Abstract
The dual rAAVrh8-HEXA and rAAVrh8-HEXB vector can restore central nervous system hexosaminidase (Hex) enzyme activity, decrease GM2 levels in cerebrospinal fluid and rescue phenotypic consequences of GM2 gangliosidosis, Tay–Sachs and Sandhoff diseases in animal models following simultaneous bi-thalamic (BiT) injections. Following up on an n = 2 expanded access trial, we initiated a phase 1/2, single-dose, dose-escalation of combined BiT, intra-cisterna magna and intrathecal infusion in children with Tay–Sachs and Sandhoff diseases (six infantile, three juvenile). The BiT injection volume and vector dose were doubled between four cohorts, with the lowest dose matching the earlier expanded access trial. Cerebrospinal fluid HexA enzyme activity, serum total Hex activity and GM2 levels showed a dose-dependent biochemical correction of the disease. Serum Hex activity surpassed 40 nmol h−1 ml−1, two times the lower limit of normal, and neuroimaging demonstrated increased fiber tracts. Correction was greatest at 12 weeks, but in decline by 24 weeks postdosing. Infantile patients experienced global clinical stabilization and prolonged oral feeding without aspiration until 3–3.5 years. Seizures had a later onset, were less frequent, less severe and more responsive to anti-convulsant medication. Adverse events were rare in infantile patients, but worsening dystonia was observed in juvenile patients, who were excluded from ongoing enrollment. ClinicalTrials.gov registration: NCT04669535 and NCT06614569.
Similar content being viewed by others
Main
Tay–Sachs and Sandhoff diseases (TSD and SD, respectively) are GM2 gangliosidoses characterized by accumulation of GM2 sphingolipids resulting from deficiency of lysosomal hexosaminidase A (HexA) activity. This deficiency is caused by mutations in either the alpha subunit (encoded by HEXA) in TSD or the beta subunit (encoded by HEXB) in SD. Severe deficiency (<0.1% enzyme activity) results in infantile forms of these diseases in which patients initially show normal neurodevelopmental progress, but then begin to inexorably regress around 5–6 months of age leading to global neurological deficits, inability to swallow, seizures and ultimately a semi-vegetative state with death at 3–6 years1,2. Juvenile patients have later onset of disease compared with the infantile form and acquire the ability to walk, but also experience disease progression that increasingly impacts motor and cognitive abilities over time with death in the second decade of life. At present, there is no effective treatment for either form of childhood GM2 gangliosidosis.
Adeno-associated virus (AAV) gene therapy has resulted in dramatic improvement in spinal muscular atrophy type 1 (ref. 3) and aromatic l-amino acid decarboxylase deficiency4,5,6,7,8. However, global central nervous system (CNS) diseases, including TSD and SD, require more widespread distribution of therapeutic proteins across all brain and spinal cord regions. In preclinical models, AAV delivery to the thalamus and cerebrospinal fluid (CSF) is an effective approach to achieve broad CNS distribution through axonal transport between interconnected structures and CSF flow9. In mouse, sheep and cat models, the combination of intraparenchymal and CSF delivery of AAV vectors showed impressive therapeutic effects9,10,11. These studies also demonstrated that axonal transport is an evolutionarily conserved mechanism for widespread distribution of lysosomal enzymes in larger mammalian brains.
We previously reported on an expanded access clinical trial with two children with infantile TSD treated by a similar approach using a combination of AAVrh8-HEXA and AAVrh8-HEXB vectors through a mixture of bi-thalamic (BiT) and CSF delivery. Here we describe a phase 1/2 clinical trial (NCT04669535 and NCT06614569) of combined BiT, intra-cisterna magna (CM) and intrathecal (IT) delivery in nine patients escalated through four different thalamic volume or dosage levels (the study design is shown in Extended Data Fig. 1). Safety findings, biochemical correction and functional outcomes were tracked. These studies showed a dose–effect relationship with levels of CSF HexA reaching ~13% of normal (0.59 nmol h−1 ml−1; normal range: 3.31–7.25 nmol h −1 ml−1, mean: 4.57 ± 1.80 nmol h−1 ml−1), total serum Hex activity reaching 40 nmol h−1 ml−1 (twice the lower limit of normal) and neural tract number increasing by diffusion tensor imaging (DTI). These effects peaked at 12 weeks and remained above baseline at 24 weeks dosing. We observed a subsequent slowing of some aspects of neurodevelopmental regression, which may provide important insights into the use of biomarkers to evaluate dosing in future studies of ‘all-in-one’ (that is, bicistronic) vectors expressing both HEXA and HEXB. The differences in outcomes between infantile and juvenile participants may also inform future trial design.
Results
Description of study participants
The characteristics and genotypes of the patients enrolled in the study are shown in Table 1. A total of nine patients were enrolled in the trial (two with SD and seven with TSD; NCT04669535 and NCT06614569). There were two screen failures: one because of a temporary hold that was placed on enrollment, which was eventually lifted and the participant was re-enrolled as Patient 005; and one because of rapid clinical worsening of the patient’s condition immediately before planned enrollment. Our study design did not exclude patients who showed seropositivity for the AAVrh8 capsid. We noted that only Patient 002 showed seropositivity, but we did not assess whether this affected patient outcomes. Because this trial was designed to study both safety and biological activity in infantile patients with GM2 disease, bioactivity and functional outcome measures in the infantile patients were the primary focus. Because of safety events in juvenile patients, they were excluded from further enrollment and not included in further analysis of outcomes.
General safety
With respect to general safety, there were a total of 171 adverse events (AEs) recorded during the study, of which only 15 were found to be possibly or definitely related to the vector (see Supplementary Table 1 for a complete list of AEs and clinical laboratory findings). Distribution of the 15 potentially vector-related AEs by dose and severity is shown in Table 2. Of these AEs, nine consisted of elevated liver enzymes and/or elevated anti-capsid interferon-ɣ enzyme-linked immunosorbent spot (ELISpot) responses, and these responses were seen at low, medium and high dose levels. All AEs of this type responded to increased steroid dosages with subsequent reduction in both transaminase levels and numbers of spot-forming units by ELISpot12. Two patients who were enrolled in this study died after being treated. Patient 001 died at study day 805 from disease progression and patient 002 died at study day 163 from fulminant, hemorrhagic Clostridioides difficile infection secondary to prolonged hospitalizations and antibiotic use because of underlying disease progression. This was deemed to be unrelated to the vector, surgery or protocol medications.
