4 controls follicular helper T-cell differentiation by regulating the strength of CD28 engagement. Proc. Natl Acad. Sci. USA 112, 524–529 (2015).
Rafei-Shamsabadi, D., Lehr, S., Behrens, M. & Meiss, F. Additive intralesional interleukin-2 improves progression-free survival in a distinct subgroup of melanoma patients with prior progression under immunotherapy. Cancers 14, 540 (2022).
Hensel, N. et al. Memory-like HCV-specific CD8+ T cells retain a molecular scar after cure of chronic HCV infection. Nat. Immunol. 22, 229–239 (2021).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587.e3529 (2021).
Korsunsky, I. et al. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat. Methods 16, 1289–1296 (2019).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Gene Ontology Consortium. The Gene Ontology resource: enriching a GOld mine. Nucleic Acids Res. 49, D325–D334 (2021).
Ashburner, M. et al. Gene Ontology: tool for the unification of biology. Nat. Genet. 25, 25–29 (2000).
Gillespie, M. et al. The reactome pathway knowledgebase 2022. Nucleic Acids Res. 50, D687–D692 (2022).
Bengsch, B., Sagar & Zhang, Z. Single-cell RNA-seq and TCR-seq analysis of PD-1+CD8+ T-cells responding to anti-PD-1 and anti-PD-1/CTLA-4 immunotherapy in melanoma. Zenodo https://doi.org/10.5281/zenodo.13971562 (2024).
Acknowledgements
We thank the patients contributing their biomaterials to the study. We thank A. Kabdalova and P. Otto-Mora for help with cytokine titrations and flow cytometry. We thank H. Pircher for provision of the B16 cell line. We thank D. J. Tenney at Bristol Myers Squibbs (BMS) for support with the analysis of CheckMate 238 samples. BMS had no role in funding, design or interpretation of results in the present study. The study was supported by the Deutsche Forschungsgemeinschaft (DFG) research grant numbers 520992132 (Heisenberg), 441891347 (SFB1479-OncoEscape), 390939984 (CIBSS), 272983813 (TRR179), 505372148 (FOR5560) and the German Cancer Consortium (DKTK). Z.Z. and K.M. were further supported by a stipend provided by the Chinese Scholarship Council. This work was supported by the US National Institutes of Health (grant numbers CA244936 and P50CA225450 to R.S.H. and J.W. and P30CA016087 to R.S.H.) and through a Career Enhancement Program award from the NYU SPORE program (grant number P50CA225450) to R.S.H.
Author information
Authors and Affiliations
Contributions
B.B. provided the study concept and design. A.C.V., I.G., J.S., K.M., K.Z., S.S., Sagar and Z.Z. carried out the human experiments and procedures. M.L., R.Z. and V.F. carried out the murine experiments and procedures. D.R.S., F.M., J.S.W., N.B., R.D. and S.L. recruited patients and acquired clinical data. E.S., F.W., J.S., K.M., M.B., N.R., R.S.H., Sagar and Z.Z. did the bioinformatics and statistical analysis. B.B. and Z.Z. interpreted the data and drafted the paper. C.N.H., F.M., F.W., M.H., R.E.V., R.H., R.T., R.Z. and T.B. revised the paper for important intellectual content. B.B. supervised the study.
