Abstract
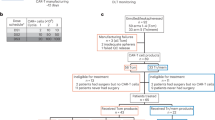
Glioblastoma (GBM) is the most common primary brain cancer in adults and carries a median overall survival (OS) of 12–15 months. Effective therapy for recurrent GBM (rGBM) following frontline chemoradiation is a major unmet medical need. Here we report the dose escalation and exploration phases of a phase 1 trial investigating intracerebroventricular delivery of bivalent chimeric antigen receptor (CAR) T cells targeting epidermal growth factor receptor (EGFR) epitope 806 and interleukin-13 receptor alpha 2 (IL-13Rα2), or CART-EGFR-IL13Rα2 cells, in patients with EGFR-amplified rGBM. Primary endpoints included dose-limiting toxicity, determination of the maximum tolerated dose and recommended dose for expansion, and occurrence of adverse events. Secondary endpoints included objective radiographic response, duration of response, progression-free survival and OS. A total of 18 patients received CART-EGFR-IL13Rα2 cells. The maximum tolerated dose was determined to be 2.5 × 107 cells. Of the 18 patients, 10 (56%) experienced grade 3 neurotoxicity; none had grade 4–5 neurotoxicity. Of 13 patients, 8 (62%) with measurable disease at the time of CAR T cell infusion experienced tumor regression, with one confirmed partial response by Modified Response Assessment in Neuro-Oncology criteria (objective radiographic response, 8%; 90% confidence interval, 0–32%) and one patient with ongoing durable stable disease lasting over 16 months. Median progression-free survival was 1.9 months (90% confidence interval, 1.1–3.4 months), and median OS was not yet reached at the time of data cut-off (median follow-up time, 8.1 months). These findings indicate that intracerebroventricular delivery of bivalent CART-EGFR-IL13Rα2 is feasible and appears safe. CART-EGFR-IL13Rα2 cells are bioactive and exhibit a signal of antitumor effect in rGBM. ClinicalTrials.gov registration: NCT05168423.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are included in the Article or may be available from the corresponding authors, recognizing that certain patient-related data not included in the paper were generated as part of the clinical trial and may be subject to patient confidentiality. It is estimated that the corresponding authors will respond to external data requests within 2 weeks of receipt of request to verify whether the request is subject to any intellectual property or confidentiality obligations. Further information on research design is available in the Nature Research Reporting Summary linked to this article. Source data are provided with this paper.
Change history
11 June 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41591-025-03824-2
References
Price, M. et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2017–2021. Neuro. Oncol. 26, vi1–vi85 (2024).
Stupp, R. et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 352, 987–996 (2005).
Taslimi, S., Ye, V. C., Wen, P. Y. & Zadeh, G. Lessons learned from contemporary glioblastoma randomized clinical trials through systematic review and network meta-analysis: part 2 recurrent glioblastoma. Neurooncol. Adv. 3, vdab029 (2021).
Reardon, D. A. et al. Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: the CheckMate 143 Phase 3 Randomized Clinical Trial. JAMA Oncol. 6, 1003–1010 (2020).
Chen, A. T. C. et al. Prospective randomized phase 2 trial of hypofractionated stereotactic radiation therapy of 25 Gy in 5 fractions compared with 35 Gy in 5 fractions in the reirradiation of recurrent glioblastoma. Int. J. Radiat. Oncol. Biol. Phys. 119, 1122–1132 (2024).
Werlenius, K. et al. Effect of disulfiram and copper plus chemotherapy vs chemotherapy alone on survival in patients with recurrent glioblastoma: a randomized clinical trial. JAMA Netw. Open 6, e234149 (2023).
Tsien, C. I. et al. NRG Oncology/RTOG1205: a randomized phase ii trial of concurrent bevacizumab and reirradiation versus bevacizumab alone as treatment for recurrent glioblastoma. J. Clin. Oncol. 41, 1285–1295 (2023).
