Abstract
Bacteriophage (phage) therapy, which uses lytic viruses as antimicrobials, is a potential strategy to address the antimicrobial resistance crisis. Cystic fibrosis, a disease complicated by recurrent Pseudomonas aeruginosa pulmonary infections, is an example of the clinical impact of antimicrobial resistance. Here, using a personalized phage therapy strategy that selects phages for a predicted evolutionary trade-off, nine adults with cystic fibrosis (eight women and one man) of median age 32 (range 22–46) years were treated with phages on a compassionate basis because their clinical course was complicated by multidrug-resistant or pan-drug-resistant Pseudomonas that was refractory to prior courses of standard antibiotics. The individuals received a nebulized cocktail or single-phage therapy without adverse events. Five to 18 days after phage therapy, sputum Pseudomonas decreased by a median of 104 CFU ml−1, or a mean difference of 102 CFU ml−1 (P = 0.006, two-way analysis of variance with Dunnett’s multiple-comparisons test), without altering sputum microbiome, and an analysis of sputum Pseudomonas showed evidence of trade-offs that decreased antibiotic resistance or bacterial virulence. In addition, an improvement of 6% (median) and 8% (mean) predicted FEV1 was observed 21–35 days after phage therapy (P = 0.004, Wilcoxon signed-rank t-test), which may reflect the combined effects of decreased bacterial sputum density and phage-driven trade-offs. These results show that a personalized, nebulized phage therapy trade-off strategy may affect clinical and microbiologic endpoints, which must be evaluated in larger clinical trials.
Similar content being viewed by others
Main
The rise of antimicrobial resistance (AMR) poses a serious global threat to human health1. By 2050, AMR-induced mortality is projected to overtake annual deaths caused by currently more common diseases (for example, cancer and diabetes) due to multidrug-resistant (MDR) and increasingly pan-drug-resistant (PDR) bacterial infections1. The ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter spp.) are particularly concerning because they can present simultaneous resistance against multiple classes of antibiotics2. Complications due to MDR and PDR pathogens are already common in people with cystic fibrosis (pwCF), which is one of the most common life-limiting monogenic diseases. The ESKAPE pathogen P. aeruginosa (PsA) is the most prevalent pathogen in adult pwCF, which significantly predicts disease morbidity and mortality3.
There is renewed interest in bacteriophage (phage) therapy as an antibacterial strategy to treat MDR and PDR pathogens4,5. Lytic phages are bacteria-specific viruses that replicate and kill (lyse) susceptible bacteria to release progeny phages, which can act as self-amplifying ‘drugs’ against infection(s)4,6. However, bacteria use a range of defense mechanisms to better resist phage infection7, which is a recognized issue with phage therapy that has historically limited its potential clinical utility. However, studies suggest that certain phages can kill target bacteria while selecting for evolved resistance that coincides with potentially clinically useful genetic trade-offs. In this case, bacteria evolve resistance to phage(s) but suffer reduced performance in a pathogenicity trait (for example, antibiotic resensitization or decreased virulence)4. Therefore, a phage therapy strategy that leverages such a trade-off could result in improved clinical outcomes by decreasing bacterial burden and by selecting for surviving bacterial mutants that are less resistant to antibiotics or less virulent8.
Using environmentally sourced lytic phages without genetic manipulation, we developed an approach to use phages that bind to bacterial cell surface receptors that contribute to functional mechanisms of antibiotic resistance or virulence (for example, efflux pumps, lipopolysaccharide (LPS) and type-IV pili (TIVP))4. In addition to killing target bacteria, these phages were chosen for their ability to predictably select for surviving bacterial mutants with reduced antibiotic resistance and/or attenuated virulence. For example, phage OMKO1 infects PsA and selects for evolved phage resistance that coincides with reduced multidrug efflux (Mex) pump function to achieve reduced antibiotic resistance9, a trade-off previously exploited in a clinical case of phage therapy10. Using this approach, the current study also deployed two additional lytic phages that bind to LPS (phage LPS-5) or to TIVP (phage TIVP-H6), which drive evolved phage resistance trade-offs that compromise bacterial virulence.
Here, we present outcomes from the first nine adult pwCF compassionate cases who were treated with nebulized phage(s) using the above approach.
Results
Treatment protocol
This cohort of eight female and one male pwCF, median age 32 years (range 22–46 years), were the first nine patients treated with compassionate phage therapy because of MDR (seven patients) or PDR (two patients) PsA that did not respond to standard cystic fibrosis (CF) therapies (Table 1). All patients had a clinical course complicated by frequent pulmonary exacerbations despite oral, inhaled and/or intravenous (IV) antibiotics without evidence of clinical benefit. When phage therapy was initiated, all patients were concurrently on IV antibiotics (n = 6) or recently completed antibiotics (n = 3; Table 2). Some patients had additional sputum pathogens (for example, Staphylococcus aureus, Achromobacter spp. and nontuberculous mycobacteria; Table 1). At the time of phage therapy, elexacaftor–tezacaftor–ivacaftor (Trikafta or Kaftrio) was not available. Two individuals were taking tezacaftor–ivacaftor (Symdeko or Symkevi; Table 1). Individuals were selected for treatment owing to a combination of the following: lack of clinical response to antibiotics, persistent symptoms and/or clinical decline despite comprehensive CF care, severity of pulmonary exacerbations, and no additional approved therapeutics available.
Phage therapy was personalized on the basis of susceptibility of the PsA sputum isolates to phages in the phage library (Table 2) at Yale’s Center for Phage Biology and Therapy, which provided phages for all participants. After suitable phage(s) were identified, a treatment protocol was reviewed with each participant’s CF physician. This protocol, phage manufacturing and the informed consent form were reviewed and approved via US Food and Drug Administration (FDA) single patient investigational new drug (SPIND) and local institutional review boards. Each participants provided written informed consent.
Inhaled phage therapy was given twice daily for inpatients (n = 4) or daily for outpatients (n = 5) for 7–10 days. Inpatients were provided jet nebulizers; outpatients used their own jet nebulizers (Table 2). Phages were delivered as mixtures (cocktails) of two or three phages (n = 6) or single-phage therapy (n = 3; Table 2), administered at a total dose of 1 × 1010 plaque-forming units (PFU). Phage nebulization was well tolerated without any adverse events. For the five outpatients, four reported subjective fevers and three reported fatigue on days 2 and 3 of phage therapy, without the need for additional treatment(s). The four inpatients did not have documented fever or report symptoms after phage therapy.