Corresponding author
Ethics declarations
Competing interests
F.M. served as consultant and/or has received honoraria or travel support from Novartis, Roche, BMS, MSD, Pierre Fabre and Sunpharma. D.R.S. served as consultant and/or has received honoraria from Roche and BMS and travel support from Sunpharma and Sanofi. R.Z. has received honoraria from Novartis, INcyte, MNK and Sanofi. R.V. has received honoraria or travel support from Abbvie, AstraZeneca, Galapagos, Janssen-Cilag, Novartis, Pfizer and Roche, and research grant support from Novartis and Pfizer. J.W. consulted for and received <US$10,000 per annum from Merck, Genentech, AstraZeneca, GSK, Novartis, Nektar, Celldex, Incyte, Biond, Moderna, ImCheck, Sellas, Evaxion, Pfizer, Regeneron and EMD Serono and received US$10,000–25,000 from BMS for membership on advisory boards. J.W. also held equity in Biond, Evaxion, OncoC4 and Instil Bio, was on the scientific advisory boards for CytomX, Incyte, ImCheck, Biond, Sellas, Instil Bio, OncoC4 and NexImmune, and was remunerated with between US$10,000 and US$50,000. In addition, J.W. is named on a patent filed by Moffitt Cancer Center on an ipilimumab biomarker and on tumor-infiltrating lymphocyte preparation, and on a PD-1 patent filed by Biodesix. J.W. received <US$6,000 in royalties. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Immunology thanks Lionel Apetoh and Samir Khleif for their contributions to the peer review of this work. Peer reviewer reports are available. Primary Handling Editor: Nick Bernard, in collaboration with the Nature Immunology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
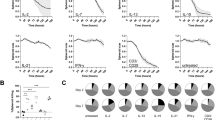
Extended Data Fig. 1 Study outline and clinical outcome of patients.
(A) Schematic of major samples and experimental techniques used in the study. (B) Tumor response of patients evaluated after 3 months. CR: Complete response; PR: partial response; SD: stable disease; PD: progressive disease. In adjuvant group, patients were evaluated either as CR or PD. (C) Kaplan-Meier progression-free survival analysis of patients stratified by therapy regimens (P = 0.045). Statistical significance was determined by Two-sided Log-rank test in (C) (* P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001). Illustrations in A were created using BioRender.com.
Extended Data Fig. 2
(A) Exemplary dot plot of surface PD-1 and IgG4 staining at baseline and post-therapy of one patient undergoing dual therapy (left); representative stainings of PD-1 and Ki-67 on CD8+ T cells (right) (B) CD8+ T cells from 10 patients (adju n = 2, mono n = 4, dual n = 4) analyzed in one batch were projected by UMAP embedding and clustered by FLOWSOM algorithm. As in Fig. 1d, 8 clusters were identified and frequencies of cluster 2-8 in patient samples across therapy regimens and time points are indicated. For (C–H), ICB therapy-treated patients within different treatment groups (adjuvant (adju) (n = 11), palliative (mono) (n = 15), and combination therapy with anti-CTLA-4 (n = 20)) were included. (C) Scatter plots depict frequencies of populations gated for different expression levels of perforin and granzyme B expression on PD-1+CD8+ T cells (Perforinint adju P = 0.2695, mono P = 0.0246, dual P = 0.0032; GZMBint adju P = 0.2061, mono P = 0.5995, dual P = 0.0002; Perforinhi adju P = 0.1475, mono P = 0.3028, dual P = 0.1769; GZMBhi adju P = 0.5195, mono P = 0.0189, dual P = 0.8480). (D) Frequencies of IL-2 (adju P = 0.7002, mono P = 0.8672, dual P = 0.6742) and CCL3/4 (adju P = 0.5566, mono P = 0.8672, dual P = 0.1991) produced by PD-1+CD8+ T cells after PMA/ ionomycin stimulation are indicated. (E) Scatter plots depicting frequencies of indicated markers within Ki-67+CD8+ T cells (Perforinint adju P = 0.2783, mono P = 0.0215, dual P = 0.0037; GZMBint adju P = 0.5771, mono P = 0.0706, dual P < 0.0001; Perforinhi adju P = 0.7002, mono P = 0.4131, dual P = 0.3683; GZMBhi adju P = 0.4648, mono P = 0.5245, dual P = 0.8408). (F) Cytokine production (IFN-γ adju P = 0.7002, mono P = 0.1395, dual P = 0.0010; TNF adju P = 0.0420, mono P = 0.6698, dual P = 0.0002; CCL3/4 adju P = 0.7646, mono P = 0.5830, dual P = 0.0441) and functional exhaustion score (FES) of Ki-67+CD8+ T cells across therapy regimens and time points (adju P = 0.4131, mono P = 0.2166, dual P < 0.0001). (G) Scatter plots depict frequencies of populations expressing indicated markers of PD-1-CD8+ T cells (Ki-67: adju P = 0.1445, mono P = 0.9012, dual P = 0.0005; CD38hi: adju P = 0.0830, mono P = 0.1102, dual P < 0.0001; CD39: adju P = 0.2783, mono P = 0.7615, dual P = 0.0006; LAG3: adju P = 0.4785, mono P = 0.3591, dual P = 0.0066; CD127: adju P = 0.6211, mono P = 0.5614, dual p = 0.0004) (H) Fold change of Ki-67, CD38hi, CD39, LAG-3, CD127, IFN-γ, TNF, IL-2, GZMBint, GZMBhi, Perforinint, Perforinhi PD-1+CD8+ T cells (++) vs. PD-1-CD8+ T cells (+-) after ICB therapy within different treatment groups. Where applicable, samples pre-therapy are represented in open circles and post-therapy in open squares in scatter plots. Bar and error bar represent median and 95% CI in scatter plots. Statistical significance was determined by two-sided paired Wilcoxon signed-rank test (B–G) or two-sided Kruskall-Wallis Test (H) (ns = non-significant, * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001).