Cappell, K. M. & Kochenderfer, J. N. Long-term outcomes following CAR T cell therapy: what we know so far. Nat. Rev. Clin. Oncol. 20, 359–371 (2023).
Peng, L., Sferruzza, G., Yang, L., Zhou, L. & Chen, S. CAR-T and CAR-NK as cellular cancer immunotherapy for solid tumors. Cell. Mol. Immunol. 21, 1089–1108 (2024).
Choi, B. D. et al. Intraventricular CARv3-TEAM-E T cells in recurrent glioblastoma. N. Engl. J. Med. 390, 1290–1298 (2024).
Bagley, S. J. et al. Intrathecal bivalent CAR T cells targeting EGFR and IL13Rα2 in recurrent glioblastoma: phase 1 trial interim results. Nat. Med. 30, 1320–1329 (2024).
Brown, C. E. et al. Locoregional delivery of IL-13Rα2-targeting CAR-T cells in recurrent high-grade glioma: a phase 1 trial. Nat. Med. 30, 1001–1012 (2024).
Monje, M. et al. Intravenous and intracranial GD2-CAR T cells for H3K27M+ diffuse midline gliomas. Nature 637, 708–715 (2025).
Vitanza, N. A. et al. Intracerebroventricular B7-H3-targeting CAR T cells for diffuse intrinsic pontine glioma: a phase 1 trial. Nat. Med. 31, 861–868 (2025).
Reilly, E. B. et al. Characterization of ABT-806, a humanized tumor-specific anti-EGFR monoclonal antibody. Mol. Cancer Ther. 14, 1141–1151 (2015).
Bartolomé, R. A. et al. IL13 receptor α2 signaling requires a scaffold protein, FAM120A, to activate the FAK and PI3K pathways in colon cancer metastasis. Cancer Res. 75, 2434–2444 (2015).
Lassman, A. B. et al. Comparison of biomarker assays for EGFR: implications for precision medicine in patients with glioblastoma. Clin. Cancer Res. 25, 3259–3265 (2019).
Joshi, B. H., Plautz, G. E. & Puri, R. K. Interleukin-13 receptor alpha chain: a novel tumor-associated transmembrane protein in primary explants of human malignant gliomas. Cancer Res. 60, 1168–1172 (2000).
Yin, Y. et al. Locally secreted BiTEs complement CAR T cells by enhancing killing of antigen heterogeneous solid tumors. Mol. Ther. 30, 2537–2553 (2022).
Schulz, K. F., Altman, D. G., Moher, D. & the, C.G. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 8, 18 (2010).
Logun, M. et al. Patient-derived glioblastoma organoids as real-time avatars for assessing responses to clinical CAR-T cell therapy. Cell Stem Cell 32, 181–190.e184 (2025).
Lee, D. W. et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol. Blood Marrow Transplant. 25, 625–638 (2019).
Mahdi, J. et al. Tumor inflammation-associated neurotoxicity. Nat. Med. 29, 803–810 (2023).
Brudno, J. N. & Kochenderfer, J. N. Current understanding and management of CAR T cell-associated toxicities. Nat. Rev. Clin. Oncol. 21, 501–521 (2024).
Grant, S. J. et al. Clinical presentation, risk factors, and outcomes of immune effector cell-associated neurotoxicity syndrome following chimeric antigen receptor T cell therapy: a systematic review. Transplant. Cell. Ther. 28, 294–302 (2022).
Kirouac, D. C., Zmurchok, C. & Morris, D. Making drugs from T cells: the quantitative pharmacology of engineered T cell therapeutics. NPJ Syst. Biol. Appl. 10, 31 (2024).
Turicek, D. P., Giordani, V. M., Moraly, J., Taylor, N. & Shah, N. N. CAR T-cell detection scoping review: an essential biomarker in critical need of standardization. J. Immunother. Cancer 11, e006596 (2023).