Inhaled phage therapy decreases sputum PsA
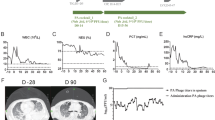
To test the hypothesis that phage therapy would reduce sputum PsA in each participant, spontaneously expectorated sputum collected before and after therapy was processed for PsA colony-forming unit (CFU) quantification. Sputum PsA CFU before therapy was greater than after therapy for all participants (Fig. 1a and Table 2). Across all participants, sputum PsA CFUs decreased after phage therapy from a median (first quartile, third quartile) 2.6 × 108 (5.0 × 107, 5.7 × 108) or mean 3.0 × 108 (±1.0 × 108 s.e.m.) CFU ml−1 before therapy, to median 2.6 × 104 (1.2 × 104, 3.1 × 106) or mean 7.7 × 106 (±6.9 × 106 s.e.m.) CFU ml−1 after completion of therapy (5–18 days (median 7, mean 8.4)), which is a 104 median or 102 mean difference, respectively (P = 0.006, two-way analysis of variance (ANOVA) with Dunnett’s multiple-comparisons test). A later time point (15–42 days (median 20, mean 22.7)) after completion of phage therapy found decreased PsA CFU median 7.8 × 105 (2.0 × 104, 2.7 × 107) or mean 1.1 × 107 (±7.5 × 106 s.e.m.) CFU ml−1, which is a 103 median, or 101 mean difference, respectively (P = 0.0112, two-way ANOVA with Dunnett’s multiple-comparisons test). Thus, sputum PsA decreased after phage therapy in each patient and across the cohort, regardless of individualized differences in the phage treatment strategies (Fig. 1a and Table 2).
a, Sputum analysis was performed before and after phage therapy. PsA CFU ml−1 from each patient’s sputum (n = 9) were counted in replicates of three and averaged. CFU ml−1 was measured before therapy (Pre) and at two time points after therapy (Post (14 days), 5–18 days (average 8.4, median 7), and Post (30 days), 15–42 days (average 22.7, median 20)); **P = 0.006 and *P = 0.0112; two-way ANOVA with Dunnett’s multiple-comparisons test. Two participants did not provide sputum samples after Post (14 days). b, Spirometry was performed before and after (days 21–35) phage therapy and reported as ppFEV1% best single measurement from at least three tests per the American Thoracic Society standards of acceptability and repeatability; **P = 0.004; Wilcoxon signed-rank two-tailed t-test. PT, patient.
Inhaled phage therapy improves lung function
In CF the most salient outcome is lung function (also known as spirometry), and therapies that decrease PsA may be associated with this clinical benefit11. Therefore, we compared lung function (percent predicted forced expiratory volume in 1 s (ppFEV1)) before versus after phage therapy (days 21–35). Results for each participant showed an increase in ppFEV1 after versus before therapy (Table 2 and Fig. 1b), and an analysis across the entire cohort showed an overall improvement in ppFEV1 from a median (first quartile, third quartile) 36 (23, 49), or mean 37 ± 5.5 s.e.m., before therapy to a median 42 (27, 67), or mean 45 ± 6.9 s.e.m., after therapy, which is median or mean ppFEV1 difference of 6 and 8, respectively (P = 0.004, Wilcoxon signed-rank two-tailed t-test). Analysis of participants with pre-therapy ppFEV1 >30 showed increased ppFEV1 after phage therapy (mean difference of 11 ± 3.2 s.e.m. (P = 0.06) Wilcoxon signed-rank two-tailed t-test), while participants with pre-therapy ppFEV1 <30 improved ppFEV1 to a lesser extent (mean difference 3.5 ± 0.96 s.e.m. (P = 0.1) Wilcoxon signed-rank two-tailed t-test). Therefore, phage therapy was associated with improved lung function, regardless of individualized differences in phage treatment strategies (Table 2 and Fig. 1b).
Phage therapy reduces PsA virulence
In addition to bacterial killing (Fig. 1a), we hypothesized that our personalized strategy to use specific phages to target AMR or virulence factors should select for surviving bacterial mutants with evolved phage resistance that results in decreased virulence. First, PsA resistance to phage therapy was investigated by studying purified PsA clones from participant sputum, and each isolate was tested for susceptibility to phage(s) used in inhaled therapy (Table 2). For each participant, results showed that post-therapy sputum PsA contained one or more bacterial clones with resistance to phage(s) used in therapy (Table 2), which showed that inhaled phage therapy exerted selection for PsA to evolve resistance to phage(s) used in treatment.
Next, we examined whether evolved phage resistance after therapy coincided with predicted trade-offs with antibiotic resistance or virulence. Phage OMKO1 selects for evolved phage resistance that coincides with increased sensitivity to various antibiotics9,10, which may be explained by this phage binding to Mex efflux pumps9,10. In the two patients who received OMKO1 (Table 2), post-therapy sputum isolates evaluated in clinical laboratories for antibiotic sensitivity showed PsA with increased susceptibility to imipenem (patient 1; Fig. 2a) and piperacillin–tazobactam, ceftazidime, amikacin, gentamicin and tobramycin (patient 3; Fig. 2b). Additional pre- and post-therapy PsA isolates were examined in our research laboratory for changes in susceptibility (minimum inhibitory concentration; MIC) to antibiotics. For patients who did not receive OMKO1, fewer PsA isolates showed changes to antibiotic susceptibility (Extended Data Fig. 1b,d–i). These results suggest that the predicted resensitivity to antibiotics occurred in the two patients who received OMKO1 (Fig. 2a,b). As expected, patients who did not receive OMKO1 did not show antibiotic resensitization in most isolates (Extended Data Fig. 1).
a,b, Antibiotic susceptibility results from clinical microbiology laboratory testing for sputum isolates taken before and after therapy from patient 1 (a) and patient 3 (b) are shown (R, resistant; S, susceptible; I, intermediate; X, not reported). c, Production of pyocyanin (µg ml−1) was measured from PsA isolates before therapy (N = 6) and after (N = 10) therapy from patients who received TIVP-H6 phage therapy (**P = 0.0047; Mann–Whitney test). d, Attachment of PsA to CF airway epithelial cells before (N = 6) and after (N = 6) therapy in duplicate from patient 2 (***P = 0.0005; Mann–Whitney test). e, LPS (ng CFU−1) quantification from PsA sputum isolates taken before therapy (N = 14) and after therapy (N = 25) from patients who received phage therapy with LPS-5 (P = 0.6963; Mann–Whitney test). f, Secreted elastase activity (U ml−1) from PsA sputum isolates taken before therapy (N = 8) and after therapy (N = 15) from patients who received phage therapy with LPS-5 (**P = 0.002; Mann–Whitney test). Data presented as mean values ± s.d.
The function of PsA TIVP includes twitching motility, secretion of pyocyanin (a virulence factor that causes cellular inflammation and oxidative stress12) and bacterial surface attachment13. Phage TIVP-H6 binds to PsA TIVP, and we investigated the hypothesis that TIVP-H6 phage therapy selects against TIVP function. Pyocyanin was measured from PsA isolates before and after therapy. Results showed that pre-therapy isolates from patients 1, 3, 4, 5, 6, 8 and 9 showed minimal to no pyocyanin production, while isolates from patients 2 and 7 secreted pyocyanin at detectable levels before therapy (Fig. 2c and Extended Data Fig. 2a–i). However, only isolates from patient 7 showed a statistically significant reduction of pyocyanin production (Extended Data Fig. 2b,g). Finally, the potential for phage TIVP-H6 to select for reduced bacterial adherence was studied in vitro using CF airway epithelial (CFBE41o-) cells grown at an air–liquid interface (ALI) using isolates from patient 2 (the only patient to receive single-phage TIVP-H6 therapy). Compared with pre-therapy PsA, post-therapy isolates showed significantly decreased adherence to CF airway epithelium (Fig. 2d). Together these results show that TIVP-H6 phage therapy selects for a trade-off in post-therapy PsA isolates for reduced virulence via a reduction in pyocyanin (Fig. 2c) and/or decreased TIVP-mediated adherence to CF airway epithelium (Fig. 2d).