Interestingly, two of three juvenile patients noted new or worsening dystonia, and the third experienced unexpected progression of the neurologic manifestations of their GM2 gangliosidosis. This type of AE was not observed in any of the infantile patients. The worsening of dystonia developed between one and two months after thalamic injection, suggesting that it could possibly be related to the biology of transgene expression in the thalami or adjacent basal ganglia structures. Although the precise mechanism of this phenomenon is not clear, neurotransmitter metabolites were measured in the CSF to determine whether either surgical process or vector effects adjacent to the basal ganglia in the juvenile patients with GM2 gangliosidosis altered the function of neurons in basal ganglia structures (Supplementary Table 2). Once the observation of dystonia was made in juvenile patients, only infantile patients were recruited for the remainder of the study.
Safety and confirmation of BiT convection-enhanced delivery
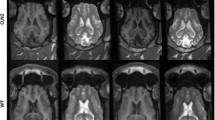
To confirm thalamic infusion and evaluate for any acute toxicity, each participant underwent cranial magnetic resonance imaging (MRI) without contrast within the first 30 min following the end of vector infusion. In eight of nine BiT infusions, there was a change in signal associated with T2-FLAIR hyperintensity in the thalamus indicative of the accuracy of the infusion sites (Fig. 1a). We noted that Patient 004 did not demonstrate an increase in T2-FLAIR signal in the thalami postinfusion, which is possibly related to backflow of infusate into the subarachnoid space. There were no clinically significant surgical complications such as surgical tract-related intracerebral hemorrhage, cerebral edema or parenchymal tissue injury in the thalamus. Both patients in the high-dose cohort (1,250 μl in each thalamus) displayed foci of T2 hyperintensity in the thalami related to the site of the catheter tip (Extended Data Fig. 2). One patient had a clinically insignificant radiological area of ischemia (as demonstrated by increase in diffusion-weighted imaging signal) in the right caudate nucleus close to the catheter trajectory, which was associated with a mild volume loss in that region in MRI data at 3 and 6 months postinfusion.
a, Immediate postoperation (postop) MRI depicting BiT injection of AAVrh8-HEXA and AAVrh8-HEXB (1,250 μl and 4.1 × 1013 vector genomes (vg) per thalamus). MRI without contrast showing T2-FLAIR hyperintensity in the thalamus at the injection site. The hyperintensity decreases over time (at 3 and 6 months). There is no evidence of hemorrhage or other major complication associated with the injection. b, Two patients demonstrated an increase in cortical T2 hypointensity, suggestive of myelin production, over the course of three months postinfusion. The effect was observed in the frontoparietal cortex, but not in the temporal cortex. c, DTI for five patients taken at baseline and 24 weeks after treatment showing neural tract fiber growth (green) and loss (red). d, Quantification of percent change in tract fiber numbers and volume from DTI data at 12 and 24 weeks posttreatment. DWI, diffusion-weighted imaging; F, female; mo, months, Pt., patient; SWI, susceptibility-weighted imaging.
Sixteen AEs were deemed to be potentially related to the BiT convection-enhanced delivery procedures and/or the associated anesthesia (Table 2). The most frequent surgical complications were related to delayed wound healing. These included superficial wound dehiscence after suture removal requiring placement of reinforcement sutures in some circumstances. After wound dehiscence was observed in the first few patients, the study protocol was amended and the time until suture removal was lengthened from 2 to 3 weeks.
Neuroimaging efficacy
The MRI data suggest a therapeutic effect of the treatment. Specifically, there was one patient in whom myelination in the frontal lobe white matter was improved (Fig. 1b), another in whom myelination was marginally improved and a more consistent finding of preservation of myelination in the cerebral cortex. The temporal lobe white matter did not show signs of this potentially beneficial effect. Vector-treated patients showed a slower increase in total brain volume than historical controls, potentially indicating a therapeutic effect on the macrocephaly, which is characteristic of this disease (Extended Data Fig. 3 and Extended Data Tables 1 and 2). There was positive correlation between lower brain volume and a lower global clinical severity score as well (Extended Data Fig. 3 and Extended Data Tables 1 and 2). These findings are consistent with a positive therapeutic effect, although this is not conclusive.
There was evidence of development of new myelinated tracts after vector treatment by DTI. Two DTI parameters, fractional anisotropy (FA) and radial diffusivity, showed trends indicative of increased myelination in the lentiform nucleus (putamen and globus pallidus), but not in other brain regions such as the temporal lobe (Extended Data Fig. 4). Poor AAV distribution to the temporal lobes has previously been reported in preclinical large animal studies9,13,14. In addition, DTI data were analyzed by differential tractography to map newly myelinated neural tracts in six of the vector-treated patients (Fig. 1c,d). As shown by the green pseudocolor in Fig. 1c, functional white tracts were observed across cerebral lobes and frequently in the corpus collosum at 12 weeks after treatment, while fewer such tracts were seen at 24 weeks posttherapy. However, all six patients had positive net fiber tract metrics at both visits, indicating improved myelination.
Clinical laboratory and immunologic assessments
As previously reported in patients with TSD, elevations in transaminase values were observed in most patients before gene transfer15. However, following gene delivery there were further significant increases from baseline in each patient. These were associated temporally with positive results on the ELISpot assay designed to evaluate effector T cell responses to AAVrh8 capsid epitopes (Fig. 2). In each case, the patients were treated by increasing the corticosteroid dosage levels, either from 1 mg kg−1 daily to 2 mg kg−1 daily prednisolone or by adding a 3-day course of methylprednisolone at 10 mg kg−1 d−1 before returning to a 2 mg kg−1 d−1 prednisolone dosage (Supplementary Table 3). Transaminase elevations from baseline ultimately resolved in all participants, as did the number of spot-forming units on capsid ELISpot assays. All participants’ serum neutralizing antibody titers to AAVrh8 increased by more than tenfold following the therapy12.