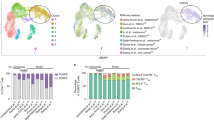
Extended Data Fig. 3
(A) Expression of selected genes by PD-1+CD8+ T cells similar to Fig. 2c are indicated on the UMAP (purple: high expression, gray: low expression). (B) Top 20 reactome pathway analysis of genes up-regulated in dual-ICB after cycle 1 (pseudobulk analysis). –log FDR of indicated gene-sets are indicated by the bar length.
Extended Data Fig. 4
Dose-response titration of IL-15 and IL-21 inducing STAT phosphorylation in PD-1+CD8+ T cells. Concentration used and % of cells positive for pSTAT1/ 3/ 5 are plotted for IL-15- and IL-21-exposed T cells, as indicated (ng/ml).
Extended Data Fig. 5 Effect of STAT inhibition and CTLA-4 blockade on IL-21 signaling.
(A) Workflow of cytokine (IL-21: 80 ng/ml)/ anti-CTLA-4/ and inhibitor treatment (PerV:1 mM, Gando:1 µM, Stattic: 5 µM, Tofa: 100 nM, Upada: 1 µM), flow cytometric staining and data analysis (n = 5). (B) Representative histograms of flow cytometric data showing pSTAT1, pSTAT3, and pSTAT5 expression in PD-1+CD8+ T cells with or without anti-CTLA-4 treatment. (C) Median fluorescence intensity (MFI) and frequencies (%) of pSTAT1 (MFI: unstim. versus IL-21 P = 0.0034, IL-21 versus IL-21+Tofa P = 0.0447, IL-21 versus IL-21+Upada P = 0.0374; frequencies: unstim. versus IL-21 P = 0.0028, IL-21 versus IL-21+Upada P = 0.0311), pSTAT3 (MFI: unstim versus IL-21 P = 0.0146, DMSO vs. IL-21 P = 0.0098, IL-21 versus IL-21+Upada P = 0.0011; frequencies: DMSO versus IL-21 P = 0.0065, IL-21 versus IL-21+Upada P = 0.0007), and pSTAT5 (MFI: IL-21 versus IL-21+Gando P = 0.0374; frequencies: IL-21 vs. IL-21+Gando P = 0.0447) on PD-1+CD8+ T cells with anti-CTLA-4 treatment. (D) Heatmaps of the MFI of pSTATs on PD-1+CD8+ T cells and PD-1-CD8+ T cells. pSTATs expression was scaled by Z-score. (E) Comparison of MFI of pSTAT1, pSTAT3, and pSTAT5 gated on PD-1+CD8+ T cells with or without anti-CTLA-4 treatment. (F) MFI and frequencies of pSTAT1, pSTAT3 and pSTAT5 on PD-1+CD8+ T cells with anti-CTLA-4 or iplimumab treatment. Two-sided Friedman analysis was performed in (C). Two-sided pairedWilcoxon signed-rank test was performed in (E). P values are indicated (P * < 0.05, P ** < 0.01, P *** < 0.005, P **** < 0.001). Error bars indicate mean ± SEM. Gando, Gandotinib; JAK, janus kinase; PerV, pervanadate; pSTATs, phosphorylated-STATs; Tofa, Tofacitinib citrate; Upada, Upadacitinib. Schematic in A was created using BioRender.com.
