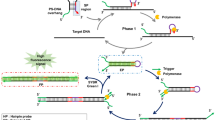
Abstract
In situ hybridization (ISH) is a powerful tool for investigating the spatial arrangement of nucleic acid targets in fixed samples. ISH is typically visualized using fluorophores to allow high sensitivity and multiplexing or with colorimetric labels to facilitate covisualization with histopathological stains. Both approaches benefit from signal amplification, which makes target detection effective, rapid and compatible with a broad range of optical systems. Here, we introduce a unified technical platform, termed ‘pSABER’, for the amplification of ISH signals in cell and tissue systems. pSABER decorates the in situ target with concatemeric binding sites for a horseradish peroxidase-conjugated oligonucleotide, enabling the localized deposition of fluorescent or colorimetric substrates. We demonstrate that pSABER effectively labels DNA and RNA targets in cultured cells and FFPE specimens. Furthermore, pSABER can achieve fivefold signal amplification over conventional signal amplification by exchange reaction (SABER) and can be serially multiplexed using solution exchange. Therefore, by linking nucleic acid detection to robust signal amplification capable of diverse readouts, pSABER will have broad utility in research and clinical settings.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Primary microscopy data, which have a substantial disk footprint, will be made available upon request. Source data are provided with this paper.
Code availability
The source code for the automated analysis of peak puncta intensities as well as example input images is available at https://github.com/beliveau-lab/SABER-Spot-Quant under an MIT license.
References
Pardue, M. L. & Gall, J. G. Molecular hybridization of radioactive DNA to the DNA of cytological preparations. Proc. Natl Acad. Sci. USA 64, 600–604 (1969).
Riegel, M. Human molecular cytogenetics: from cells to nucleotides. Genet. Mol. Biol. 37, 194–209 (2014).
Raj, A., van den Bogaard, P., Rifkin, S. A., van Oudenaarden, A. & Tyagi, S. Imaging individual mRNA molecules using multiple singly labeled probes. Nat. Methods 5, 877–879 (2008).
Solovei, I. et al. Spatial preservation of nuclear chromatin architecture during three-dimensional fluorescence in situ hybridization (3D-FISH). Exp. Cell Res. 276, 10–23 (2002).
Rosen, B. & Beddington, R. S. Whole-mount in situ hybridization in the mouse embryo: gene expression in three dimensions. Trends Genet. 9, 162–167 (1993).
Bergmann, J. H. et al. Regulation of the ESC transcriptome by nuclear long noncoding RNAs. Genome Res. 25, 1336–1346 (2015).
Tripathi, V., Fei, J., Ha, T. & Prasanth, K. V. RNA fluorescence in situ hybridization in cultured mammalian cells. In Regulatory Non-Coding RNAs: Methods and Protocols (ed. Carmichael, G. G.) 123–136 (Springer, 2015).
Chang, K.-C. et al. MaTAR25 lncRNA regulates the Tensin1 gene to impact breast cancer progression. Nat. Commun. 11, 6438 (2020).
Chen, K. H., Boettiger, A. N., Moffitt, J. R., Wang, S. & Zhuang, X. RNA imaging. Spatially resolved, highly multiplexed RNA profiling in single cells. Science 348, aaa6090 (2015).
Shah, S., Lubeck, E., Zhou, W. & Cai, L. In situ transcription profiling of single cells reveals spatial organization of cells in the mouse hippocampus. Neuron 92, 342–357 (2016).
Rudkin, G. T. & Stollar, B. D. High resolution detection of DNA–RNA hybrids in situ by indirect immunofluorescence. Nature 265, 472–473 (1977).
Bauman, J. G. J., Wiegant, J., Borst, P. & van Duijn, P. A new method for fluorescence microscopical localization of specific DNA sequences by in situ hybridization of fluorochrome-labelled RNA. Exp. Cell Res. 128, 485–490 (1980).
Langer-Safer, P. R., Levine, M. & Ward, D. C. Immunological method for mapping genes on Drosophila polytene chromosomes. Proc. Natl Acad. Sci. USA 79, 4381–4385 (1982).
Rigby, P. W. J., Dieckmann, M., Rhodes, C. & Berg, P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J. Mol. Biol. 113, 237–251 (1977).
Femino, A. M., Fay, F. S., Fogarty, K. & Singer, R. H. Visualization of single RNA transcripts in situ. Science 280, 585–590 (1998).
Yamada, N. A. et al. Visualization of fine-scale genomic structure by oligonucleotide-based high-resolution FISH. Cytogenet. Genome Res. 132, 248–254 (2011).
Boyle, S., Rodesch, M. J., Halvensleben, H. A., Jeddeloh, J. A. & Bickmore, W. A. Fluorescence in situ hybridization with high-complexity repeat-free oligonucleotide probes generated by massively parallel synthesis. Chromosome Res. 19, 901–909 (2011).
Beliveau, B. J. et al. Versatile design and synthesis platform for visualizing genomes with Oligopaint FISH probes. Proc. Natl Acad. Sci. USA 109, 21301–21306 (2012).