PsA LPS contributes to virulence by reducing antibiotic permeability, contributing to biofilm production and activating human immunity14. As phage LPS-5 binds to PsA LPS, LPS per cell (Fig. 2e and Extended Data Fig. 3) and elastase production (Fig. 2f and Extended Data Fig. 4) from pre- and post-therapy PsA isolates were measured. Across all eight patients who received phage LPS-5, there was no statistically significant change in LPS content before versus after therapy (Fig. 2e). A similar analysis for elastase activity showed a significant decrease in elastase activity across all patients who received LPS-5 and produced elastase before therapy (Fig. 2f); patients 1 and 6 (Extended Data Fig. 4a,f) had decreased elastase activity after phage therapy. While evidence for phage LPS-5-selected trade-offs was mixed, these results show that LPS-5 phage therapy selects for a trade-off in post-therapy PsA isolates for reduced elastase production (Fig. 2f).
Phage therapy does not change bacterial species diversity
Because antibiotics have secondary effects on nontarget bacteria, a similar possibility can be examined for administered phage(s). Specifically, reducing PsA in the lung may open niche(s) for other species, which were previously occupied by PsA. This is particularly important in pwCF where several pathogens can contribute to lung disease15,16. Thus, potential off-target effects were investigated in longitudinal sputum samples obtained before and after therapy. First, results from clinical laboratory sputum cultures showed no change in CF pathogens before or after therapy. Second, metagenomic analysis of longitudinal changes in alpha-diversity (species richness)17 present in pre- and post-therapy sputum from patients 2–9 (Extended Data Fig. 5a) showed no significant change(s) in: (1) Chao1 diversity index (Extended Data Fig. 5b), which is a richness metric that especially considers potentially missing (‘rare’) species18; (2) Shannon diversity index (Extended Data Fig. 5c), which accounts for the abundance and evenness of species across time19; and (3) Simpson diversity index (Extended Data Fig. 5d), which accounts for the presence of specific dominant species, aside from overall abundance20. Thus, these results showed that phage therapy did not significantly alter the composition of bacterial communities in these patients.
Pre- and post-therapy PsA strains are closely related
While the above results show that phage therapy selects for phage resistance commensurate with trade-offs in antibiotic resistance or bacterial virulence at a phenotypic level, we hypothesized that we could detect similar signal(s) at the genotypic level. Whole-genome sequencing and variant analysis of the pre- and post-therapy isolates showed that post-therapy isolates had numerous mutations not found in pre-therapy isolates (Fig. 3 and Extended Data Fig. 6). For example, patient 1 post-therapy isolate showed nonsynonymous mutations in the sequences encoding MexB (R586S), a component of a complex involved in efflux of several antibiotics; MigA (P169T), an enzyme involved in LPS biosynthesis; and multiple proteins involved in TIVP assembly, one of which (PilE P81R) was shared by all post-therapy isolates examined (Fig. 3a). Patient 4 had nonsynonymous mutations in two LPS biosynthesis genes, wzm and PA5455 (Fig. 3B) in four out of five post-therapy isolates, which might account for the lower LPS content observed in these isolates (Extended Data Fig. 3). Several mutations involved in TIVP biosynthesis were observed in all post-therapy isolates from patient 7, which correlate with the significantly lower pyocyanin measured from these isolates (Extended Data Fig. 2), although only one isolate showed a mutation in a LPS biosynthesis gene (Fig. 3c). Many mutations in isolates from these patients, as well as the other six patients (Extended Data Fig. 6), were found in genes related to the synthesis, modification or regulation of the receptors used by the phage(s) these patients received. However, nonsynonymous mutations were also found across isolate genomes at loci that do not obviously correspond with the phage treatments, which suggests that the PsA populations from which these isolates were drawn are genetically diverse and under in vivo selection pressures other than phage(s). A larger isolate set and more tailored laboratory experiments are needed to directly associate the mutations highlighted above with the measured phenotypes of interest.
a–c, Circular plots showing the distribution of variants in the post-treatment PsA isolate genomes of patient 1 (a), patient 4 (b) and patient 7 (c). Concentric circles represent single isolate genomes. Gray lines represent nonconservative variants that appear in coding sequences in one or more post-treatment isolates but are absent from all pre-treatment isolates. Orange lines represent nonconservative variants (nonsynonymous polymorphisms and frameshifts) coincident with genes expected to be under selection by the phage used in treatment. Labeled, colored arrows indicate the positions and functional categories of these genes. Vertical black lines represent the genome start in the PAO1 reference against which variants were called.
Discussion
Here, we summarize our experience with adjunctive inhaled phage therapy to treat MDR or PDR PsA in a cohort of nine pwCF. Importantly, we found that personalized phage therapy was associated with decreased sputum PsA and improved lung function, which may reflect the effects of phage-driven evolved trade-offs.
Inhaled phage therapy was first reported in 1959 (ref. 21), and so far, there have been few reports of inhaled phage therapy for PsA in smaller cohorts of pwCF22,23. For our consecutive cohort of nine CF adults, nebulized phage therapy was chosen, in favor of IV delivery, to directly target the site of infection and to limit systemic phage exposure, with the added benefit that most individuals with CF have experience with inhaled medications. Compared with IV, nebulization should decrease adaptive immune responses that neutralize phage(s), which could limit future treatments with the same phage(s)24. Sputum analysis showed that (1) nebulized phage(s) decreased PsA CFU ml−1 (Fig. 1a), irrespective of nebulizer used, and (2) post-phage therapy PsA showed evidence of phage resistance to treatment phages (Table 2), which suggests that inhaled phages affected PsA in vivo, as predicted from in vitro studies9,10.
The ability for inhaled phage therapy to decrease sputum bacteria, especially MDR and PDR pathogens, has the potential to translate into promising clinical applications. Our personalized approach reduced CFU despite each patient concurrently receiving, or recently completing, antibiotics (Tables 1 and 2). Nevertheless, decreased CFU does not always translate to clinical improvement in pwCF25. Therefore, it is notable that this cohort had improved lung function (Fig. 1b), which suggests an additional contribution of our strategy to carefully choose administered phage(s) by anticipating their selection exerted on PsA, such that evolved phage resistance would coincide with favorable trade-offs in virulence9,10. In this cohort, some post-therapy PsA isolates were resistant to phage OMKO1 (Table 2), which resulted in increased antibiotic sensitivity (Fig. 2a,b). This increased antibiotic sensitivity allowed previously unavailable antibiotics to be considered for future use, which has meaningful clinical relevance. Decreased virulence traits (pyocyanin secretion and cell adherence) in post-therapy PsA isolates (Fig. 2c,d) was observed, which may be attributed to phage TIVP-H6 targeting pili. However, despite evidence that PsA sputum isolates treated with phage LPS-5 became resistant to this phage (Table 2), there was mixed evidence for changes in LPS content and elastase production after therapy (Fig. 2e,f and Extended Data Figs. 2 and 3). It is possible that: (1) the observed post-therapy resistance to phage LPS-5 was via a mechanism independent of the phage’s putative receptor, LPS; (2) phages killed all sensitive PsA, opening niche space for pre-therapy minority variants of intrinsically phage-resistant PsA to fill in the space; or (3) techniques used to measure LPS content were not optimal to measure a trade-off between phage resistance and virulence for phage LPS-5. LPS is usually crucial for bacterial virulence, and it may be naive to expect that our in vivo evolved phage resistance results would match our predictions from in vitro evolution of phage resistance. This highlights the necessity for further translational studies to better inform clinical decisions for phage therapy.
