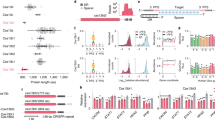
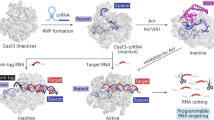
Abstract
Competitive coevolution between microbes and viruses has led to the diversification of CRISPR–Cas defense systems against infectious agents. By analyzing metagenomic terabase datasets, we identified two compact families (775 to 803 amino acids (aa)) of CRISPR–Cas ribonucleases from hypersaline samples, named Cas13X and Cas13Y. We engineered Cas13X.1 (775 aa) for RNA interference experiments in mammalian cell lines. We found Cas13X.1 could tolerate single-nucleotide mismatches in RNA recognition, facilitating prophylactic RNA virus inhibition. Moreover, a minimal RNA base editor, composed of engineered deaminase (385 aa) and truncated Cas13X.1 (445 aa), exhibited robust editing efficiency and high specificity to induce RNA base conversions. Our results suggest that there exist untapped bacterial defense systems in natural microbes that can function efficiently in mammalian cells, and thus potentially are useful for RNA-editing-based research.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All the sequencing data have been deposited in the NCBI SRA under project accession number PRJNA680488. Meta-information on all raw NGS datasets is provided in Supplementary Table 16. Source data are provided with this paper.
Code availability
Bioinformatics codes were deposited in the GitHub repository (https://github.com/yszhou2016/Cas13).
Change history
02 January 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41592-021-01379-x
References
Makarova, K. S. et al. An updated evolutionary classification of CRISPR-Cas systems. Nat. Rev. Microbiol. 13, 722–736 (2015).
Fonfara, I. et al. Phylogeny of Cas9 determines functional exchangeability of dual-RNA and Cas9 among orthologous type II CRISPR-Cas systems. Nucleic Acids Res. 42, 2577–2590 (2014).
Harrington, L. B. et al. Programmed DNA destruction by miniature CRISPR-Cas14 enzymes. Science 362, 839–842 (2018).
Pausch, P. et al. CRISPR-CasΦ from huge phages is a hypercompact genome editor. Science 369, 333–337 (2020).
Wang, D., Zhang, F. & Gao, G. CRISPR-based therapeutic genome editing: strategies and in vivo delivery by AAV vectors. Cell 181, 136–150 (2020).
Shmakov, S. et al. Discovery and functional characterization of diverse class 2 CRISPR-Cas systems. Mol. Cell 60, 385–397 (2015).
Abudayyeh, O. O. et al. C2c2 is a single-component programmable RNA-guided RNA-targeting CRISPR effector. Science 353, aaf5573 (2016).
Smargon, A. A., Shi, Y. J. & Yeo, G. W. RNA-targeting CRISPR systems from metagenomic discovery to transcriptomic engineering. Nat. Cell Biol. 22, 143–150 (2020).
Markowitz, V. M. et al. IMG: the integrated microbial genomes database and comparative analysis system. Nucleic Acids Res. 40, D115–D122 (2012).
O’Connell, M. R. Molecular mechanisms of RNA targeting by Cas13-containing type VI CRISPR-Cas systems. J. Mol. Biol. 431, 66–87 (2019).
Makarova, K. S. et al. Evolutionary classification of CRISPR-Cas systems: a burst of class 2 and derived variants. Nat. Rev. Microbiol. 18, 67–83 (2020).
Lynch, K. H., Stothard, P. & Dennis, J. J. Genomic analysis and relatedness of P2-like phages of the Burkholderia cepacia complex. BMC Genomics 11, 599 (2010).
Saridaki, A. et al. Wolbachia prophage DNA adenine methyltransferase genes in different Drosophila-Wolbachia associations. PLoS ONE 6, e19708 (2011).
Sternberg, N. & Coulby, J. Cleavage of the bacteriophage P1 packaging site (pac) is regulated by adenine methylation. Proc. Natl Acad. Sci. USA 87, 8070–8074 (1990).
Konermann, S. et al. Transcriptome engineering with RNA-targeting type VI-D CRISPR effectors. Cell 173, 665–676.e614 (2018).