Extended Data Fig. 6
(A) t-SNE dimension reduction was performed on influenza (FLU)-specific CD8+ T cells cultured with (0 ng/ml, 30 ng/ml, or 80 ng/ml of IL-21) after 7 d of culture. Overlay dot plot (left) and normalized colored-marker expression (right) of indicated cytotoxic, activation and exhaustion-associated genes are visualized on the t-SNE map. (B) Production of IL-2 (adju P = 0.5928, mono P = 0.4921, dual P = 0.0259), TNF (adju P = 0.1299, mono P = 0.1040, dual P = 0.9452), IFN-γ (adju p = 0.4922, mono p = 0.0107, dual p = 0.1160) and CCL-3/4 (adju P = 0.7148, mono P = 0.9785, dual P = 0.6762) was determined by CD4+ T cells after PMA/ ionomycin stimulation. Scattered dot plots indicate frequency of cytokine-positive cells by patient groups (adjuvant (adju) (n = 11), palliative (mono) (n = 14), and combination therapy with anti-CTLA-4 (n = 20)) and time point. (C) IgG (adju P = 0.7145, mono P = 0.3281, dual P = 0.0026), IgA (adju P = 0.2101, mono P = 0.8796, dual P = 0.0064) and IgM (adju P = 0.3031, mono P = 0.9645, dual P = 0.6387) concentration in patient plasma was measured by nephelometry (adjuvant (adju) (n = 14), palliative (mono) (n = 15), and combination therapy with anti-CTLA-4 (n = 15)). Samples pre-therapy are represented in open circles and post-therapy in open squares in scatter plots. Bar and error bar represent median and 95% CI in scatter plots. Statistical significance was determined by two-sided paired Wilcoxon signed-rank test (* P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001).
Extended Data Fig. 7
CD8+ T-cell responses during anti-PD-1 (nivolumab) or anti-CTLA-4 (ipilimumab) monotherapy in the CheckMate 238 cohort were analyzed by scRNASeq at week 1 and week 3 (n = 4 patients per treatment). (A) Pseudobulk PCA analysis indicates differential transcriptomic programing at week 3 between treatment conditions. PC1 and PC2 are shown, samples are colored by treatment regimen. (B) UMAP visualization of CD8+ T cells per analysis time point and treatment regimen. Clusters are indicated by color. (C) Expression of selected cytotoxic genes and IL-21 are depicted by violin plot stratified by treatment regimen. (D) Selected cytotoxic genes are visualized for cluster 3 cells by violin diagram. (E) Heatmap indicating differential gene expression is shown for clusters at week 3 time point.
Extended Data Fig. 8
C57/Bl6 WT mice were irradiated and transplanted with either WT or Il21r-/- bone marrow for 30 days, followed by intravenous inoculation of B16.F10 melanoma cell line. Mice were treated with anti-PD-1- or anti-CTLA-4 monotherapy, dual anti-PD-1- and anti-CTLA-4 therapy or isotype control on day 1, 4, 8, 16 and 22. Survival was monitored for up to 80 days. Kaplan-Meier plots of mice plots grouped according to chimerism genotype are shown, WT/WT chimera (A) and WT/IL21R-/- (B).
Extended Data Fig. 9 Gating strategies.
(A) TEX panel, (B) Surface panel, (C) Cytokine panel, (D) Melan-A panel.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Z., Langenbach, M., Sagar, S. et al. Efficacy of CTLA-4 checkpoint therapy is dependent on IL-21 signaling to mediate cytotoxic reprogramming of PD-1+CD8+ T cells. Nat Immunol 26, 92–104 (2025). https://doi.org/10.1038/s41590-024-02027-0
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41590-024-02027-0
This article is cited by
-
Suppression of multiple mouse models of refractory malignancies by reprogramming IL-18 ligand-receptor interaction
Nature Communications (2025)
-
Combination of low-intensity pulsed ultrasound irradiating immune organs with immune checkpoint blockade augments systemic anti-tumor immunity on low tumor burden 4T-1 breast cancer
Cancer Immunology, Immunotherapy (2025)