Normal ranges are indicated for liver enzymes in dotted lines. Positive and negative ELISpot responses are denoted by red and blue shading, respectively. Complete immune response data are presented in the companion publication12. ALT, alanine transaminase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transferase; IFNɣ, interferon-ɣ; M, male; NA, not applicable (no sample collected); yr, years.
Bioactivity outcomes
The combined rAAV8-HexA and rAAV8-HexB vector was designed to express both subunits of the functional HexA enzyme. HexA activity was measured in CSF samples taken from all patients before dosing on day 2 (after BiT delivery, just before intra-CM and IT delivery), at 12 weeks and at 24 weeks after gene therapy to determine whether enzyme activity was increased in the CNS. Patients in the earlier cohort provided samples at additional time points.
As shown in Fig. 3a, there was a clear dose–response relationship for HexA enzyme activity among the infantile patients with TSD (the CSF HexA assay is uninformative in patients with SD, who are deficient in the beta subunit of HEXA, because homodimers of the alpha subunit of the HEXA gene product are able to hydrolyze the substrate used in the assay), with the highest CSF HexA enzyme activity being 0.59 nm h−1 ml−1 in the final participant (Patient 011) at the 12-week time point, which is 13% of the normal value. Overall, HexA activity levels peaked in most participants at 12 weeks and declined somewhat thereafter, but remained above pretreatment levels throughout the duration of the study (Fig. 3a). Similarly, the total Hex levels in the serum of infantile patients with TSD increased after vector treatment, peaking between day 22 (exceeding 40 nm h−1 ml) and week 8, and then partially declining (Fig. 3b). As with the CSF HexA activity, the highest Hex levels in the serum were observed in the high-dose cohort, indicating a dose–response relationship for biological activity. Finally, C18:0 and C20:0 GM2 levels were quantified by mass spectrometry in CSF and correlated with HexA levels at the same time points. As observed in previous studies16, the C20:0 GM2 levels were more consistently lowered in vector-treated participants than C18:0 GM2 (Fig. 3c and Supplementary Table 4).
a, HexA enzyme activity in CSF collected at the indicated time points. Analysis for HexA enzyme was only interpretable in patients with TSD. b, Total serum Hex levels collected at the indicated time points. The dashed line indicates normal Hex levels. c, C20:0 GM2 quantification by mass spectrometry. We note that juvenile patients have lower levels of GM2 as predicted by their disease subtype. d, CSF HexA activity levels from a generally correlated with GM2 20:0 levels.
Measurement of CSF GM2 20:0 by mass spectrometry did not show consistent dose-related differences from pre- to posttreatment. However, of the six infantile patients in the study, four showed a downward trend in GM2 20:0 (Extended Data Table 1). The percentage decrease of CSF GM2 20:0 from the baseline to its lowest point ranged from a 9.1% decrease in one patient in the low-dose cohort to a 52.5% decrease in one patient in the mid-dose cohort and 49.5% in one patient in the high-dose cohort. Two participants reached the lowest level (nadir) of recorded concentration of GM2 20:0 at 12 weeks and two reached their minimum value at 24 weeks; one showed two nadirs at 12 and 48 weeks. This time frame was generally concurrent with the timing of the highest levels of CSF HexA activity (Fig. 3d).
Neurologic and developmental outcomes
Patients with GM2 gangliosidosis have a substantial increase in both brain and ventricle volume over time, which is thought to be caused by the accumulation of GM2 gangliosides17. The patients in this study demonstrated a slower rate of brain volume growth (measured in ml per year) compared with historical data of age-matched patients with GM2 gangliosidosis, potentially indicating a therapeutic effect on the macrocephaly, which is characteristic of this disease (Extended Data Figs. 3 and 5, Extended Data Table 2 and Supplementary Table 5). There was no significant decrease in growth of ventricle volume for the patients in this study, and so the change cannot be explained by atrophy alone. As with other biomarkers, there was a correlation between lower brain volume and less-severe Clinical Global Inventory (CGI) scores. We noted that the two patients from the high-dose cohort showed an overall decrease in cerebellar cortex volume following treatment, while the remaining study participants did not (Extended Data Fig. 5). These findings are consistent with a positive therapeutic effect, although this is not conclusive.
The complete set of assessments including neurologic and developmental outcomes is presented in Extended Data Fig. 6. Changes in GCI score from baseline for infantile patients are shown in Fig. 4a. Patients 007 and 008 (both mid dose) showed improvement from baseline at 4 weeks. Patient 010 (high dose) maintained baseline score at the 4- and 12-week time points, but then declined. Patient 011 (high dose) improved from baseline at 12 weeks and maintained that improvement at 24 weeks (which was the end of the study for this participant). Of note, this patient had the highest posttreatment CSF HexA activity. As shown in Fig. 4a, there was a strong negative correlation between vector dose and the last recorded CGI value. All participants were stable on this score at 4 weeks, with Patients 007 and 008 worsening at week 12 and thereafter. All other patients maintained the same severity score at week 24.
Infantile neurologic exam scores for seizures and swallowing
Two important aspects of infantile disease progression include the frequency and severity of seizures and retaining the ability to suck and/or swallow so that patients may maintain oral feeding. As shown in Fig. 4b, Patient 011 was seizure-free for the entire duration of the study (24 weeks), but developed seizures shortly after the end of the study period. Patient 010 had an electroencephalogram (EEG) showing seizure activity before treatment but was placed on Keppra and remained stable on Keppra alone to the end of the study (24 weeks). With respect to the ability to suck and/or swallow without aspiration, all participants were either stable or improved (Patients 010 and 011) (Fig. 4c). For both patients in the high-dose cohort, the ability to feed orally was maintained at the end of the study (24 weeks).
Discussion
This study demonstrated a dose-related increase in bioefficacy of rAAVrh8-HexA and rAAVrh8-HexB when delivered by bilateral intrathalamic convection-enhanced delivery up to a maximum intrathalamic dose of 4.1 × 1013 vg in 1.25 ml of infusate, along with intra-CM and IT injection. This was evident by CSF HexA activity in patients with TSD, and all participants also showed an increase in total Hex activity in the serum. The CSF HexA activity peaked at ~0.6 nmol h−1 ml−1 in the highest dose group, or ~13% of the average of normal CSF, and the serum total Hex activity was above the lower limit of the normal range in most vector-treated patients. Vector-mediated enzyme activities were highest at the 12-week time point and generally showed some decline thereafter. We did not assess levels of anti-HexA antibodies, but it is possible that patient immune systems produced neutralizing antibodies in the serum that contributed to reducing the efficacy of the therapy.
