Abstract
Spatial transcriptomics (ST) technologies have advanced to enable transcriptome-wide gene expression analysis at submicron resolution over large areas. However, analysis of high-resolution ST is often challenged by complex tissue structure, where existing cell segmentation methods struggle due to the irregular cell sizes and shapes, and by the absence of segmentation-free methods scalable to whole-transcriptome analysis. Here we present FICTURE (Factor Inference of Cartographic Transcriptome at Ultra-high REsolution), a segmentation-free spatial factorization method that can handle transcriptome-wide data labeled with billions of submicron-resolution spatial coordinates and is compatible with both sequencing-based and imaging-based ST data. FICTURE uses the multilayered Dirichlet model for stochastic variational inference of pixel-level spatial factors, and is orders of magnitude more efficient than existing methods. FICTURE reveals the microscopic ST architecture for challenging tissues, such as vascular, fibrotic, muscular and lipid-laden areas in real data where previous methods failed. FICTURE’s cross-platform generality, scalability and precision make it a powerful tool for exploring high-resolution ST.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The full results from the five real datasets are available in Zenodo via https://doi.org/10.5281/zenodo.10070621 (ref. 44). The Seq-Scope mouse colon data are publicly available in the Gene Expression Omnibus with the identifier GSE266556. The Stereo-seq pixel-level expression data were downloaded from the ‘Bin1 matrix’ section in the STOmicsDB (https://db.cngb.org/stomics/mosta/download/) for the embryo E16.5_E1S3. ‘Cell_bin’ matrix was also downloaded to obtain the raw expression data. The published annotations of segmented cells were obtained from the ‘Embryo data’ section (E16.5_E1S3_cell_bin.h5ad). The Xenium breast cancer dataset was downloaded from https://www.10xgenomics.com/resources/datasets/ffpe-human-breast-with-pre-designed-panel-1-standard/. The Xenium healthy lung preview dataset was downloaded from https://www.10xgenomics.com/resources/datasets/xenium-human-lung-preview-data-1-standard/. The MERSCOPE mouse liver data were downloaded from https://info.vizgen.com/mouse-liver-access/ (sample L1R1 was used for the analysis).
Code availability
The source code and Python package for FICTURE method are publicly available in the GitHub repository at https://github.com/seqscope/ficture/.
References
Bressan, D., Battistoni, G. & Hannon, G. J. The dawn of spatial omics. Science 381, eabq4964 (2023).
Moses, L. & Pachter, L. Museum of spatial transcriptomics. Nat. Methods https://doi.org/10.1038/s41592-022-01409-2 (2022).
Kang, H. M. & Lee, J. H. Spatial single-cell technologies for exploring gastrointestinal tissue transcriptome. Compr. Physiol. https://doi.org/10.1002/cphy.c210053 (2023).
Ståhl, P. L. et al. Visualization and analysis of gene expression in tissue sections by spatial transcriptomics. Science 353, 78–82 (2016).
Liu, Y. et al. High-spatial-resolution multi-omics sequencing via deterministic barcoding in tissue. Cell 183, 1665–1681.e18 (2020).
Stickels, R. R. et al. Highly sensitive spatial transcriptomics at near-cellular resolution with Slide-seqV2. Nat. Biotechnol. 39, 313–319 (2021).
Vickovic, S. et al. High-definition spatial transcriptomics for in situ tissue profiling. Nat. Methods 16, 987–990 (2019).
Cho, C.-S. et al. Microscopic examination of spatial transcriptome using Seq-Scope. Cell 184, 3559–3572.e22 (2021).
Chen, A. et al. Spatiotemporal transcriptomic atlas of mouse organogenesis using DNA nanoball-patterned arrays. Cell https://doi.org/10.1016/j.cell.2022.04.003 (2022).
Fu, X. et al. Polony gels enable amplifiable DNA stamping and spatial transcriptomics of chronic pain. Cell 185, 4621–4633.e17 (2022).
Zhang, M. et al. Spatially resolved cell atlas of the mouse primary motor cortex by MERFISH. Nature 598, 137–143 (2021).
Janesick, A. et al. Hiigh resolution mapping of the tumor microenvironment using integrated single-cell, spatial and in situ analysis. Nat. Commun. 14, 8353 (2022).