While PsA is currently the most prevalent pathogen observed in CF adult sputum, polymicrobial infections (for example, S. aureus) are common and evident by studying CF lung microbiome utilizing culture-independent techniques that detect multiple pathogens26,27. Given the potential for phage therapy to decrease PsA while increasing the opportunity for competitively inferior pathogens to thrive, an important aspect of our study was to investigate how phage therapy might alter the CF lung microbiome, which was examined using longitudinal sputum analysis of metagenomics data. We found no evidence that phage therapy altered species compositions in the CF lung microbiome (Extended Data Fig. 4). These observations were consistent with clinical laboratory analyses of post-therapy samples, which also showed no changes in the number of sputum pathogens.
Many aspects of our study addressed possible safety concerns for phage therapy, which does not currently have US FDA approval for general use. This study used environmentally sourced phages that were not genetically manipulated. Safety was addressed by restricting administered phages to those with strictly lytic replication cycles, thus avoiding temperate phages capable of host lysogeny. Genomes of each phage were sequenced before therapy and screened for presence of lysogeny genes to further ensure phages were strictly lytic. Purification techniques were used to minimize endotoxin levels in phage therapy doses to meet US FDA requirements (<5 endotoxin units kg−1 h−1). Because pre-therapy sputum PsA was on average ~3 × 108 CFU ml−1, a phage concentration was chosen to exceed sputum bacterial density by at least tenfold. Although this method was successful in the current study, a unique benefit of lytic phage therapy is phage self-amplification when viruses productively infect and kill bacterial host cells, which suggests that lower concentrations could be considered with future inhaled phage therapy approaches.
Phage cocktails are often assumed to be the superior phage therapy approach, although this has not been studied in humans. While phage cocktails can increase the spectrum of activity, this popular approach may have limitations: (1) different phages co-infecting the same bacterial cell can suffer reduced reproductive fitness due to phage competition for limited intracellular resources28,29; (2) cocktails can select for unanticipated mutations compared with single phages30,31, which is concerning for unexpected changes that increase bacterial virulence after phage therapy; (3) cocktails may stimulate greater adaptive immune responses compared with single phages32; and (4) cocktails have the potential to select for cross-resistance in bacteria, which more quickly depletes libraries of potentially useful phages4. We observed microbiological (Table 2) and associated clinical improvement (Fig. 1) with single phages TIVP-H6 and LPS-5. This approach is attractive considering the usual necessity to treat PsA, or other bacteria, for prolonged durations because of the difficulties to eradicate bacteria within CF bronchiectatic lungs. In addition, a sequential, single-phage therapy approach could be deployed such that alternating phages would target different bacterial surface receptors to drive specific trade-offs in PsA virulence after therapy. This serial approach would leverage the same phage discovery platform that we used to discover phages OMKO1, LPS-5 and TIVP-H6 to search for PsA phages that target additional virulence factors (for example, secretion systems, flagella and siderophores). Sequentially nebulized monophage therapy may also limit adaptive immune responses to phage, although this requires rigorous study. While we favor the above approaches, some naturally occurring phages are capable of broadly killing genotypes of host bacteria, including PsA33, and a general goal of phage biotechnology is often to identify, or engineer, viruses with broad host range34. Clinical trials using single or cocktail-based approaches of inhaled phage therapy in pwCF will provide useful data to address these issues. An investigator-initiated clinical trial CYstic fibrosis bacterioPHage at Yale (CYPHY; NCT04684641) showed no safety concerns, but although there was evidence for decreased PsA after phage therapy, the trial did not meet its prespecified microbiologic efficacy endpoint. The Armata Pharmaceutical (NCT04596319) trial included dose escalation and multiple doses of a nebulized phage cocktail. While safety data are available without evidence for significant safety concerns, no microbiologic endpoint data are available. Presently, there are no data available for the BiomX (NCT05010577) multicenter inhaled phage cocktail trial.
Limitations
Because of the nature of compassionate phage therapy, there was no control group for comparison. PwCF had differences in CF genotype, clinical phenotype and CF-specific treatments (for example, access to CF transmembrane conductance regulator (CFTR) modulators). Across the cohort, there were differences in the number of phages used (for example, single versus cocktail), duration of phage therapy (7–10 days) and frequency of treatment (inpatient and outpatient). In addition, the nebulizer used was not standardized. Each patient used a nebulizer provided while inpatient or used their personal one at home. However, we confirmed that different nonmesh nebulizers had no impact on phage viability35, which suggests that nebulizer choice had no adverse effects on phage particles from two virus families of differing morphology (myoviruses and podoviruses), which include phages OMKO1, TIVP-H6 and LPS-5.
While each patient had MDR or PDR sputum PsA that had not responded to prior antibiotics, another limitation of this study was unknown variability among strains (lineages) infecting each participant. In these compassionate cases, a small number of PsA isolates from sputum were randomly selected to be representative of the PsA sputum population. While the isolates probably represent the most abundant lineages in the lung community, it is unlikely that they reflect all lineages present in the lungs of pwCF, and we cannot be sure that all post-therapy isolates are direct descendants of the sampled pre-therapy isolates despite supportive evidence from the reported genome sequence analyses; this is a focus of ongoing studies. Additional compassionate cases and clinical trials are using protocols to improve sputum standardization and processing (for example, induced sputum), increase sampling of PsA isolates and measuring treatment phages in sputum to begin to assess phage amplification, pharmacokinetics and pharmacodynamics.
In summary, this study evaluated the impact of adjunctive nebulized phage therapy for MDR and PDR PsA on a cohort of compassionate cases in pwCF. While the number of patients is limited, phage nebulization was well tolerated, and results showed that a personalized phage therapy approach was associated with reduced PsA burden and improved lung function. In addition to bacterial killing, this phage therapy approach was associated with trade-offs in PsA that resulted in decreased antibiotic resistance and reduced virulence in post-therapy isolates. Thus, in a chronic lung disease such as CF, inhaled phage therapy may offer an effective therapeutic option. Further studies of this new therapeutic are currently underway in clinical trials, and our personalized phage therapy approach will similarly require larger clinical trials.
Methods
Identification of patients suitable for phage therapy
For this cohort, we received unsolicited requests from the patient or their physician for assistance to treat sputum MDR or PDR PsA refractory to standard, approved CF therapies. For each case, we reviewed patient clinical history, antibiotic treatments and immunologic status with a multidisciplinary team that includes phage biologists, pulmonologists with expertise in CF, and infectious disease physicians. Spontaneously expectorated sputum was sent to what is now Yale’s Center for Phage Biology and Therapy laboratory for microbiological characterization. PsA was isolated from sputum, and susceptibility to three phages OMKO1, LPS-5 and TIVP-H6 was determined (see ‘Sputum processing’ and ‘Phage sensitivity testing and preparation’). Susceptibility to phages was measured by titering (enumerating) phages on each clinical PsA isolate and comparing this with the phage titer on permissive PsA laboratory strains PAO1 or PA14 (ref. 36).