The cause of the reduction in Hex activity in the CSF and serum from 24 weeks onward is unclear. Effector T cell responses directed against AAVrh8 capsid epitopes were detected in the peripheral blood of all patients, and this could have resulted in elimination of vector-transduced cells. However, each such instance was treated with increasing doses of corticosteroids (ranging from 2 to 10 mg kg−1 d−1) and the concomitant transaminase elevations responded to this intervention. Furthermore, the number of spot-forming units in the AAVrh8 capsid ELISpot assay declined after treatment with steroids as well12, suggesting that the immune response was effectively suppressed. Finally, the CSF analysis at 24 weeks and beyond did not show pleocytosis or elevation in protein concentration (Supplementary Table 6) that would have been predicted to occur if there had been substantial cytotoxic T cell-mediated killing of the vector-transduced neurons.
Despite the consistent increase in CSF enzyme activity across our study, there were significant differences in our cohorts based on phenotype. The worsening of dystonia in our juvenile cohort led us to exclude other juvenile patients from further recruitment and outcome analysis. In infantile patients, increases in HexA enzyme activity were associated with clinical stabilization or slowing of the typical pattern of regression. The higher dose cohorts of patients maintained the ability to feed orally for considerably longer than the lower dose cohorts, and patients in all cohorts showed at least partial or temporary stabilization or improvement. Indeed, whereas in an historical cohort gastrostomy tube placement had occurred in half of patients by 13–18 months of age18, half of our cohort remained on full oral feeds without gastrostomy tube placement until after 25 months and the two high-dose participants (Patients 010 and 011) remained on full oral feeds at the end of the study (at 27 and 20 months, respectively). This is encouraging because eating by mouth is an important quality-of-life outcome for the families of these patients19. Despite partial or temporary stabilization in disease course, there was no clear effect on the development of seizures. Although Patient 011 remained seizure-free for the duration of the study, she developed seizures shortly after completing participation. Patient 010 showed abnormal movements and seizure-like activity on EEG exam, but was maintained on Keppra and we cannot diagnose whether she truly experienced seizures during this study; if she did, it occurred at a young age. Seizures were reported to begin at an average of 17.9 months in an historic cohort1 and we observed them to begin at an average of 19 months in this cohort of patients.
Potentially corresponding to these clinical changes, we also observed new development of white matter tracts by DTI tractography at 3 months posttherapy. Neurodegeneration in GM2 is one of the most devastating and diffuse pathologies that occur during early infancy. The accumulation of storage material resulting from enzyme deficiency causes the brain size to markedly increase by 24 months of life17,20. Use of this early increase in brain size as a single prognostic marker is difficult, however, because the loss of viable neurons during development leads to a subsequent decrease in brain volume in this disease. Following gene therapy, our cohort attenuated brain growth (with the rate of increase dropping more than half) and increasing net fiber tract number, both suggesting less storage accumulation and improved connectivity. A recent study showed that patients with infantile GM2 gangliosidosis had a sharp increase in cerebellar cortex volume17. Interestingly, the two patients in our high-dose cohort showed an overall decrease in cerebellar cortical volume by 10%–15%, while the others in the study did not. Nonetheless, the partial effects of the therapy as administered here still left patients well below normal in neurocognitive function, indicating the need for continued improvements in the gene therapy.
By contrast, juvenile patients experienced the unexpected toxicity of a worsening movement disorder that appeared to be related to administration of gene therapy. Although movement disorders are known to occur in juvenile GM2, they are usually a less-common feature of overall decline (extrapyramidal signs were reported in 38% of juvenile patients with GM2 gangliosidosis)21. In juvenile GM2 gangliosidosis, the neuronal pathology in the CNS is generally less severe than that in the infantile form and tends to be more prominent in the hypothalamus, cerebellum, brain stem and spinal cord than in the cerebral cortex. In addition, neuronal storage material in late-onset cases tends to be more heterogenous, which may be impacting our outcomes and explain the emergence of dystonia22. It is unclear whether worsening dystonia is related to the vector, transgene expression and/or thalamic administration.
This emergence of a movement disorder following gene therapy is reminiscent of the symptoms encountered in patients with aromatic amino acid decarboxylase deficiency after bilateral intraputaminal infusions of eladocagene exuparvovec, an AAV2-aromatic amino acid decarboxylase vector8. In that study, 24 of 26 patients experienced dyskinesia, with most beginning within 1 month after intraputaminal infusion and resolving within the first 3 to 9 months. Of the three patients in this study who were noted to have movement disorder symptoms, one was initially described as ‘worsening of neurologic progression’ and ultimately succumbed to an intercurrent infection at 5 months post-vector before total resolution, one has persisted through the end of the study, and one has progressively improved and essentially had resolved by 29 months posttherapy. It is reassuring that this was not observed in the infantile patients with GM2 gangliosidosis in this study. Although the overall safety of the BiT infusion route of vector administration in infants supports the concept that this method of gene delivery could be useful for other genetic disorders that affect the CNS globally, questions remain around altered deep brain nuclei connectivity in juvenile patients and whether concentration of vector administration here could exacerbate symptoms.
One of the fundamental issues with gene therapy for global CNS disorders is the inability to deliver vector to all of the affected regions of the brain and spinal cord. Most recent gene therapy trials for genetic diseases with global CNS defects have involved either intravenous (IV) infusion of vector or delivery into the CSF space (IT, intra-CM or intraventricular). IV infusion of an AAV9 vector has been particularly efficacious in young infants with SMA1, crossing the blood–brain barrier at least to some extent23. However, SMA1 predominantly affects lower motor neurons, which appear to be more accessible by the IV route compared with the deep brain structures affected in GM2 gangliosidosis. CSF infusions of AAV vectors are being used in several other global CNS genetic disorders, and this route appears to be very promising based on preclinical and clinical data from our group and others9,14,24. However, where therapeutic efficacy requires transduction of neurons with two separate AAV constructs (for example, separate HEXA and HEXB vectors), the preclinical data favor the intrathalamic route because this route provides a high local concentration of both vectors in a relatively small volume of distribution11,13,14,25,26,27.