Rotte, A. et al. Dose–response correlation for CAR-T cells: a systematic review of clinical studies. J. Immunother. Cancer 10, e005678 (2022).
Baur, K. et al. CD4+ CAR T-cell expansion is associated with response and therapy related toxicities in patients with B-cell lymphomas. Bone Marrow Transplant. 58, 1048–1050 (2023).
Ellingson, B. M. et al. Objective response rate targets for recurrent glioblastoma clinical trials based on the historic association between objective response rate and median overall survival. Neuro. Oncol. 25, 1017–1028 (2023).
Rhee, J. Y., Ghannam, J. Y., Choi, B. D. & Gerstner, E. R. Labeling T cells to track immune response to immunotherapy in glioblastoma. Tomography 9, 274–284 (2023).
Barajas, R. F. Jr. et al. [18F]-fluoromisonidazole (FMISO) PET/MRI hypoxic fraction distinguishes neuroinflammatory pseudoprogression from recurrent glioblastoma in patients treated with pembrolizumab. Neurooncol. Pract. 9, 246–250 (2022).
Ellingson, B. M. et al. Hypothetical generalized framework for a new imaging endpoint of therapeutic activity in early phase clinical trials in brain tumors. Neuro. Oncol. 24, 1219–1229 (2022).
Thokala, R. et al. High-affinity chimeric antigen receptor with cross-reactive scFv to clinically relevant EGFR oncogenic isoforms. Front. Oncol. 11, 664236 (2021).
Yin, Y. et al. Checkpoint blockade reverses anergy in IL-13Rα2 humanized scFv-based CAR T cells to treat murine and canine gliomas. Mol. Ther. Oncolytics 11, 20–38 (2018).
Louis, D. N. et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro. Oncol. 23, 1231–1251 (2021).
O’Rourke, D. M. et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci. Transl. Med. 9, eaaa0984 (2017).
Maude, S. L. et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 371, 1507–1517 (2014).
Acknowledgements
This trial was originally funded by Tmunity Therapeutics, which was acquired by Kite Pharma (a Gilead company). The trial has subsequently been funded by Kite Pharma. Additional funding sources included the Abramson Cancer Center Glioblastoma Translational Center of Excellence to D.M.O., the Templeton Family Initiative in Neuro-Oncology to D.M.O. and the Maria and Gabriele Troiano Brain Cancer Immunotherapy Fund to D.M.O. We would like to thank the patients who participated in this study and their families for their dedication to furthering GBM treatment. We also thank the Neurosurgery Clinical Research Division, the Safety, Monitoring, and Data Management teams of the Center for Cellular Immunotherapy, the Translational and Correlative Sciences Laboratory and the Clinical Cell and Vaccine Production Facility at the University of Pennsylvania Perelman School of Medicine for all of their clinical trial contributions and support. Kite Pharma had an advisory role in the design of the study and review of the final paper but had no role in data collection, analysis, decision to publish or preparation of the paper.
Author information
Authors and Affiliations
Contributions
Study design: S.J.B., A.M., W.T.-H., E.O.H., Z.A.B. and D.M.O. Patient recruitment and treatment: S.J.B., A.S.D., A.N., L.J.B., R.M., N.S., E.M., S.C., B.S.O., G.P., A.B., F.C., D.L.S. and D.M.O. Data generation, curation and analyses: S.J.B., J.A.F., D.L.S., A.N., L.J.B., J.P., D.J., R.M., N.S., C.S., R.M.L., J.K.J., V.G., M.P.N., W.T.-H., C.A., N.F.F., D.C., D.L.S., Z.A.B. and D.M.O. Writing—original draft: S.J.B., N.F.F., D.C. and Z.A.B. Writing—review and editing: S.J.B., J.A.F., D.S., D.B., W.T.-H., C.A., C.H.J., E.O.H., Z.A.B. and D.M.O. Supervision: S.J.B., J.A.F., E.M., L.L., C.S., A.M., J.K.J., G.P., A.B., D.L.S., C.H.J., E.O.H., Z.A.B. and D.M.O. Funding support: D.B.