He, S. et al. High-plex imaging of RNA and proteins at subcellular resolution in fixed tissue by spatial molecular imaging. Nat. Biotechnol. 40, 1794–1806 (2022).
Beucher, S. The watershed transformation applied to image segmentation. Scanning Microsc. 6, 299–314 (1992).
Stringer, C., Wang, T., Michaelos, M. & Pachitariu, M. Cellpose: a generalist algorithm for cellular segmentation. Nat. Methods 18, 100–106 (2021).
Palla, G. et al. Squidpy: a scalable framework for spatial omics analysis. Nat. Methods 19, 171–178 (2022).
Dries, R. et al. Giotto: a toolbox for integrative analysis and visualization of spatial expression data. Genome Biol. 22, 78 (2021).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587.e29 (2021).
Long, Y. et al. Spatially informed clustering, integration, and deconvolution of spatial transcriptomics with GraphST. Nat. Commun. 14, 1155 (2023).
Gruner, H. N. & McManus, M. T. Examining the evidence for extracellular RNA function in mammals. Nat. Rev. Genet. 22, 448–458 (2021).
Sacher, F., Feregrino, C., Tschopp, P. & Ewald, C. Y. Extracellular matrix gene expression signatures as cell type and cell state identifiers. Matrix Biol. Plus 10, 100069 (2021).
Park, J. et al. Cell segmentation-free inference of cell types from in situ transcriptomics data. Nat. Commun. 12, 3545 (2021).
Xi, J., Lee, J. H., Kang, H. M. & Jun, G. STtools: a comprehensive software pipeline for ultra-high-resolution spatial transcriptomics data. Bioinform. Adv. 2, vbac061 (2022).
Petukhov, V. et al. Cell segmentation in imaging-based spatial transcriptomics. Nat. Biotechnol. 40, 345–354 (2022).
Littman, R. et al. Joint cell segmentation and cell type annotation for spatial transcriptomics. Mol. Syst. Biol. 17, e10108 (2021).
Miller, B. F., Huang, F., Atta, L., Sahoo, A. & Fan, J. Reference-free cell type deconvolution of multi-cellular pixel-resolution spatially resolved transcriptomics data. Nat. Commun. 13, 2339 (2022).
Wolf, F. A., Angerer, P. & Theis, F. J. SCANPY: large-scale single-cell gene expression data analysis. Genome Biol. 19, 15 (2018).
Birkl, D. et al. TNF-α promotes mucosal wound repair through enhanced platelet activating factor receptor signaling in the epithelium. Mucosal Immunol. 12, 909–918 (2019).
Leoni, G., Neumann, P.-A., Sumagin, R., Denning, T. L. & Nusrat, A. Wound repair: role of immune-epithelial interactions. Mucosal Immunol. 8, 959–968 (2015).
Schaum, N. et al. Single-cell transcriptomics of 20 mouse organs creates a Tabula Muris. Nature 562, 367–372 (2018).
Blondel, V. D., Guillaume, J.-L., Lambiotte, R. & Lefebvre, E. Fast unfolding of communities in large networks. J. Stat. Mech. Theory Exp. 2008, P10008 (2008).
Traag, V. A., Waltman, L. & van Eck, N. J. From Louvain to Leiden: guaranteeing well-connected communities. Sci. Rep. 9, 5233 (2019).
Bora, P. & Majumdar, A. S. Adipose tissue-derived stromal vascular fraction in regenerative medicine: a brief review on biology and translation. Stem Cell Res. Ther. 8, 145 (2017).
Choi, J., Cha, Y. J. & Koo, J. S. Adipocyte biology in breast cancer: from silent bystander to active facilitator. Prog. Lipid Res. 69, 11–20 (2018).
Anderson, N. M. & Simon, M. C. The tumor microenvironment. Curr. Biol. 30, R921–R925 (2020).
Liu, C. C. et al. Robust phenotyping of highly multiplexed tissue imaging data using pixel-level clustering. Nat. Commun. 14, 4618 (2023).
Blei, D. M., Kucukelbir, A. & McAuliffe, J. D. Variational inference: a review for statisticians. J. Am. Stat. Assoc. 112, 859–877 (2017).
Chen, D. et al. Measuring and relieving the over-smoothing problem for graph neural networks from the topological view. AAAI 34, 3438–3445 (2020).