High-level transduction of the thalamus is intended to allow for axonal transport of both alpha and beta Hex subunits along thalamo-cortical tracts that are very broadly distributed throughout the cerebral cortex. This clinical trial demonstrates the feasibility and relative safety of the BiT approach, but further studies are needed to determine whether this route can achieve sufficient distribution in the CNS and therapeutic levels of transduction. Thalamic injection represents a potential approach to broad distribution in global CNS disorders, but uneven coverage may also exacerbate altered connectivity in disease states. Interestingly, the intracerebral GDNF gene therapy for Parkinson disease has found it difficult to achieve full coverage of the globus pallidus, and therefore altered the surgical approach to a posterior route of administration28. In our small pediatric population, we achieved precise targeting of the thalami bilaterally, but dystonic reactions in juvenile patients with residual enzyme activity suggests that high concentrations of gene delivery may be less well-tolerated.
The increase in serum total Hex activity into the normal range is another interesting finding. While the vector was delivered directly into the thalamus and CSF spaces, the fact that transaminase values were elevated in concert with T cell responses against the vector capsid suggests that a significant quantity of vector was distributed to the liver via the circulation. This, in turn, might suggest that the relatively high levels of total Hex activity in the serum could be attributable, at least in part, to vector expression in the liver. The vector transgene is driven by a ubiquitous promoter, so it is likely that liver cells were transduced and expressed HEXA and HEXB. In addition to the neurologic phenotypes, GM2 gangliosidosis affects peripheral tissues including the liver (hepatosplenomegaly is a feature of the disease)29, so systemic expression of the vector could produce additional benefits for patients.
It is interesting to note that despite a three-part immune suppressive regimen with rituximab, sirolimus and corticosteroids, each participant was observed to develop effector T cell responses with specificity for AAVrh8 capsid epitopes. In most instances these responses were accompanied by transaminase elevations above baseline values. In each case, an increase in corticosteroid dosage was sufficient to reverse transaminase elevations. None of the patients with these findings showed evidence of jaundice or of more-severe or persistent liver disease. This was quite reassuring, given the progressive nature of vector-induced liver abnormalities in patients with X-linked myotubular myopathy, another genetic disease in which baseline transaminase abnormalities are common. More detailed characterization of the immune responses to the AAVrh8 capsid and the transgene products are described a companion paper12.
Although encouraging, the results of this study also demonstrate important limitations of this therapeutic approach. First, the infusion of 1.25 ml of vector material into the parenchyma of the thalamus is likely at or near the maximum feasible dose. With less than complete clinical efficacy and a limited ability to increase the dose further, the clinical utility of this approach is far from optimal. The fact that this product was a mixture of two separate AAV vectors, one encoding HEXA and the other HEXB, prompted the BiT intraparenchymal approach based on the assumption that delivering the vectors together in a small physical structure would facilitate dual-vector transduction of individual neurons. Importantly, BiT delivery of both HEXA and HEXB was superior to delivery of HEXA alone in preclinical data in a Tay–Sachs sheep model9. Other possible approaches, such as IV or CSF-only delivery would entail a degree of dilution of the infusate that would disfavor dual transduction by the two vectors. In the interim, a single bicistronic AAV vector encoding both HEXA and HEXB has already been developed and shown to have robust clinical benefit in a Sandhoff mouse model, which has the potential to improve delivery efficiency30. We anticipate that the results of the trial published here will provide a basis for further examination of correlations between CSF biochemical markers, CNS imaging biomarkers and clinical benefits, including those of greatest value to patients and families such as alleviating seizures and maintaining eating by mouth.
Methods
Study design
This was originally designed to be a two-stage trial with stage 1 being a phase 1/2 dose-escalation, safety and bioefficacy study and stage 2 being a phase 2/3 clinical efficacy and safety study at the maximum tolerated dose. However, the corporate sponsor withdrew from the program after enrollment of the first five patients (one at the mid-dose level and none at the high-dose level), so the stage 1 portion of the study was completed under an investigator–sponsor of an investigational new drug (IND; IND no. 19314). All versions of the study as amended were approved by the Institutional Biosafety Committee of UMass Chan Medical School, WCG Institutional Review Board (IRB) and Massachusetts General Hospital IRB. Following modification of the trial, the outcomes were only measured over 24 weeks post-vector delivery. A Data and Safety Monitoring Committee (DSMC) was formed and coordinated by Advarra (a clinical research consultant). The DSMC and an independent medical monitor were provided with data updates as indicated in the study design (shown in Extended Data Fig. 1) and were informed promptly of all serious AEs.
The trial as performed was an open label, nonrandomized two-stage, dose-escalation and safety study of a single, two-part administration of rAAVrh8-HexA and rAAVrh8-HexB (the sequence of the vector is provided in the Supplementary Information). The vector was delivered by convection-enhanced injection into each thalamus bilaterally on day 1 followed by CSF delivery on day 2 using an IT catheter placed through the L3–L4 interspace, with 75% of the CSF dose injected with the catheter tip in the CM and 25% at the thoraco-lumbar junction. The trial cohorts were designed based on the assumption that the volume of infusion into the thalamus would be most likely to present dose-limiting AEs. The starting dose was equivalent to the dose given in a previous expanded access study16 with 180 μl of vector per thalamus, the low dose with 360 μl per thalamus, the mid dose with 720 μl per thalamus and the high dose with 1,250 μl per thalamus. The dose range was from 5.9 × 1012 to 4.1 × 1013 vg per thalamus and the total dose ranged from 1.42 × 1014 to 3.6 × 1014 vg per patient.
Patients were recruited into this study by referrals from physicians, patient advocacy groups or foundations and clinicaltrials.gov. Cohort sizes were determined based on the anticipated enrollment in consultation with the US Food and Drug Administration and patients were assigned to dose cohorts as they were enrolled in the study (the first patients were enrolled into the low-dose cohort and subsequent patients were enrolled into the mid- and high-dose cohorts). Both male and female patients were enrolled into this study, but sex was not considered when assigning patients to cohorts.