Corresponding authors
Ethics declarations
Competing interests
S.J.B. has received consulting fees from Modifi Bio, Telix, Servier, Kiyatec, Novocure and Bayer and has received research funding from Kite (a Gilead Company) related to the submitted work and from Incyte, Novocure, GSK and Eli Lilly, all outside of the submitted work. J.A.F. is a member of the scientific advisory boards of Cartography Bio and Shennon Biotechnologies Inc and has patents, royalties and other intellectual property. A.J.R. is a cofounder and shareholder in Cellformatica. J.J. has received consulting fees from Bluewhale Bio, outside of the submitted work. D.B. is an employee of Kite Pharma (a Gilead company). D.L.S. holds founder’s equity and has licensed intellectual property to Verismo Therapeutics and Vetigenics, Inc. and has intellectual property licensing to Chimeric Therapeutics, Ltd. C.H.J. and the University of Pennsylvania have patents pending or issued related to the use of gene modification in T cells for adoptive T cell therapy. C.H.J. is a cofounder of Tmunity (acquired by Kite Pharma, a Gilead company); is a scientific cofounder and holds equity in Capstan Therapeutics, Dispatch Biotherapeutics and Bluewhale Bio; serves on the board of AC Immune; is a scientific advisor to BluesphereBio, Cabaletta, Carisma, Cartography, Cellares, Cellcarta, Celldex, Danaher, Decheng, ImmuneSensor, Kite, Poseida, Verismo, Viracta, Vittoria Bio and WIRB-Copernicus group; and is an inventor on patents and/or patent applications licensed to Novartis Institutes of Biomedical Research and Kite and may receive license revenue from such licenses. Z.A.B. has received research funding from Kite Pharma, has inventorship interest in intellectual property owned by the University of Pennsylvania and has received royalties related to CAR T therapy in solid tumors. D.M.O. reports previous or active roles as consultant and scientific advisory board member for Celldex Therapeutics, Prescient Therapeutics, Century Therapeutics and Chimeric Therapeutics, and has an advisory role and holds equity in Kiragen and Cellula Therapeutics. He has received research funding from Celldex Therapeutics, Novartis, Tmunity Therapeutics and Gilead Sciences/ Kite Pharma. D.M.O. is an inventor of intellectual property (US patent numbers 7,625,558 and 6,417,168 and related families) and has received royalties related to targeted ErbB therapy in solid cancers previously licensed by the University of Pennsylvania. D.M.O. is also an inventor on multiple patents related to CART cell therapy in solid tumors that have been licensed by the University of Pennsylvania and has received royalties from these license agreements. The other authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks Jasia Mahdi and Sheila Singh for their contribution to the peer review of this work. Primary Handling Editor: Saheli Sadanand, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Immunofluorescence images of representative pre-infusion tumor samples.
Patient slides demonstrated a range of target staining, including tissue with subjective positivity for both targets (16321-04, 16321-40, 16321-23, 16321-52), for EGFR (16321-18), and for IL13Ra2 (16321-11). Given the trial inclusion criterion of EGFR amplification, the negative staining for EGFR seen in 16321-11 is a relevant example of CAR target spatial heterogeneity. All images taken at 20x magnification. Experiments were performed in duplicate.
Extended Data Fig. 2 Individual CSF pharmacokinetics for dose level -1.
CAR copies per ug of genomic DNA (Top). CAR copies per mL of CSF (Bottom).
Extended Data Fig. 3 Individual CSF pharmacokinetics for dose level 1.
CAR copies per ug of genomic DNA (Top). CAR copies per mL of CSF (Bottom).
Extended Data Fig. 4 Individual CSF pharmacokinetics for dose level 2.
CAR copies per ug of genomic DNA (Top). CAR copies per mL of CSF (Bottom).