Hoffman, M. D., Blei, D. M., Wang, C. & Paisley, J. Stochastic variational inference. J. Mach. Learn. Res. 14, 1303–1347 (2013).
Blei, D. M., Ng, A. Y. & Jordan, M. I. Latent Dirichlet allocation. J. Mach. Learn. Res. 3, 993–1022 (2003).
Do, T. H. et al. TREM2 macrophages induced by human lipids drive inflammation in acne lesions. Sci. Immunol. 7, eabo2787 (2022).
Xu, Z. et al. STOmicsDB: a comprehensive database for spatial transcriptomics data sharing, analysis and visualization. Nucleic Acids Res. 52, 1053–1061 (2023).
Hafemeister, C. & Satija, R. Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol. 20, 296 (2019).
Si, Y. Data from “FICTURE: scalable segmentation-free analysis of sub-micron resolution spatial transcriptomics” (1.0). Zenodo https://doi.org/10.5281/zenodo.10070621 (2023).
Acknowledgements
The work was supported by the Taubman Institute (to W.C., Y.H., C.C., H.M.K. and J.H.L.), an endowment fund from the Dr. and Mrs. James Robert Spencer Family (to W.Z.), DOD/CDMRP grant W81XWH2110005 (to W.Z.) and the National Institutes of Health grants R01HG011031 (to Y.S., H.M.K. and S.Z.), UH3CA268091 (to J.H.L.), R01DK133448 (to J.H.L.), R01AG079163 (to J.H.L.), CA269661 (to W.Z.), CA290792 (to W.Z.) and CA260239 (to W.Z.).
Author information
Authors and Affiliations
Contributions
Y.S., G.J., J.H.L. and H.M.K. formulated the study. Y.S. developed the method, implemented the FICTURE package and performed simulation studies, with feedback from G.J., S.Z., J.H.L. and H.M.K. M.Q., A.N., C.C. and J.H.L. generated the Seq-Scope mouse colon dataset. C.H.L., J.H.Y., W.Z., A.N. and J.H.L. provided detailed feedback on the individual results of real datasets performed by Y.S., Y.H. and W.C., and H.M.K. implemented and executed software tools to process raw data and visualize the results, contributing to improving the method. Y.S., J.H.L. and H.M.K. drafted the manuscript, with contributions by C.H.L., J.H.Y., G.J. and S.Z. All authors provided suggestions and comments on the manuscript text.
Corresponding authors
Ethics declarations
Competing interests
J.H.L. is an inventor on a patent and pending patent applications related to Seq-Scope, which is indirectly relevant to the work presented in this paper. The following disclosures are unrelated to the current study: H.M.K. owns stock in Regeneron Pharmaceuticals; J.H.Y. serves as a consultant for Bridge Biotherapeutics and as a scientific advisory board member for Genentech; C.H.L. began employment with Somite Therapeutics after the initial submission of this manuscript; Y.H. began employment with Samsung Semiconductor after the initial submission of this manuscript. All other authors declare no competing interests.
Peer review
Peer review information
Nature Methods thanks Zhangsheng Yu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available. Primary Handling Editor: Rita Strack, in collaboration with the Nature Methods team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Generative Models used in FICTURE.
(a) The generative model for pixel-level inference used in FICTURE. Shaded circles represent observed data. (\({x}_{j},{x}_{i}\)) represent spatial locations of anchors and pixels; \(\widetilde{{y}_{i}}\) represents gene counts for pixel i. The black-outlined circles (\({{\eta }},\,{\rm{\alpha }}\)) represent hyperparameters for the Dirichlet priors. The orange-outlined circles (\({{{\beta }}}_{k}\)) represent factor level expression distribution and the green-outlined circles (\({{\rm{\theta }}}_{j}\)) represent the anchor level factor proportions. The blue-outlined circles (\({z}_{i},{c}_{i}\)) represent latent factor and latent anchor assignments for each pixel. We provide the typical range of the number of factors (K), anchor points (n), and pixels (N) next to the corresponding boxes. (b) Latent Dirichlet Allocation model used in the fully unsupervised FICTURE. The standard LDA model is used to infer factors (\({{{\beta }}}_{k}\)) to model gene counts for each ‘spot’ and the learned factors are used as input to part (A). Each ‘spot’ level gene count (\(\widetilde{{y}_{i,j}}\)) is generated using a fixed-sized hexagonal grid. \({\Theta }_{j}\) represents the probabilistic distribution over K factors for spot j. \({z}_{{ij}}\) represents the latent factor of pixel i in spot j.