The study included both infantile (age 6–20 months) and juvenile (age 2–12 years) patients with TSD or SD, as confirmed by identification of two mutant alleles in either the HEXA (Tay–Sachs) or HEXB (Sandhoff) genes, along with clinical presentation consistent with the diagnosis and low Hex activity in serum. For inclusion, infantile patients were required to currently have or have previously acquired the ability to maintain a sitting position unassisted and to track and reach for objects. Based on the concern about the BiT infusion volumes, juvenile patients with GM2 gangliosidosis were the first enrolled after each dose-escalation step because of their larger brain volumes. However, the first three juvenile patients in the study experienced an unexpected worsening of dystonic movements after vector infusion, which was not seen with the infantile patients enrolled in this study. After noting this association and with the permission of the DSMC and the US Food and Drug Administration, we excluded juvenile patients from the remainder of the study.
Patients were treated with immune suppressive agents starting approximately 2 weeks before vector infusion consisting of an initial infusion of 10 mg kg−1 of methylprednisolone and infusion of the anti-CD20 monoclonal antibody rituximab at 375 mg m−2, followed by initiation of oral sirolimus at 1.5 mg m−2 d−1 and prednisolone at 1–2 mg kg−1 d−1 after receiving the infusion. The daily sirolimus dose was titrated to achieve a trough level between 7 and 12 ng ml−1 and it was continued at the full dose for 24 weeks, followed by a 4-week taper and discontinuation. Patients were treated with oral trimethoprim–sulfamethoxazole prophylaxis while on sirolimus. Oral prednisolone was maintained at 1 to 2 mg kg−1 d−1 to week 12, then tapered over 4 weeks before discontinuation. Patients received lansoprazole for peptic ulcer prophylaxis while on steroids. The adequacy of B cell depletion was confirmed by flow cytometry indicative of CD19 and CD20 cell numbers less than 5% of total peripheral blood lymphocytes. After vector delivery, patients were given intravenous immune globulin at a dose of 1,000 mg kg−1 as needed to maintain total immunoglobulin G serum levels at or above 500 mg dl−1.
The immune suppressive regimen, the two-part vector infusion, and the safety outcomes were managed at UMass Chan Medical School (the site of the trial) while the efficacy outcomes were assessed at Massachusetts General Hospital. The clinical efficacy assessment tools included those designed to measure neurologic, developmental and global clinical status (Supplementary Table 5). Neuroimaging outcomes including MRI, magnetic resonance spectroscopy and DTI were acquired and analyzed at both sites. Biochemical and molecular analyses were performed at UMass Chan Medical School and Washington University in St. Louis.
Outcomes
Primary endpoints
Part A.
-
The incidence, severity, seriousness and relatedness to treatment of treatment-emergent AEs were graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events v.5.0
Part B.
-
Surrogate biologic marker: Serum or CSF HexA activity change from baseline to visit 7 (month 3)
Clinical function:
-
Infantile-onset participants: Bayley III Scale of Infant Development Motor Score change from baseline to visit 9 (month 12)
-
Juvenile-onset participants: Six-minute walk test change from baseline to visit 9 (month 12)
-
Part C.
-
Adverse events
Secondary endpoints
Part A.
-
Patient health:
-
Changes in vital signs including weight
-
Changes in physical and neurologic examination
-
Changes in clinical laboratory tests including complete blood count with auto-differential, comprehensive metabolic panel and high sensitivity-C reactive protein
-
CD20 count
-
Electrocardiogram, EEG
-
Cellular responses and neutralizing antibody titers to the AAVrh8 capsid and HEXA and HEXB proteins as well as antibody levels to HEXA and HEXB proteins.
-
Part B.
-
Biologic markers of disease:
-
CSF HexA activity levels change from baseline to visit 9 (month 12)
-
Serum HexA activity levels change from baseline to visits 4, 5, 6, 7, 8 and 9
-
CSF GM2 ganglioside levels change from baseline (by liquid chromatography–tandem mass spectrometry) to visit 9 (month 12)
-
CSF levels of lactate dehydrogenase and aspartate aminotransferase change from baseline to visits 7 (month 3) and 9 (month 12)
-
-
Clinical function:
-
CHOP-INTEND Total Score change from baseline to visits 5, 7, 8 and 9
-
Bayley III Scale of Infant Development Composite score change from baseline to visits 5, 7, 8 and 9
-
Vineland-3 Adaptive Behavior composite standard score change from baseline to visits 5, 7, 8 and 9
-
Change from baseline in neurological symptoms based on clinical and developmental rating scale scores and a structured videotaped neurologic exam
-
Change from baseline in brainstem auditory evoked response, visual evoked potential and somatosensory evoked potential responses
-
MRI brain volume and DTI indices of myelination and brain water change from baseline to visits 7 and 8
-
Magnetic resonance spectroscopy indices of metabolite accumulation change from baseline to visits 7 and 8.
-
HexA enzyme assay
HexA activity was measured using a synthetic 4-methylumbelliferyl (4MU) fluorogenic substrate (4MU-6-sulfo-2-acetamido-2-deoxy-β-d-glucopyranoside; Toronto Research Chemicals) and a standard curve of 4MU (Sigma). Levels are expressed as nmol 4MU h−1 ml−1 of CSF. Samples (no dilution) were incubated at 37 °C for 3 h and reactions were stopped by addition of 0.2 ml of 1 M sodium carbonate, pH 10.7. Fluorescence was measured using a Synergy-HTX multimode reader (BioTek) using 360 and 460 nm excitation and emission filters, respectively.
Analysis of C18:0 and C20:0 ganglioside GM2
C18:0 and C20:0 GM2 gangliosides in CSF were analyzed using validated liquid chromatography–tandem mass spectrometry assay. The lower limit of quantification was 1 ng ml−1. Intra-assay precision (percent coefficient of variation) was 1.9% to 4.7%. Intra-assay accuracy (percent relative error) was −2.8% to 8.8%. Interassay precision (percent coefficient of variation) was 3.9% to 4.0%. Interassay accuracy (percent relative error) was −0.4% to 6.7%. As in previous studies16, the C20:0 GM2 levels were indicative of dynamic changes in ganglioside accumulation after gene therapy, so only C20:0 GM2 levels were used to assess treatment effects.