Extended Data Fig. 5 CAR T cell expansion from day+1 to day+7.
Fold change in CAR copies/ug DNA in the CSF from Day +1 to Day +7 by dose level (two-sided p = 0.107, Wilcoxon test).
Extended Data Fig. 6 Mean fold increase of key inflammatory cytokines in the CSF from Day 0 through Day 28 across all patients by dose level.
Mean fold increase of cytokines interferon-gamma (IFNg), interleukin-2 (IL2), interleukin-6 (IL6), and tumor necrosis factor alpha (TNFa) in the CSF from Day 0 through Day 28 across all patients by dose level (n = 6 per dose level). Data are presented as mean values +/− SEM.
Extended Data Fig. 7 T1 post-contrast (top panels) and T2/FLAIR (bottom panels) MRI images for the 8 patients with measurable disease who experienced any degree of tumor regression following CAR T cell infusion.
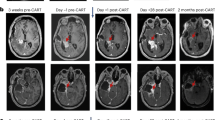
Timepoints shown include (1) the post-operative MRI scan taken on post-operative day 1 following the surgery that was performed for maximal safe tumor resection and Ommaya reservoir placement, (2) the immediate pre-CART MRI scan taken within 1-2 days prior to CAR T cell infusion, and (3) the 1-month post-CART MRI scan. No anticancer therapies were administered between scan 1 and 2, and no anticancer therapies other than CART-EGFR-IL13Ra2 cells were administered between scan 2 and scan 3. In Patient-07, an increase in enhancing tumor on the 1-month MRI was followed by spontaneous regression on the 2-month MRI, consistent with pseudo-progression.
Extended Data Fig. 8 Individual CSF pharmacokinetics patients who underwent retreatment with CAR T cells.
CAR copies per ug of genomic DNA (Top). CAR copies per mL of CSF (Bottom).
Supplementary information
Supplementary Information
Full study protocol.
Supplementary Tables 1–9
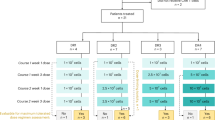
Supplementary Table 1: Optimized CAR T expression values on the patient infusion product. Supplementary Table 2: CRS grading system. Supplementary Table 3: University of Pennsylvania modified CAR neurotoxicity grading system for patients with glioblastoma. Supplementary Table 4: All treatment-emergent AEs by dose level. Supplementary Table 5: Administration and timing of dexamethasone and anakinra. Supplementary Table 6: Comparison of peak ICANS and peak TIAN grade for CAR neurotoxicity. Supplementary Table 7: Longitudinal CAR copies per microgram of genomic DNA data in the CSF for each dose level and for retreatment patients. Supplementary Table 8: Longitudinal CAR copies per microgram of genomic DNA data in the peripheral blood for each dose level and for retreatment patients. Supplementary Table 9: Longitudinal CAR copies per milliliter of CSF data for each dose level and for retreatment patients.
Source data
Source Data Fig. 3
Full CSF and blood cytokine data for all patients (n = 18).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bagley, S.J., Desai, A.S., Fraietta, J.A. et al. Intracerebroventricular bivalent CAR T cells targeting EGFR and IL-13Rα2 in recurrent glioblastoma: a phase 1 trial. Nat Med 31, 2778–2787 (2025). https://doi.org/10.1038/s41591-025-03745-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41591-025-03745-0
This article is cited by
-
The development of CAR T cells for patients with CNS malignancies
Nature Reviews Clinical Oncology (2026)
-
Emerging therapies for glioblastoma
Journal of Neuro-Oncology (2026)
-
Obstacles and improvement strategies for CAR-T cell therapy in solid tumors
Discover Oncology (2026)
-
Immunotherapy and targeted therapy for high grade gliomas: current and future directions
Journal of Neuro-Oncology (2026)
-
MiR-148a-3p/SIRT7 axis promotes glioma progression and regulates temozolomide chemosensitivity
Journal of Neuro-Oncology (2026)