Extended Data Fig. 2 Overview of the Datasets Analyzed.
A tabular summary of the datasets analyzed in this manuscript, in order of appearance. Large datasets ( > 10mm2) were analyzed with FICTURE only since they are not analyzable by other methods. Simulated datasets and smaller subset of real datasets were analyzed with multiple methods. For simulated datasets, only the values from the largest dataset to which the corresponding methods are applied was presented in this table.
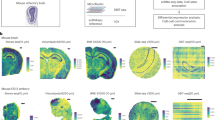
Extended Data Fig. 3 Additional analysis on the Seq-scope mouse colon dataset.
(a) H&E (top) and RNA density (bottom) of the full dataset including 9 tissue sections. (b) A dot plot visualizing the marker genes in the Seq-Scope mouse colon dataset. The x-axis represents the marker genes identified from Seurat, and the y-axis represent different clusters with their annotated cell types. The colors represent the fold enrichment of average expression levels of each gene compared to the rest of the cell types. The size of dot represents the relative expression of the marker gene among all genes in each cluster. (c) FICTURE’s pixel-level decoding result of the whole data (top) and the magnified view in each section (bottom). The full color codes and marker genes of each factor are shown in Supplementary Table 1. These analyses were performed once as the results are deterministic given identified factors.
Extended Data Fig. 4 Benchmarking analysis under various simulation settings.
(a) Cell shape parameters used in all benchmarking simulations for comparison between FICTURE, Baysor and GraphST. (b) The pixel level error rate under two scenarios with different cell packing settings across 20 simulations with randomly sampled cell types. Apart from the three methods compared in the main results, we included grid-based LDA analysis performed on hexagons. (c-h) A visualization similar to Fig. 3b, but instead of simulating transcripts assuming sparse background outside cell boundaries, this simulation uses the uniform transcript density across the entire region. We refer to this simulation as ‘densely packed’ while ‘sparse background’ represents the simulation in Fig. 3b. (c-e): All pixels are visualized with colors representing their inferred factors from (c) FICTURE, (d) Baysor, and (e) Graph ST. (f-h): Pixels where the factors inferred from (f) FICTURE, (g) Baysor, and (h) GraphST differ from the ground truth are visualized with colors representing their true generating factors.
Extended Data Fig. 5 The effect of anchor density on FICTURE performance.
(a) A representative example of the simulated spatial pattern with random cell type assignment. Colors indicate cell types. (b) Sliding hexagonal grids that generate the anchors. Top left shows a regular hexagonal tiling of the space. Black lines represent the axes along which we will slide the grid the yellow line segment marks the distance (d) between the centers of adjacent hexagons. The other three subplots on the left show the three possible tiling when we slide the grid with a step size s = d/2. The subplot on the right shows the resulting anchors, which are centers of the hexagons, where the distance between adjacent anchors equals to s. (c) The pixel level cell type assignment error rate (y-axis, with different ranges across rows) across analyses with different hexagon sizes (color) and distances between adjacent anchors (x-axis), stratified by the transcript density of the simulation (rows) and the shape of the simulated cells where the error is located (columns). ‘Background’ means the pixels located outside of the simulated cell boundaries. The transcript density outside cells is 20% of that within cells. The transcript densities indicated in the figure are the within-cell densities. (d) Total runtime (in seconds) of FICTURE when using different number of anchors in a 0.5 mm x 0.5 mm area, stratified by the transcript density. Data are presented as mean +/- SD with n = 10 in (C, D).
Extended Data Fig. 6 Comparison between FICTURE and JSTA on simulated data.
(a) We simulated the data using the simulation code provided in JSTA publication. We simulated a 200 µm x 200 µm area with 2 pixels per µm. We simulated 8 cell types from the single cell reference data as used in JSTA publication. (b) Runtime (left, wall time) and maximum memory usage (right) of the two methods, presented as mean +/- SD. We used 4 CPUs for both methods and repeated the simulation 20 times. (c) Visualization of the pixel level cell type assignment errors of FICTURE (left) and JSTA (middle). Pixels classified correctly are left as white while the errors are visualized with colors corresponding to true cell types. (d) Visualization of the pixel level cell (nuclei) assignment errors of FICTURE (left) and JSTA (middle). The mean +/- SD of the cell type and cell assignment error rate over n = 20 simulations are shown on the right side of (C) and (D), respectively.