DTI analysis
DTI data were analyzed by differential tractography to identify neural tracts with improving myelination. Differential tractography is a participant-level analysis technique in which longitudinal FA throughout the entire brain is compared to calculate fiber tracts with substantial myelination or demyelination31. Differential tractography was performed in DSI Studio using a 20% FA threshold, the angular threshold was 60 and the step size was 1 mm, tracks <20 mm or >200 mm were discarded, and 1,000,000 seeds were placed32. A baseline whole brain fiber tractography was also performed to calculate a relative percentage change in fiber tract number and volume from the baseline evaluation. Net fiber tract metrics were calculated as the growth minus the loss32. In addition, region of interest analysis was performed by generating FA and radial diffusivity maps to further assess myelination changes.
Volumetric analysis
Brain and ventricular volumes were calculated for the patients with GM2 gangliosidosis based on a semiautomated segmentation method33. GM2 natural history data was incorporated in this analysis based on the data from ref. 17. The rate of volume growth was calculated for all patients and a linear mixed model analysis was performed to assess significant differences between the treated patients with GM2 gangliosidosis and the natural history data. In addition, for the GM2 treated patients, correlation analysis was performed between the volumetrics and the CGI scores.
Ethics statement
Ethical oversight of this study was provided by Western Institutional Review Board (Western IRB). The vector is being studied under IND no.19314. All versions of the study as amended were approved by the Institutional Biosafety Committee of UMass Chan Medical School, WCG IRB, and Massachusetts General Hospital IRB. All patients were enrolled into this study after informed consent was obtained from their parents/guardians. These trials are registered on clinicaltrials.gov under identifiers NCT04669535 and NCT06614569.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Individual datapoints are presented for all relevant data collected for each patient. All data from each patient that were used to draw conclusions from this study are included in Figs. 1–4, Tables 1 and 2 and Supplementary Information.
References
Bley, A. E. et al. Natural history of infantile GM2 gangliosidosis. Pediatrics 128, e1233–e1241 (2011).
Regier, D. S., Proia, R. L., D’Azzo, A. & Tifft, C. J. The GM1 and GM2 gangliosidoses: natural history and progress toward therapy. Pediatr. Endocrinol. Rev. 13, 663–673 (2016).
Day, J. W. et al. Onasemnogene abeparvovec gene therapy for symptomatic infantile-onset spinal muscular atrophy in patients with two copies of SMN2 (STR1VE): an open-label, single-arm, multicentre, phase 3 trial. Lancet Neurol. 20, 284–293 (2021).
Hwu, W.-L. et al. Gene therapy for aromatic l-amino acid decarboxylase deficiency. Sci. Transl. Med. 4, 134ra61 (2012).
Pearson, T. S. et al. Gene therapy for aromatic L-amino acid decarboxylase deficiency by MR-guided direct delivery of AAV2-AADC to midbrain dopaminergic neurons. Nat. Commun. 12, 4251 (2021).
Muramatsu, S. et al. A phase I study of aromatic L-amino acid decarboxylase gene therapy for Parkinson’s disease. Mol. Ther. 18, 1731–1735 (2010).
Kojima, K. et al. Gene therapy improves motor and mental function of aromatic l-amino acid decarboxylase deficiency. Brain 142, 322–333 (2019).
Tai, C.-H. et al. Long-term efficacy and safety of eladocagene exuparvovec in patients with AADC deficiency. Mol. Ther. 30, 509–518 (2022).
Gray-Edwards, H. L. et al. Adeno-associated virus gene therapy in a sheep model of Tay–Sachs disease. Hum. Gene Ther. 29, 312–326 (2018).
Bradbury, A. M. et al. Biomarkers for disease progression and AAV therapeutic efficacy in feline Sandhoff disease. Exp. Neurol. 263, 102–112 (2015).
Gray-Edwards, H. L. et al. Mucopolysaccharidosis-like phenotype in feline Sandhoff disease and partial correction after AAV gene therapy. Mol. Genet. Metab. 116, 80–87 (2015).
Flotte, T. R. & Keeler, A. M. Characterization of immune responses to rAAVrh8 gene therapy for GM2 gangliosidosis in phase 1/2 trial. Preprint at medRxiv https://doi.org/10.1101/2025.05.07.25327170 (2025).
McCurdy, V. J. et al. Therapeutic benefit after intracranial gene therapy delivered during the symptomatic stage in a feline model of Sandhoff disease. Gene Ther. 28, 142–154 (2021).
Rockwell, H. E. et al. AAV-mediated gene delivery in a feline model of Sandhoff disease corrects lysosomal storage in the central nervous system. ASN Neuro. 7, 175909141556990 (2015).
Schneck, L., Maisel, J. & Volk, B. W. The startle response and serum enzyme profile in early detection of Tay–Sachs’ disease. J. Pediatr. 65, 749–756 (1964).
Flotte, T. R. et al. AAV gene therapy for Tay–Sachs disease. Nat. Med. 28, 251–259 (2022).
Nestrasil, I. et al. Distinct progression patterns of brain disease in infantile and juvenile gangliosidoses: volumetric quantitative MRI study. Mol. Genet. Metab. 123, 97–104 (2018).
Jarnes Utz, J. R. et al. Infantile gangliosidoses: mapping a timeline of clinical changes. Mol. Genet. Metab. 121, 170–179 (2017).
Flynn, K. & Jussila, D. Voice of the patient report on GM2 gangliosidosis (Tay–Sachs and Sandhoff). Hum. Gene Ther. 35, 869–881 (2024).
Toro, C., Zainab, M. & Tifft, C. J. The GM2 gangliosidoses: unlocking the mysteries of pathogenesis and treatment. Neurosci. Lett. 764, 136195 (2021).
Maegawa, G. H. B. et al. The natural history of juvenile or subacute GM2 gangliosidosis: 21 new cases and literature review of 134 previously reported. Pediatrics 118, e1550–e1562 (2006).
Neudorfer, O. et al. Late-onset Tay–Sachs disease: phenotypic characterization and genotypic correlations in 21 affected patients. Genet. Med. 7, 119–123 (2005).
Mendell, J. R. et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N. Engl. J. Med. 377, 1713–1722 (2017).