Phage therapy
Each phage therapy protocol was submitted as an individual US FDA emergency investigational drug or SPIND application. After suitable phage(s) were identified, a treatment protocol was reviewed with each participant’s CF physician. This protocol, phage manufacturing and the informed consent form were reviewed and approved by FDA and the local institutional review board for each institution where phage therapy took place (Rutgers University, Texas Tech University and Yale University). Each participant provided written informed consent. The Yale Center for Phage Biology and Therapy prepared and provided phages for all patients at no cost to patients. Phage therapy was delivered by nebulization using a nonmesh jet nebulizer (Table 2). Nebulization was chosen because (1) several CF therapies are nebulized and well tolerated by pwCF, (2) nebulization delivers phage(s) directly to the site of infection and (3) nebulization may limit systemic toxicity. In a separate set of experiments, it was confirmed that different nonmesh nebulizers had no impact on phage viability using a Next Generation Cascade Impactor (MSP)35. All patients received a test dose of nebulized phage in either an inpatient or outpatient clinical setting where they were monitored for at least 30 min after nebulization to ensure that inhalation was well tolerated. For inpatients (n = 4), phage therapy was continued twice daily, while outpatients (n = 5) received phage therapy once daily; three participants transitioned from inpatient to outpatient. The total duration of phage therapy was 7–10 days. Participants were treated at their local institution (n = 4) or traveled to Yale University/Yale New Haven Hospital (n = 5) for treatment. Outpatients with initial ppFEV1 <30% had clinical follow-up within 72 h of treatment. After phage therapy, participants returned to their CF physician(s) for care, and we remained in contact to assess potential adverse events for a total of 30 days. Patients and their care teams were asked to report new symptoms and changes in CF therapies. Because each case was a SPIND, standardized CF symptom diaries or questionnaires were not used and there was no safety monitoring board. Sputum samples were collected at different intervals over the subsequent 28 days (see ‘Sputum processing’), and spirometry was performed after phage therapy (21–30 days) at each patient’s CF center (see ‘Spirometry’).
Spirometry
Forced expiratory volume in 1 s (FEV1) was obtained from each patient by spirometry per each CF clinic’s protocol. All clinics used American Thoracic Society standards of acceptability and repeatability; reported ppFEV1 reflects patients best effort from at least three attempts per American Thoracic Society guidelines. Pre-phage therapy FEV1 is consistent with each patient’s best FEV1 over the preceding 6 months, and no significant difference was found compared with pre-phage therapy values.
Sputum processing
All sputum samples were spontaneously expectorated. For standard microbiology analysis, samples were sent to each institution’s clinical microbiology laboratory. All of these institutions are CF centers accredited by the US CF Foundation, which provides guidelines for microbiology laboratories. For additional analysis, at Yale’s Center for Phage Biology and Therapy laboratory, sputum was weighed and combined with 1 ml phosphate-buffered saline (PBS) supplemented with 10 mM magnesium sulfate (PBS-M) per gram and manually homogenized with a syringe and 16-gauge needle37. Samples were used for bacterial isolation (see ‘Bacterial isolation, culture and quantification’) and immediately stored in DNA/RNA Shield (Zymo Research) per the manufacturer’s protocol before metagenomic sequencing (see ‘Metagenomic sequencing’).
Bacterial isolation, culture and quantification
Bacteria were isolated by direct plating dilutions of sputum homogenate on PsA isolation agar (Millipore) and used to determine CFU ml−1. Phenotypically distinct strains were isolated for further characterization (n = 1–5 per sputum sample). For subsequent experiments, all bacteria were grown for 16 h at 37 °C from glycerol stock in lysogeny broth (LB; Sigma-Aldrich) plates (1.5% agar). Individual colonies were used to initiate cultures grown overnight in LB with shaking (200 rpm) and incubation at 37 °C. Isolates were preserved at −80 °C in glycerol for future studies.
Metagenomic sequencing
DNA from sputum was extracted and metagenomic sequencing was performed on the Illumina HiSeq4000 platform (Illumina). Raw reads were preprocessed for quality control using bowtie38. Contigs were assembled with metaSPAdes39, and metaBAT240 was used to assign contigs to the most common bacterial genera found in CF lungs. Contigs were assessed for quality, quantity, completeness and coverage with prokka41, quast42 and checkM43. Comparisons are made between sputum samples collected before, during and after phage therapy.
Phage sensitivity testing and preparation
Phage sensitivity testing for OMKO1, TIVP-H6 and LPS-5 was completed by preparing a homogenate of sputum that was plated on selective medium to isolate PsA. Up to five PsA morphotypes were isolated, grown and tested for phage sensitivity with an efficiency of plating (EOP) relative to phage amplification host (for example, laboratory strains PAO1 or PA14) 0.1 floor for sensitivity (that is, ≥0.1 EOP was considered sensitive). Initial EOP testing was completed for OMKO1, TIVP-H6 and LPS-5, which were found to have an acceptable EOP for sputum PsA for this patient cohort. Phage selection for cocktails met EOP criteria for each phage, and single phages were selected on the basis of best EOP ≥0.1.
Bacterial killing, or time-kill, assays were not required by the FDA and were not completed.
Phage lysates were prepared by growing PAO1 or PA14 to exponential phase in non-animal-origin tryptic soy broth (Sigma-Aldrich). Phage(s) were then added to a fresh culture of bacteria at a multiplicity of infection (ratio of phage particles to bacterial cells) of ~0.01 and incubated for 6 h at 37 °C with shaking (200 rpm). Amplifications were centrifuged and filtered (pore size 0.22 µm). Lysates were then concentrated with Centricon small pore concentrators (100 kDa molecular weight cutoff) and dialyzed in 1,000× volume PBS-M. Endotoxin concentration was then determined with Hyglos EndoNext kits (bioMerieux). Per FDA requirements, USP 71 testing was completed by a third-party laboratory (Accugen Laboratories) on all phage preparations that were used for phage therapy. Purified phages were diluted in 3 ml PBS-M for nebulization to a final concentration of 1.0 × 1010 PFU ml−1 for individual phages. If phages were used in a cocktail (two or more phages), the total phage concentration remained ≤1.0 × 1010 PFU (for example, two phages ~5.0 × 109 PFU; three phages ~3.33 × 109 PFU). If shipping was required, phages were shipped on ice and refrigerated before use.
Antibiotic resistance measurements
Sputum samples were sent to each institution’s CF Foundation-accredited clinical laboratory for standard microbiology analysis that included antibiotic susceptibility testing. Limitations include the following: (1) only one or two PsA isolate results are reported for each sputum sample, (2) sensitivity or resistance is reported on the basis of Clinical Laboratory Improvement Amendment standards and (3) MICs are not provided. Therefore, we tested MICs for ten antibiotics via ETEST strips (bioMerieux) per the manufacturer’s guidelines on additional PsA isolates. In brief, an overnight culture of bacteria was diluted to a McFarland standard of 0.5 and then spread on Mueller–Hinton agar (2 g beef extract, 17.5 g casein hydrolysate, 1.5 g starch and 17 g agar per liter) using a sterile L-shaped cell spreader. The bacterial lawn was allowed to dry, and an ETEST strip was placed on top of the lawn. After overnight incubation at 37 °C, the plate was scored by recording the lowest concentration of antibiotic that inhibited growth of the bacterial lawn.