Smargon, A. A. et al. Cas13b is a type VI-B CRISPR-associated RNA-guided RNase differentially regulated by accessory proteins Csx27 and Csx28. Mol. Cell 65, 618–630.e617 (2017).
Abudayyeh, O. O. et al. RNA targeting with CRISPR-Cas13. Nature 550, 280–284 (2017).
Wang, Q. et al. The CRISPR-Cas13a gene-editing system induces collateral cleavage of RNA in glioma cells. Adv. Sci. (Weinh.) 6, 1901299 (2019).
Abbott, T. R. et al. Development of CRISPR as an antiviral strategy to combat SARS-CoV-2 and influenza. Cell https://doi.org/10.1016/j.cell.2020.04.020 (2020).
Freije, C. A. et al. Programmable inhibition and detection of RNA viruses using Cas13. Mol. Cell 76, 826–837.e811 (2019).
Nguyen, T. M., Zhang, Y. & Pandolfi, P. P. Virus against virus: a potential treatment for 2019-nCov (SARS-CoV-2) and other RNA viruses. Cell Res. 30, 189–190 (2020).
Hulo, C. et al. ViralZone: a knowledge resource to understand virus diversity. Nucleic Acids Res. 39, D576–D582 (2011).
de Wit, E. et al. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc. Natl Acad. Sci. USA 117, 6771–6776 (2020).
Ruch, T. R. & Machamer, C. E. The coronavirus E protein: assembly and beyond. Viruses 4, 363–382 (2012).
Harding, A. T., Heaton, B. E., Dumm, R. E. & Heaton, N. S. Rationally designed influenza virus vaccines that are antigenically stable during growth in eggs. mBio https://doi.org/10.1128/mBio.00669-17 (2017).
Abudayyeh, O. O. et al. A cytosine deaminase for programmable single-base RNA editing. Science 365, 382–386 (2019).
Kushawah, G. et al. CRISPR-Cas13d induces efficient mRNA knockdown in animal embryos. Dev. Cell 54, 805–817 (2020).
He, B. et al. Modulation of metabolic functions through Cas13d-mediated gene knockdown in liver. Protein Cell https://doi.org/10.1007/s13238-020-00700-2 (2020).
Zhou, H. et al. Glia-to-neuron conversion by CRISPR-CasRx alleviates symptoms of neurological disease in mice. Cell https://doi.org/10.1016/j.cell.2020.03.024 (2020).
Zhou, C. et al. CasRx-mediated RNA targeting prevents choroidal neovascularization in a mouse model of age-related macular degeneration. Natl Sci. Rev. https://doi.org/10.1093/nsr/nwaa033 (2020).
Mehta, A. & Merkel, O. M. Immunogenicity of Cas9 protein. J. Pharm. Sci. 109, 62–67 (2020).
East-Seletsky, A. et al. Two distinct RNase activities of CRISPR-C2c2 enable guide-RNA processing and RNA detection. Nature 538, 270–273 (2016).
East-Seletsky, A., O’Connell, M. R., Burstein, D., Knott, G. J. & Doudna, J. A. RNA targeting by functionally orthogonal type VI-A CRISPR-Cas enzymes. Mol. Cell 66, 373–383.e373 (2017).
Cox, D. B. T. et al. RNA editing with CRISPR-Cas13. Science 358, 1019–1027 (2017).
Edgar, R. C. PILER-CR: fast and accurate identification of CRISPR repeats. BMC Bioinformatics 8, 18 (2007).
Hyatt, D. et al. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics 11, 119 (2010).
Katoh, K. & Standley, D. M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evolution 30, 772–780 (2013).
Kumar, S., Stecher, G. & Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evolution 33, 1870–1874 (2016).
Zhang, Y. I-TASSER server for protein 3D structure prediction. BMC Bioinformatics 9, 40 (2008).
Biswas, A., Gagnon, J. N., Brouns, S. J., Fineran, P. C. & Brown, C. M. CRISPRTarget: bioinformatic prediction and analysis of crRNA targets. RNA Biol. 10, 817–827 (2013).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. https://doi.org/10.14806/ej.17.1.200 (2011)
Clement, K. et al. CRISPResso2 provides accurate and rapid genome editing sequence analysis. Nat. Biotechnol. 37, 224–226 (2019).