Taghian, T. et al. A safe and reliable technique for CNS delivery of AAV vectors in the cisterna magna. Mol. Ther. 28, 411–421 (2020).
Johnson, A. K. et al. Life‐limiting peripheral organ dysfunction in feline Sandhoff disease emerges after effective CNS gene therapy. Ann. Neurol. 94, 969–986 (2023).
Bradbury, A. M. et al. AAV-mediated gene delivery attenuates neuroinflammation in feline Sandhoff disease. Neuroscience 340, 117–125 (2017).
McCurdy, V. J. et al. Widespread correction of central nervous system disease after intracranial gene therapy in a feline model of Sandhoff disease. Gene Ther. 22, 181–189 (2015).
Rocco, M. T. et al. Long-term safety of MRI-guided administration of AAV2-GDNF and gadoteridol in the putamen of individuals with Parkinson’s disease. Mol. Ther. 30, 3632–3638 (2022).
Moriwaki, S. et al. Histological observation of the brain of Tay–Sachs disease with seizure and chronic dph intoxication: report of an autopsy case. Acta Pathol. Jpn 27, 387–407 (1977).
Lahey, H. G. et al. Pronounced therapeutic benefit of a single bidirectional AAV vector administered systemically in Sandhoff mice. Mol. Ther. 28, 2150–2160 (2020).
Yeh, F.-C. et al. Differential tractography as a track-based biomarker for neuronal injury. NeuroImage 202, 116131 (2019).
Lewis, C. J., et al. Differential tractography: a biomarker for neuronal function in neurodegenerative disease. Preprint at medRxiv https://doi.org/10.1101/2024.08.25.24312255 (2024).
Zoppo, C. et al. Quantitative reliability assessment of brain MRI volumetric measurements in type II GM1 gangliosidosis patients. Front. Neuroimaging 3, 1410848 (2024).
Acknowledgements
We are grateful to the National Tay-Sachs & Allied Diseases Association (NTSAD), Mathew Forbes Rober Foundation (MFRF), Cure Tay-Sachs Foundation (CTSF), Blu Genes Foundation, and UMass Chan Horae Gene Therapy Center for funding this study. We thank S. Parajuli for assistance with carrying out this study, K. Strauss for expert medical consultation during this study, and B. Barton for consultation and advice before and during this study. We are especially grateful to the patients and families who volunteered for this trial.
Author information
Authors and Affiliations
Contributions
Conceptualization: F.E., O.I.C., M.S.-E., H.L.G.-E., T.R.F. Data curation: R.A., D.K. Formal analysis: F.E., O.I.C., R.D., T.T., X.J., M.S.S., C.J.L., B.V., M.B., E.-M.R., C.J.T., A.M.K., T.R.F. Funding acquisition: M.S.-E., H.L.G.-E., T.R.F. Investigation: F.E., O.I.C., R.D., A.S.P., T.T., X.J., A.K., A.H., H.C., Z.V., R.A., A.N., R.T., S.B., J.P., S.G.S., M.B., M.-A.A., E. D′Ambrosio, D.K., E. Drummond, E.L.T., H.M., T.R.F. Methodology: F.E., O.I.C., R.D., A.S.P., H.L.G.-E., X.J. Project administration: K.P., E.D.B., T.A.V. Resources: X.J., A.M.K. Supervision: F.E., O.I.C., C.J.T., A.M.K., M.S.-E., H.L.G.-E., T.R.F. Validation: F.E. Visualization: M.S.S., C.J.L. Writing—original draft: F.E., H.L.G.-E., T.R.F. Writing—review and editing: T.G., R.D., H.L.G.-E., T.R.F.
Corresponding authors
Ethics declarations
Competing interests
M.S.-E. may be entitled to receive licensing revenue from the patents covering the technologies used in this study. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks Guilherme Baldo and Steven Gray for their contribution to the peer review of this work. Primary Handling Editors: Anna Ranzoni; Jerome Staal, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Diagram of study design.
The study was designed to test the safety and bioactivity of a single two-part delivery of rAAVrh8-HexA/HexB (bilateral thalamic plus CSF) with the dose escalation based on the volume of vector delivered by convection-enhanced delivery to the thalamus on each side. The Starting Dose was equivalent to that in the previously published Expanded Access study. The dose per thalamus ranged from 5.9E + 12 to 4.1E + 13 vg/thalamus and the total dose ranged from 1.42E + 14 to 3.6E + 14 vg/patient.
Extended Data Fig. 2 Thalamic infusion dose escalation ranged from starting dose (180 mcl) to high dose (1250 mcl).
Infusion accuracy was seen in thalami bilaterally as displayed with T2/Flair hyperintensity. There was no significant bleeding, as demonstrated by susceptibility-weighted imaging (SWI). There was no significant stroke, as demonstrated by DWI imaging, except for one clinically insignificant caudate area of ischemia immediately post-operation from cannula placement (bottom right).
Extended Data Fig. 3 Volumetric measurements.
Volumetric measurements are shown for the brain and ventricles in GM2 infantile patients (Patients 001, 004, 007, 008, 010, 011) in the first column.
Extended Data Fig. 4 DTI measurements.
DTI measurements of the lentiform nucleus are shown for the infantile patients (Patients 001, 004, 007, 008, 010, 011).
Extended Data Fig. 5 Cerebellar cortex volumes.
Cerebellar cortex volumes of GM2 patients at the time of treatment and subsequently compared to natural history data of GM2 patients.
Extended Data Fig. 6 Neurologic and developmental outcomes.
Clinical Global Impression severity and change from baseline are shown for each patient.
Supplementary information
Supplementary Information
Supplemental Tables 1–6.
Vector Sequence 1
Vector sequence.
Vector Sequence 2
Vector sequence.
Study Protocol
Full study protocol.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Eichler, F., Cataltepe, O.I., Daci, R. et al. Dual-vector rAAVrh8 gene therapy for GM2 gangliosidosis: a phase 1/2 trial. Nat Med 31, 2927–2935 (2025). https://doi.org/10.1038/s41591-025-03822-4
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41591-025-03822-4
This article is cited by
-
Location matters for brain gene therapy in lysosomal disorders
Nature Medicine (2025)