Pyocyanin measurements
Pre- and post-phage therapy PsA isolates were grown in LB for 48 h at 37 °C with shaking (200 rpm). Cultures were serially diluted and plated on LB for enumeration. Pyocyanin was extracted from the remaining culture as described previously44. In brief, bacterial supernatant was mixed with chloroform and allowed to separate. The blue layer was then mixed with 0.2 M hydrochloric acid and allowed to separate again. The pink layer was transferred to a 96-well plate, and an absorbance measurement was taken at 520 nm. The pyocyanin concentration was calculated and normalized to total PsA CFU.
Bacteria attachment assay
Immortalized CF ΔF508/ΔF508 bronchial epithelial (CFBE41o-) cells (generously provided by Dr. J. Bomberger) were cultured at 37 °C and 5% CO2 in minimal essential medium (MEM; Gibco) with 10% fetal bovine serum (Gemini Bio-Products), supplemented with 2 mM l-glutamine, 5 U ml−1 penicillin, 5 μg ml−1 streptomycin (Sigma-Aldrich) and 0.5 μg ml−1 plasmocin (InvivoGen)45. For the attachment assay, cells were seeded at confluence on transwell Falcon Permeable Supports (Corning) and differentiated at an ALI for 7 days before use46. PsA cultures were grown 12–24 h in tryptic soy broth, pelleted via centrifugation, washed with MEM and diluted to an OD600 of 0.1. Normalized bacteria were then added to CFBE41o- cells at a multiplicity of infection of 5–10 and co-cultured at 37 °C for 1 h, as previously described46. The apical medium was removed, and ALI airway epithelium was washed thoroughly with MEM. Attached bacteria were dispersed with MEM containing 0.1% Triton X-100 solution, serially diluted and spread on LB plates for enumeration. Results are expressed as CFU percentage of the original PsA inoculum.
LPS measurements
Pre- and post-phage therapy PsA isolates were grown on LB plates for 24 h. All growth was scraped into 1 ml of PBS and serially diluted to enumerate bacteria. The remaining bacteria were lysed via sonication and LPS was extracted from bacteria using an LPS extraction kit (Abcam ab239718). LPS was quantified using a carbohydrate quantification assay (Abcam ab155891). LPS was extracted, and the total carbohydrate content (g CFU−1) was quantified following the manufacturer’s instructions. The total carbohydrate content (g CFU−1) was normalized by cell density47.
Elastase measurements
Pre- and post-phage therapy PsA isolates were grown in LB for 48 h at 37 °C with shaking (200 rpm). Cultures were serially diluted and plated on LB for enumeration. Bacterial supernatant was filtered to remove cells, and elastase activity was measured with a fluorometric assay following the manufacturer’s instructions (Molecular Probes E12056).
Isolate sequencing and comparative genomics
Libraries were prepared from sputum-derived PsA isolates using the Nextera XT DNA Library Prep Kit and sequenced on an Illumina NextSeq platform. Paired-end reads were filtered and trimmed with fastp v0.23.2 using default settings, and clean reads were used to call variants against the Pseudomonas aeruginosa PAO1 reference genome (RefSeq NC_002516.2) with breseq v0.38.1. Variant calls, reference annotations and the reference genome sequence were imported into R v4.2.0, and variant consequences were determined for coding sequences using functions from the VariantAnnotation (v1.44.1) and GenomicFeatures (v1.50.4) packages. Variant data were processed and plotted using functions from the tidyverse suite of packages (v2.0.0).
Statistics
GraphPad Prism (GraphPad Software) was used for all statistical analysis. Two-way ANOVA with Dunnett’s multiple-comparisons test and Wilcoxon signed-rank t-test for paired samples was used for the statistical analysis of bacterial density and spirometry before and after therapy, respectively. Welch’s t-test, for normally distributed data, or Mann–Whitney test (both two-sided) was used for statistical analysis of antibiotic sensitivity, pyocyanin production, bacterial attachment, LPS content and elastase activity in isolates before and after therapy. Statistical analysis of longitudinal alpha-diversity was done with ANOVA with Tukey correction for multiple comparisons. Except for spirometry, all N values indicate the number of biological replicates.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Sequencing data have been made available at the National Center for Biotechnology Information Sequence Archive (accession no. PRJNA1182815). Additional data (for example, bacterial density, antibiotic sensitivity, pyocyanin production, bacterial attachment, LPS content and elastase activity) are available via Dryad at https://doi.org/10.5061/dryad.pc866t1t0 (ref. 48).
References
O’Neill, J. in Review on Antimicrobial Resistance 1–14 (HM Government & Wellcome Trust, 2014).
De Oliveira, D. M. P. et al. Antimicrobial resistance in ESKAPE pathogens. Clin. Microbiol. Rev. 33, e00181-19 (2020).
Durda-Masny, M. et al. The determinants of survival among adults with cystic fibrosis—a cohort study. J. Physiol. Anthropol. 40, 19 (2021).
Kortright, K. E., Chan, B. K., Koff, J. L. & Turner, P. E. Phage therapy: a renewed approach to combat antibiotic-resistant bacteria. Cell Host Microbe 25, 219–232 (2019).
Pirnay, J. P. et al. Personalized bacteriophage therapy outcomes for 100 consecutive cases: a multicentre, multinational, retrospective observational study. Nat. Microbiol. 9, 1434–1453 (2024).
Roach, D. R. & Donovan, D. M. Antimicrobial bacteriophage-derived proteins and therapeutic applications. Bacteriophage 5, e1062590 (2015).
Torres-Barceló, C., Turner, P. E. & Buckling, A. Mitigation of evolved bacterial resistance to phage therapy. Curr. Opin. Virol. 53, 101201 (2022).
Rodriguez-Gonzalez, R. A., Leung, C. Y., Chan, B. K., Turner, P. E. & Weitz, J. S. Quantitative models of phage–antibiotic combination therapy. mSystems 5, e00756-19 (2020).
Chan, B. K. et al. Phage selection restores antibiotic sensitivity in MDR Pseudomonas aeruginosa. Sci. Rep. 6, 26717 (2016).
Chan, B. K. et al. Phage treatment of an aortic graft infected with Pseudomonas aeruginosa. Evol. Med. Public Health 2018, 60–66 (2018).
Regelmann, W. E., Elliott, G. R., Warwick, W. J. & Clawson, C. C. Reduction of sputum Pseudomonas aeruginosa density by antibiotics improves lung function in cystic fibrosis more than do bronchodilators and chest physiotherapy alone. Am. Rev. Respir. Dis. 141, 914–921 (1990).
Muller, M. Pyocyanin induces oxidative stress in human endothelial cells and modulates the glutathione redox cycle. Free Radic. Biol. Med. 33, 1527–1533 (2002).
Mattick, J. S. Type IV pili and twitching motility. Annu. Rev. Microbiol. 56, 289–314 (2002).