Kluesner, M. G. et al. EditR: a method to quantify base editing from Sanger sequencing. CRISPR J. 1, 239–250 (2018).
Cox, M. P., Peterson, D. A. & Biggs, P. J. SolexaQA: at-a-glance quality assessment of Illumina second-generation sequencing data. BMC Bioinformatics 11, 485 (2010).
Kim, D., Langmead, B. & Salzberg, S. L. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods 12, 357–360 (2015).
Anders, S., Pyl, P. T. & Huber, W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Picardi, E. & Pesole, G. REDItools: high-throughput RNA editing detection made easy. Bioinformatics 29, 1813–1814 (2013).
Smigielski, E. M., Sirotkin, K., Ward, M. & Sherry, S. T. dbSNP: a database of single nucleotide polymorphisms. Nucleic Acids Res. 28, 352–355 (2000).
Gootenberg, J. S. et al. Nucleic acid detection with CRISPR-Cas13a/C2c2. Science 356, 438–442 (2017).
Tambe, A., East-Seletsky, A., Knott, G. J., Doudna, J. A. & O’Connell, M. R. RNA binding and HEPN-nuclease activation are decoupled in CRISPR-Cas13a. Cell Rep. 24, 1025–1036 (2018).
Acknowledgements
We thank M. Poo for discussions and comments on this manuscript; and the FACS facility, H. Wu and L. Quan at ION. This work was supported by the Basic Frontier Scientific Research Program of Chinese Academy of Sciences From 0 to 1 original innovation project (grant no. ZDBS-LY-SM001), the R&D Program of China (grant nos. 2017YFC1001300 and 2018YFC2000100), the CAS Strategic Priority Research Program (grant no. XDB32060000), the National Natural Science Foundation of China (grant nos. 31871502, 31925016, 91957122, 31901047, 82021001), the Shanghai Municipal Science and Technology Major Project (grant no. 2018SHZDZX05), the Shanghai City Committee of Science and Technology Project (grant nos. 18411953700, 18JC1410100, 19XD1424400, 19YF1455100) and the International Partnership Program of Chinese Academy of Sciences (grant no. 153D31KYSB20170059).
Author information
Authors and Affiliations
Contributions
C.X., Y.Z. and H.Y. conceived the project. C.X., Y.Z., Q.X., B.H. and G.G. designed and conducted experiments. Y.Z. and J.L. performed bioinformatics analysis. Z.W. and B.C. assisted with plasmids construction and RNA analysis. T.Y. assisted with cell experiments. X.W., D.Z. and X.H. assisted with virus experiments. H.Y. designed experiments and supervised the whole project. C.X., Y.Z. and H.Y. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
H.Y. is a founder of Hui-Gene Therapeutics. H.Y., C.X., Y.Z., and Q.X. are co-inventors on US patent application 16/864,982 relating to the Cas proteins described in this manuscript. The remaining authors declare no competing interests.
Additional information
Peer review information Nature Methods thanks the anonymous reviewers for their contribution to the peer review of this work. Lei Tang was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Computational pipeline for the identification of new CRISPR RNA-Targeting System Cas13 and protein schematics for Cas13 effectors.
a, Schematic describing a fast and classical computational pipeline for CRISPR system identification. In fast pipeline, a minimal definition for a putative class 2 CRISPR locus was used, requiring only a CRISPR repeat array and a nearby protein of 400 to 900 aa in length. b, Distribution of RNxxxH, RHxxxH, RQxxxH motifs among Cas13 proteins. All values are presented as percentage of motif occurrence (n = 180). c, Schematics describing protein-size and HEPN motif position of previously identified Cas13a/b/c/d and Cas13X/f proteins used in this study. The RxxxxH HEPN motif is highlighted. d, Similarity analysis between type E and F effectors by position-specific iterated BLAST. E value of >1e-10 defined as cutoff for non-significant similarity. e, HEPN domain alignment between type VI-X/Y and previously reported Cas13 effectors. Conserved residuals for Cas13X and Cas13Y were highlighted in pink and previously reported Cas13 in green.