Lubeck, E., Coskun, A. F., Zhiyentayev, T., Ahmad, M. & Cai, L. Single-cell in situ RNA profiling by sequential hybridization. Nat. Methods 11, 360–361 (2014).
Wang, S. et al. Spatial organization of chromatin domains and compartments in single chromosomes. Science 353, 598–602 (2016).
Jensen, E. Technical review: in situ hybridization. Anat. Rec. 297, 1349–1353 (2014).
McNicol, A. M. & Farquharson, M. A. In situ hybridization and its diagnostic applications in pathology. J. Pathol. 182, 250–261 (1997).
Player, A. N., Shen, L. P., Kenny, D., Antao, V. P. & Kolberg, J. A. Single-copy gene detection using branched DNA (bDNA) in situ hybridization. J. Histochem. Cytochem. 49, 603–611 (2001).
Wang, F. et al. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J. Mol. Diagn. 14, 22–29 (2012).
Beliveau, B. J. et al. Single-molecule super-resolution imaging of chromosomes and in situ haplotype visualization using Oligopaint FISH probes. Nat. Commun. 6, 7147 (2015).
Choi, H. M. T. et al. Programmable in situ amplification for multiplexed imaging of mRNA expression. Nat. Biotechnol. 28, 1208–1212 (2010).
Rouhanifard, S. H. et al. ClampFISH detects individual nucleic acid molecules using click chemistry-based amplification. Nat. Biotechnol. 37, 84–89 (2018).
Dardani, I. et al. ClampFISH 2.0 enables rapid, scalable amplified RNA detection in situ. Nat. Methods 19, 1403–1410 (2022).
Kerstens, H. M., Poddighe, P. J. & Hanselaar, A. G. A novel in situ hybridization signal amplification method based on the deposition of biotinylated tyramine. J. Histochem. Cytochem. 43, 347–352 (1995).
Schulte-Merker, S., Ho, R. K., Herrmann, B. G. & Nüsslein-Volhard, C. The protein product of the zebrafish homologue of the mouse T gene is expressed in nuclei of the germ ring and the notochord of the early embryo. Development 116, 1021–1032 (1992).
Lizardi, P. M. et al. Mutation detection and single-molecule counting using isothermal rolling-circle amplification. Nat. Genet. 19, 225–232 (1998).
Kishi, J. Y. et al. SABER amplifies FISH: enhanced multiplexed imaging of RNA and DNA in cells and tissues. Nat. Methods 16, 533–544 (2019).
Kishi, J. Y., Schaus, T. E., Gopalkrishnan, N., Xuan, F. & Yin, P. Programmable autonomous synthesis of single-stranded DNA. Nat. Chem. 10, 155–164 (2017).
Sarrab, R. M. et al. Establishment of conditionally immortalized human glomerular mesangial cells in culture, with unique migratory properties. Am. J. Physiol. Renal Physiol. 301, F1131–F1138 (2011).
Hershberg, E. A. et al. PaintSHOP enables the interactive design of transcriptome- and genome-scale oligonucleotide FISH experiments. Nat. Methods 18, 937–944 (2021).
Rouquette, J., Choesmel, V. & Gleizes, P.-E. Nuclear export and cytoplasmic processing of precursors to the 40S ribosomal subunits in mammalian cells. EMBO J. 24, 2862–2872 (2005).
Uhlén, M. et al. Proteomics. Tissue-based map of the human proteome. Science 347, 1260419 (2015).
Yildirim, E., Sadreyev, R. I., Pinter, S. F. & Lee, J. T. X-chromosome hyperactivation in mammals via nonlinear relationships between chromatin states and transcription. Nat. Struct. Mol. Biol. 19, 56–61 (2011).
Prasanth, K. V. et al. Nuclear organization and dynamics of 7SK RNA in regulating gene expression. Mol. Biol. Cell 21, 4184–4196 (2010).
Otsu, N. A threshold selection method from gray-level histograms. IEEE Trans. Syst. Man Cybern. 9, 62–66 (1979).
Krainer, F. W. & Glieder, A. An updated view on horseradish peroxidases: recombinant production and biotechnological applications. Appl. Microbiol. Biotechnol. 99, 1611–1625 (2015).
Radtke, A. J. et al. IBEX: a versatile multiplex optical imaging approach for deep phenotyping and spatial analysis of cells in complex tissues. Proc. Natl Acad. Sci. USA 117, 33455–33465 (2020).
Moffitt, J. R. et al. High-throughput single-cell gene-expression profiling with multiplexed error-robust fluorescence in situ hybridization. Proc. Natl Acad. Sci. USA 113, 11046–11051 (2016).
Xiao, L., Labaer, J. & Guo, J. Highly sensitive and multiplexed in situ RNA profiling with cleavable fluorescent tyramide. Cells 10, 1277 (2021).
Green, N. M. Avidin. In Advances in Protein Chemistry Vol. 29 (eds Anfinsen, C. B. et al.) 85–133 (Academic Press, 1975).