Balibar, C. J. & Grabowicz, M. Mutant alleles of lptD increase the permeability of Pseudomonas aeruginosa and define determinants of intrinsic resistance to antibiotics. Antimicrob. Agents Chemother. 60, 845–854 (2016).
2020 Annual Data Report (Cystic Fibrosis Foundation Patient Registry, 2020).
Chan, B. K., Stanley, G., Modak, M., Koff, J. L. & Turner, P. E. Bacteriophage therapy for infections in CF. Pediatr. Pulmonol. 56, S4–S9 (2021).
Whittaker, R. H. Vegetation of the Siskiyou Mountains, Oregon and California. Ecol. Monogr. 30, 279–338 (1960).
Chao, A. Nonparametric estimation of the number of classes in a population. Scand. J. Stat. 11, 265–270 (1984).
Shannon, C. E. A mathematical theory of communication. Bell Syst. Tech. J. 27, 379–423 (1948).
Simpson, E. H. Measurement of diversity. Nature 163, 688 (1949).
Delacoste, P. Considerations on the treatment of common respiratory diseases by means of bacteriophages. Rev. Med. Suisse Romande 79, 552–563 (1959).
Kutateladze, M. & Adamia, R. Phage therapy experience at the Eliava Institute. Med. Mal. Infect. 38, 426–430 (2008).
Kvachadze, L. et al. Evaluation of lytic activity of staphylococcal bacteriophage Sb-1 against freshly isolated clinical pathogens. Microb. Biotechnol. 4, 643–650 (2011).
Dedrick, R. M. et al. Potent antibody-mediated neutralization limits bacteriophage treatment of a pulmonary Mycobacterium abscessus infection. Nat. Med. 27, 1357–1361 (2021).
Lam, J. C. et al. Reduction in Pseudomonas aeruginosa sputum density during a cystic fibrosis pulmonary exacerbation does not predict clinical response. BMC Infect. Dis. 15, 145 (2015).
Muhlebach, M. S. et al. Initial acquisition and succession of the cystic fibrosis lung microbiome is associated with disease progression in infants and preschool children. PLoS Pathog. 14, e1006798 (2018).
Quinn, R. A. et al. Biogeochemical forces shape the composition and physiology of polymicrobial communities in the cystic fibrosis lung. mBio 5, e00956–13 (2014).
Turner, P. E. & Chao, L. Prisoner’s dilemma in an RNA virus. Nature 398, 441–443 (1999).
Turner, P. E. & Chao, L. Escape from prisoner's dilemma in RNA phage phi6. Am. Nat. 161, 497–505 (2003).
Wright, R. C. T. et al. Resistance evolution against phage combinations depends on the timing and order of exposure. mBio 10, e01652–19 (2019).
Wright, R. C. T. et al. Cross-resistance is modular in bacteria–phage interactions. PLoS Biol. 16, e2006057 (2018).
Dedrick, R. M. et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat. Med. 25, 730–733 (2019).
Bono, L. M. et al. Advancing phage therapy through the lens of virus host-breadth and emergence potential. Adv. Virus Res. 111, 63–110 (2021).
Kortright, K. E., Chan, B. K. & Turner, P. E. High-throughput discovery of phage receptors using transposon insertion sequencing of bacteria. Proc. Natl Acad. Sci. USA 117, 18670–18679 (2020).
Thompson, D. L. et al. Particle size distribution of viable nebulized bacteriophage for the treatment of multi-drug resistant Pseudomonas aeruginosa. Respir. Med. Res. 86, 101133 (2024).
Clokie, M. R. J. & Kropinski, A. M. (eds) Bacteriophages: Methods and Protocols, Vol. 1 (Humana, 2009).
Khanal, S. et al. SPLUNC1: a novel marker of cystic fibrosis exacerbations. Eur. Respir. J. 58, 2000507 (2021).
Langmead, B. et al. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25 (2009).
Nurk, S. et al. metaSPAdes: a new versatile metagenomic assembler. Genome Res. 27, 824–834 (2017).
Kang, D. D. et al. MetaBAT 2: an adaptive binning algorithm for robust and efficient genome reconstruction from metagenome assemblies. PeerJ 7, e7359 (2019).
Seemann, T. Prokka: rapid prokaryotic genome annotation. Bioinformatics 30, 2068–2069 (2014).
Gurevich, A. et al. QUAST: quality assessment tool for genome assemblies. Bioinformatics 29, 1072–1075 (2013).
Parks, D. H. et al. CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 25, 1043–1055 (2015).
Frank, L. H. & Demoss, R. D. On the biosynthesis of pyocyanine. J. Bacteriol. 77, 776–782 (1959).
Cozens, A. L. et al. CFTR expression and chloride secretion in polarized immortal human bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 10, 38–47 (1994).
Melvin, J. A. et al. Pseudomonas aeruginosa contact-dependent growth inhibition plays dual role in host–pathogen interactions. mSphere 2, e00336-17 (2017).
Kortright, K. E. et al. Selection for phage resistance reduces virulence of Shigella flexneri. Appl. Environ. Microbiol. 88, e0151421 (2022).
Chan, B. et al. Personalized inhaled bacteriophage therapy for treatment of multidrug-resistant Pseudomonas aeruginosa in cystic fibrosis. Dryad https://doi.org/10.5061/dryad.pc866t1t0 (2025).
Acknowledgements
We thank J. Bomberger (University of Pittsburgh) for assistance with the attachment assay; P. Caudill (Yale University) for kindly ensuring phage manufacturing compliance; A. Hummel (Yale University) and the Yale Center for Clinical Investigation for assistance with the FDA IND process; M. Baranoski at the Yale University Institutional Review Board; and C. Fiore (FDA) for invaluable advice and assistance in these cases. Funding was provided by the Cystic Fibrosis Foundation (CFF) Clinical Research Scholars Program Award (KOFF19Y5) to J.L.K.; CFF 3rd/4th/5th Year Fellowships (STANLE20D0) and NIH Loan Repayment Program (STANLEY OYPU4195) to G.L.S.; and CFF Research Award (TURNER19P0) to B.K.C. and P.E.T. Furthermore, P.E.T. acknowledges support for genetic sequencing from Illumina, Inc. The funders had no role in the design and execution of the study.
Author information
Authors and Affiliations
Contributions
B.K.C., J.L.K. and P.E.T. developed the concept and designed the experiments. A.C.V., C.N.G., B.I.K., B.K.C., G.L.S., G.R., I.M.O., J.L.K., K.E.K., M.M., P.E.T., Q.-A.M., S.W., V.S. and Y.S. performed and contributed to experiments, and analyzed experimental data. A.K., B.I.K., B.S.Q., C.R.A., C.C.T., C.N.G., C.J.B., G.L.S., J.L.K., J.M.R., R.J., J.S., J.S.T., M.M., N.C., S.K.J. and Z.M.H. analyzed clinical data. A.C.V., B.K.C., G.L.S., J.L.K., K.E.K. and P.E.T. wrote and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The following authors declare no competing interests: G.L.S., A.C.V., M.M., I.M.O., Y.S., S.W., C.N.G., B.I.K., G.R., Z.M.H., C.J.B., J.S., J.S.T., C.R.A., N.C., S.K.J., R.J., A.K., B.S.Q., J.M.R., C.C.T., Q.-A.M., V.S. and J.L.K. The following authors declare a competing interest: B.K.C., K.E.K. and P.E.T. for US Provisional Patent Application No. 62/844,515, filed 7 May 2019, and US Provisional Patent Application No. 63/017,369, filed 29 April 2020, for bacteriophages (OMKO1, LPS-5 and TIVP-H6) that have been studied in this project.