Extended Data Fig. 2 Pre-crRNA processing mechanism of Cas13X.1 and effect of crRNA length and PFS on Cas13X.1 activity.
a, Procedure diagram of mCherry reporter inhibition assay for testing Cas13 activity in HEK293T. b, Schematics describing HEPN-active and -inactive Cas13X.1. The wild-type and inactivated mutant RxxxxH HEPN motifs are highlighted. c, Schematic diagram of potential mechanisms for pre-crRNA to mature crRNA processing by Cas13X.1. d, Reporter inhibition assay revealed ribonuclease-activity dependence on HEPN domains and crRNAs with 3’DR. e, Top, Schematic diagram showing the mCherry specific crRNAs with different spacer lengths. Bottom, Changes of mCherry fluorescence intensity for mCherry specific crRNAs of different lengths relative to non-targeting (NT) crRNA, as measured by flow cytometry. f, PFS analysis with crRNA targeting sequences flanked by different PFS. All values are presented as mean ± s.e.m (n = 3). P values are by two-sided unpaired t-test.
Extended Data Fig. 3 Comparison of knockdown activity between previously reported Cas13 and Cas13X.1/Cas13Y.1.
a, Relative target RNA knockdown by individual position-matched Cas13X.1 and RfxCasRx crRNAs. b, Cas13X.1 targeting efficiency of 12 endogenous transcripts, each with 3 guides and a non-targeting (NT) crRNA in HEK293T cells. c, Comparison of mCherry knockdown activity among Cas13X.1, Cas13Y.1, LwaCas13a, PspCas13b and RfxCas13d (n = 3 for each protein). d, Comparison of endogenous genes knockdown activity among Cas13X.1, Cas13Y.1, LwaCas13a, PspCas13b and RfxCas13d (n = 12 for each protein). *P < 0.05, ***P < 0.001, two-sided unpaired t-test. All values are presented as mean ± s.e.m (n = 3). P values are by two-sided unpaired t-test.
Extended Data Fig. 4 Genome-wide search for similar sequences with crRNA target from both B4GALNT1 and EZH2 gene predicted several potential off-target genes and volcano plot showed their differential expression significance.
Red and blue dot indicate significantly upregulated and downregulated off-target genes. Grey dot depicts non-significantly regulated off-target genes. Large blue dot indicates target gene, B4GALNT1 and EZH2.
Extended Data Fig. 5 Collateral activity comparison between Cas13X.1, RfxCas13d and LwaCas13a in HEK293T cells.
a, Schematics describing collateral activity detection system based on EGFP-transgenic HEK293T cells. b-e, Target knockdown and collateral activity for Cas13X.1, RfxCas13d and LwaCas13a with mCherry, endogenous ANXA4, B4GALNT1 and EZH2-targeting crRNAs. All values are presented as mean ± s.e.m (n = 3). P values are by two-sided unpaired t-test.
Extended Data Fig. 6 Biochemical characterization of on-target and collateral ribonuclease activity.
a, On-target ribonuclease activity comparison among LwaCas13a, RfxCas13d, Cas13X.1 and Cas13Y.1. b, Collateral ribonuclease activity comparison among LwaCas13a, RfxCas13d, Cas13X.1 and Cas13Y.1. AU, arbitrary unit. All Values shown are mean (n = 3).
Extended Data Fig. 7 Bioinformatics analysis for identifying minimal number of coronaviruses-targeting crRNAs.
a, Schematic describing a computational pipeline for identifying the minimal number of crRNAs targeting all coronaviruses by analyzing available coronavirus genomes. b,c, Histograms showing the predicted minimal number of 22-nt and 30-nt crRNAs with zero mismatch to target all sequenced 3,137 coronavirus genomes. d, Seventeen 30-nt crRNAs with single-nt mismatch in the minimal pool that targets all coronaviruses and their similarity with human transcriptome.