Extended Data Fig. 7 Limitations of DAPI-based cell segmentation in Stereo-seq dataset.
(a) DAPI staining images of Stereo-seq E16.5 (E1S3) mouse embryo. (b) Spatial distribution of erythrocyte from the published 25-cluster annotation based on cell segmentation. (c) Spatial distribution of erythrocyte factor from FICTURE. (d) A magnified view of DAPI image focusing on the heart and blood vessel. (e) A corresponding magnified view of published annotation. (f) A corresponding magnified view of FICTURE factors. (g) Proportion of transcripts assigned to cells within each tissue area. The 23 tissue areas are annotated based on the published grid-based segmentation. The full color codes and marker genes of each factor are shown in Supplementary Table 1. These analyses were performed at least three times independently, producing qualitatively similar results.
Extended Data Fig. 8 Proportion of transcripts unassigned to any cell during staining-based cell segmentation.
Each gene is plotted based on the total number of transcripts in the whole dataset (x-axis) and the proportion of the transcripts excluded after the cell segmentation (y-axis). Each gene is colored by the tissue or factor that it is most enriched in. (a) Stereo-seq E16 mouse embryo data across 30,050 genes. The 23 tissue annotations are from the published grid-based segmentation. The top 40 most excluded genes (with >10,000 transcripts) are enriched in liver (Hba-a2, Alas2, Hba-a1, Hbb-bs, Hbb-bt, Ube216, Mkrn1, Apex2, Trim71, Bpgm, Snca, Fam220a, Hbb-bh1), heart (Myl7,Tnni3, Myh6, Myl3, Myl2, Myh2, Pln, Tnnt2, Csrp3, Sh3bgr, Cox7a1, Ankrd1, Mybpc3), blood vessel (Hba-x, Hba-y), epidermis (Krt77), and muscle (Ckm, Cox8b, Tnni2, Myl4, Cox6a2, Tceal7, Acta1, Myh8, Mylpf, Synpo2l, Tnnt3). (b) 10x Xenium human breast cancer data across 313 genes analyzed with 20 factors inferred from FICTURE. (c) Vizgen mouse liver data across 347 genes analyzed with 24 factors inferred from FICTURE. The factors in (B) and (C) are annotated based on marker genes and spatial distribution.
Extended Data Fig. 9 Comparison of FICTURE and other methods on the Vizgen mouse liver dataset.
A small (0.46mm2) subset of the Vizgen mouse liver data containing both central and portal veins are selected to compare FICTURE with Baysor and GraphST. All methods are run with 12 factors/clusters. (a) Pixel-level output from FICTURE, (b) Pixel-level output from Baysor, and (c) Cell-level output from GraphST, rendered at pixel resolution based on cell segmentation. Magnified views of portal veins for (d) FICTURE, (e) Baysor, and (f) GraphST. Magnified views of central veins for (g) FICTURE, (h) Baysor, and (i) GraphST.
Extended Data Fig. 10 Analysis of Human Lung Preview Dataset.
(a) Pixel-level visualization of inferred factor by FICTURE on a healthy human lung tissue assayed by 10x Xenium platform. An upper-left subsection was magnified in the inset. (b) Further magnified view of FICTURE’s pixel-level inference on a peribronchial region showing a signature of immune infiltration (orange in the lower center). (c) Corresponding view in the 10x Xenium Explorer based on staining-based cell segmentation, each segmented cell colored by its assigned cluster. (d) DAPI image of the same region. (e) Gene-level visualization of the same region, focusing on CD8A, CD27, IL7R, and CD28 in different colors. (f) Gene level visualization of GZMK. The full color codes and marker genes of each factor are shown in Supplementary Table 1.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Si, Y., Lee, C., Hwang, Y. et al. FICTURE: scalable segmentation-free analysis of submicron-resolution spatial transcriptomics. Nat Methods 21, 1843–1854 (2024). https://doi.org/10.1038/s41592-024-02415-2
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41592-024-02415-2
This article is cited by
-
High-definition spatial transcriptomic profiling of immune cell populations in colorectal cancer
Nature Genetics (2025)
-
Spatial architecture of development and disease
Nature Reviews Genetics (2025)