Peer review
Peer review information
Nature Medicine thanks Jean-Paul Pirnay, Hiran Selvadurai and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Alison Farrell and Joao Monteiro, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 PsA Antibiotic Sensitivity and Susceptibility.
MIC and antibiotic susceptibility results from sputum isolates taken pre- and post-therapy for (A) patient 1 (Npre=1, Npost=3, p = NA), (B) patient 2 (Npre=4, Npost=5, p=0.6905, 0.2063, 0.4444, 0.4444, >0.9999, 0.7222, 0.0476, 0.7619, 0.3968, 0.7857), (C) patient 3 (Npre=1, Npost=1, p=NA), (D) patient 4 (Npre=1, Npost=4, p=NA), (E) patient 5(Npre=2, Npost=4, p = 0.733, >0.9999, >0.9999, >0.9999,> 0.9999, >0.9999, >0.9999, 0.4667, 0.6667, 0.8667), (F) patient 6 (Npre=3, Npost=2, p=0.6000, 0.2000, 0.9000, 0.8000, 0.5000, 0.6000, 0.1000, 0.1000, 0.4000, 0.1000), (G) patient 7 (Npre=2, Npost=5, p=>0.9999, >0.9999, >0.9999, >0.9999, >0.9999, >0.9999, 0.1429, 0.1429, 0.3333, 0.0476), (H) patient 8 (Npre=3, Npost=1, p=NA), and (I) patient 9 (Npre=1, Npost=4, p=NA), to aztreonam (ATM), piperacillin/tazobactam (PIP/TAZ), cefepime (CEF), ceftazidime (CAZ), doripenem (DOM), meropenem (MEM), ciprofloxacin (CIP), levofloxacin (LEV), tobramycin (TOB) and colistin (COL). Antibiotic susceptibility determined to be resistant, intermediate, or sensitive according to CLSI. Data presented as mean +/- SD (Mann-Whitney test).
Extended Data Fig. 2 Effect of TIVP-H6 Phage Therapy on Pyocyanin Production.
Production of pyocyanin (µg/mL) from cultures of bacterial isolates taken pre-and post-therapy from (A) patient 1 (Npre=1, Npost=3, p=NA), (B) patient 2 (Npre =4, Npost =5, p=0.1759), (C) patient 3 (Npre =1, Npost =1, p=NA), (D) patient 4 (Npre =1, Npost =6, p=NA), (E) patient 5 (Npre =2, Npost =4, p > 0.9999), (F) patient 6 (Npre =3, Npost =3, p > 0.9999), (G) patient 7 (Npre =2, Npost =5, p=0.0476), (H) patient 8 (Npre =3, Npost =1, p=NA), and (I) patient 9 (Npre =1, Npost =3, p=NA). Data representative of two independent experiments; error bars represent median (Mann-Whitney test).
Extended Data Fig. 3 Effect of LPS-5 Phage Therapy on LPS content.
Quantification of extracted LPS (ng/CFU) from PsA sputum isolates taken pre- and post-therapy from (A) patient 1 (Npre =1, Npost =3, p=NA), (B) patient 2 (Npre =4, Npost =5, p=0.2857), (C) patient 3 (Npre =1, Npost =1, p=NA), (D) patient 4 (Npre =1, Npost =5, p=NA), (E) patient 5 (Npre =2, Npost =4, p=0.5333), (F) patient 6 (Npre =3, Npost =3, p=0.0403), (G) patient 7 (Npre =2, Npost =5, p=0.0952), (H) patient 8 (Npre =3, Npost =1, p=NA), and (I) patient 9 (Npre =1, Npost =3, p=NA). Data representative of two independent experiments; error bars represent median (Welch’s t test or Mann-Whitney test).
Extended Data Fig. 4 Effect of LPS-5 Phage Therapy on PsA Elastase Production.
Secreted elastase activity (U/mL) from PsA sputum isolates taken pre- and post-therapy from (A) patient 1 (Npre =1, Npost =3, p=NA), (B) patient 2 (Npre =4, Npost =5, p=0.2857), (C) patient 3 (Npre =1, Npost =1, p=NA), (D) patient 4 (Npre =1, Npost =6, p=NA), (E) patient 5 (Npre =2, Npost =4, p=0.3333), (F) patient 6 (Npre =3, Npost =3, p=0.2032), (G) patient 7 (Npre =2, Npost =5, p=0.2381), (H) patient 8 (Npre =3, Npost =1, p=NA), and (I) patient 9 (Npre =1, Npost =3, p=NA). Data representative of two independent experiments; error bars represent median (Mann-Whitney test).
Extended Data Fig. 5 Effect of Phage Therapy on Sputum Microbiome.
(A) Relative abundance of bacteria genera (greater than 0.1%) in sputum samples over time: pre-therapy (pre), during therapy (d0), and post-therapy (d7-14, d14+). Analysis of alpha diversity via (B) Chao-1 richness, (C) Shannon Evenness, and (D) Simpson [not significant (ns); ANOVA with Tukey Correction for multiple comparisons]. Box and whisker plots presented as min, max, 25% and 75% quartile and median.
Extended Data Fig. 6 Nonsynonymous Variants in Post-Treatment Isolate Genomes.
Circular plots showing the distribution of variants in the post-treatment isolate genomes for the patients not shown in Fig. 3. Concentric circles represent single isolate genomes. Gray lines represent nonconservative variants that appear in coding sequences in one or more post-treatment isolates but are absent from all pre-treatment isolates. Yellow lines represent nonsynonymous polymorphisms coincident with genes expected to be under selection by the phage used in treatment. Red lines represent frameshifts for the same genes. Labeled, colored arrows indicate the positions and functional categories of these genes. Vertical black lines represent the genome start in the PAO1 reference against which variants were called.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chan, B.K., Stanley, G.L., Kortright, K.E. et al. Personalized inhaled bacteriophage therapy for treatment of multidrug-resistant Pseudomonas aeruginosa in cystic fibrosis. Nat Med 31, 1494–1501 (2025). https://doi.org/10.1038/s41591-025-03678-8
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41591-025-03678-8
This article is cited by
-
Hospital-adapted inhaled phage therapy for ventilator-associated pneumonia caused by multidrug-resistant Klebsiella pneumoniae: a comparative pilot study
Critical Care (2026)
-
Combined bacteriophage and antibiotic therapy for refractory peritoneal dialysis-related peritonitis caused by Klebsiella pneumoniae
Nature Communications (2026)
-
Quorum sensing in bacteria: insights into communication and inhibition strategies—a review
Archives of Microbiology (2026)
-
A review on antibiotic and non-antibiotic decolonization strategies of multidrug-resistant bacteria in the gastrointestinal tract
Infection (2026)
-
Characterization of a lytic phage and its efficacy against carbapenem-resistant Pseudomonas aeruginosa infection in mice
BMC Microbiology (2025)