Extended Data Fig. 8 Verification of eABE function with mCherry* reporter system and predicted Cas13X.1 protein structure.
a, Schematic procedure of testing eABE with the mCherry* reporter system. b, Predicted protein structure of Cas13X.1. Yellow denote N terminal; green indicate C terminal; red represent HEPN motif.
Extended Data Fig. 9 Effect of mismatched base position on xABE activity and Sanger sequencing results of editing human endogenous transcripts by xABE.
a, Mismatch positions in different crRNAs targeting the mCherry amber mutation. b, Effect of mismatched base position with 50-nt spacer on A-to-I editing efficiency by both full and mini xABE editors. All values are presented as mean ± s.e.m (n = 3). c, Sanger sequencing results showing representative A-to-I conversion on endogenous transcripts by full and mini xABE editors. Red triangles indicate mutation sites.
Extended Data Fig. 10 Off-target RNA editing effect for xABE, mxABE, xCBE, mxCBE and REPAIR system.
a, Transcriptome-wide off-target sites numbers for GFP/mCherry (control), xABE, mxABE, xCBE and mxCBE transfection experiments in HEK293T cells. All values are presented as mean ± s.e.m (n = 3). b, Manhattan plots of transcriptome-wide off-target RNA editing analysis for REPAIR transfection experiments in HEK293T cells (A-to-I editor targeting endogenous SMAD4 RNA). The x and y axis are proportionally enlarged with each Manhattan plot to make the axis legend clear. Non-DR, guide RNA without direct repeats. NT, non-targeting crRNA.
Supplementary information
Supplementary Information
Supplementary gating strategy and experimental protocol.
Supplementary Table 1
Genomic positions and habitat information of Cas13X.1-X.2 and Cas13Y.1-Y5
Supplementary Table 2
Cas13X.1-X.2 and Cas13Y.1-Y.5 protein sequences used in the study
Supplementary Table 3
Type VI-X and VI-Y classification criteria
Supplementary Table 4
HEPN domains sequence alignment of type VI-A to VI-Y Cas13 proteins
Supplementary Table 5
Spacer sequences in Cas13X and Cas13Y associated CRISPR arrays
Supplementary Table 6
Natural crRNA targets
Supplementary Table 7
Significant differentially downregulated and upregulated genes by EZH2 knockdown with Cas13X.1
Supplementary Table 8
Significant differentially downregulated and upregulated genes by EZH2 knockdown with RfxCas13d
Supplementary Table 9
Significant differentially downregulated and upregulated genes by B4GALNT1 knockdown with Cas13X.1
Supplementary Table 10
Significant differentially downregulated and upregulated genes by B4GALNT1 knockdown with RfxCas13d
Supplementary Table 11
Primers used in the study
Supplementary Table 12
Predicted off-target sequences from hg38 genome for B4GALNT1 crRNA
Supplementary Table 13
Predicted off-target sequences from hg38 transcriptome for B4GALNT1 crRNA
Supplementary Table 14
Predicted off-target sequences from hg38 genome for EZH2 crRNA
Supplementary Table 15
Predicted off-target sequences from hg38 transcriptome for EZH2 crRNA
Supplementary Table 16
RNA-seq datasets information
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
About this article
Cite this article
Xu, C., Zhou, Y., Xiao, Q. et al. Programmable RNA editing with compact CRISPR–Cas13 systems from uncultivated microbes. Nat Methods 18, 499–506 (2021). https://doi.org/10.1038/s41592-021-01124-4
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41592-021-01124-4
This article is cited by
-
De novo design of hypercompact transcript degraders by engineering substrate-specific toxins and Cas6-CBS system
Nature Communications (2025)
-
RNA chemistry and therapeutics
Nature Reviews Drug Discovery (2025)
-
A high-fidelity CRISPR-Cas13 system improves abnormalities associated with C9ORF72-linked ALS/FTD
Nature Communications (2025)
-
Integrative analysis identifies the atypical repressor E2F8 as a targetable transcriptional activator driving lethal prostate cancer
Oncogene (2025)
-
Near-cognate tRNAs increase the efficiency and precision of pseudouridine-mediated readthrough of premature termination codons
Nature Biotechnology (2025)