Liao, R. et al. Highly sensitive and multiplexed protein imaging with cleavable fluorescent tyramide reveals human neuronal heterogeneity. Front. Cell Dev. Biol. 8, 614624 (2020).
Zhou, X. et al. Highly sensitive spatial transcriptomics using FISHnCHIPs of multiple co-expressed genes. Nat. Commun. 15, 2342 (2024).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Acknowledgements
We thank members of the Shechner, Shendure, Lieberman, Schweppe, Akilesh and Beliveau laboratories for helpful discussion about this work. We thank N. Peters of the UW W.M. Keck Microscopy Center and D. Fong of Nikon for assistance with microscopy during the development of pSABER and S. Jiang for helpful advice about working with HRP in tissues. This work was supported by a Damon Runyon Dale F. Frey Breakthrough Award (32-19 to B.J.B.), the National Institutes of Health (under grants 1R35GM137916 to B.J.B, 1UM1HG011586 to J.S. and B.J.B., 1R01DK130386 to S. Akilesh and 1R01GM138799-01 to D.M.S.), the Andy Hill Cancer Research Endowment (under a COVID-19 Response Grant Award to B.J.B. and S. Akilesh) and the Diabetic Complications Consortium (under grant 19AU3987 to S. Akilesh and B.J.B.). This project was also supported in part by a Building Bridges Award to J.A.L. and S. Akilesh from the Department of Laboratory Medicine and Pathology. A.F.T. was supported by NIH training grant T32GM007750.
Author information
Authors and Affiliations
Contributions
S. Attar, S. Akilesh and B.J.B. conceived the study. V.E.B. wrote and optimized software code. S. Attar, M.K., Y.L., V.E.B., E.K.N. and A.F.T. performed experiments. S. Attar, V.E.B., M.K., S. Akilesh and B.J.B. wrote the paper. All authors edited and approved the paper. D.M.S., J.S., D.K.S., J.A.L., S. Akilesh and B.J.B. supervised the work.
Corresponding authors
Ethics declarations
Competing interests
S. Attar, A.F.T., D.M.S., S. Akilesh and B.J.B. have filed a patent application covering pSABER. B.J.B. is listed as an inventor on patent applications related to the SABER technology. The other authors declare no competing interests.
Peer review
Peer review information
Nature Methods thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Rita Strack, in collaboration with the Nature Methods team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Visualization of PER extension products relevant to Fig. 4.
a, Gel electrophoresis shows the lengths of E1 and E2 from Fig. 4a. E1 is ~450 nt and E2 is ~700 nt. b, Gel electrophoresis shows the lengths of probes used for Fig. 4c. Probe length without the spacer is 65 nt. c, Gel electrophoresis shows the length of the mouse minor satellite PER used for Fig. 4e. Extension length is ~800 nt.
Extended Data Fig. 2 Quantification of the variability of HRP-oligo activity.
Absorbance values representing the activity of three HRP oligos over the course of three days.
Extended Data Fig. 3 Comparison of SABER and pSABER on a slide-scanning microscope.
a, SABER (left) and pSABER (right) visualization of mouse major satellite DNA imaged using a 10x air objective. b, SABER (left) and pSABER (right) visualization of mouse major satellite DNA imaged using a 20x air objective. c, SABER (left) and pSABER (right) visualization of mouse major satellite DNA imaged using a 10x air objective. Each image is presented at the indicated high (top) and low (bottom) contrast settings to allow for visual comparisons between signals occupying different portions of the 16-bit dynamic range (0–65535). Images are maximum projections in Z. Scale bars, 20 µm (insets) or 100 µm (fields of view). Each experiment was repeated ≥3 times and yielded similar results.
Extended Data Fig. 4 Efficiency and fidelity of Exchange-pSABER.
a, Schematic of Exchange-pSABER in which exchange occurred after hybridization with an HRP-oligo but before localized label deposition to assess exchange efficiency. b, Representative images of DNA pSABER targeting mouse minor satellite in EY.T4 mouse embryonic fibroblasts using three different combinations of HRP-oligo and fluorescent tyramide. The mock condition proceeded through pSABER as normal whereas the exchange condition developed after displacing the HRP-oligos. Images are displayed at the indicated high (top) and low (bottom) contrast settings to illustrate exchange efficiency. Scale bars, 10 µm. Each experiment was repeated ≥3 times and yielded similar results.
Extended Data Fig. 5 Biotin-ss-tyramide unlocks multiplexing for small targets.
a, Schematic of a pSABER experiment using biotin-ss-tyramide as the substrate and fluorescent streptavidin to visualize label deposition. Deposited biotin-ss-tyramide was reduced to remove the signal. b, Representative images of DNA pSABER targeting telomeres in HCT-116 CTCF-AID human colorectal cancer cells. The mock samples (left) incubated in PBS while the reduced (right) samples incubated in DTT. Images are displayed at the indicated high (top) and low (bottom) contrast settings to show the decrease in signal as a result of reduction. c, Images of DNA pSABER targeting alpha satellites in HCT-116 CTCF-AID human colorectal cancer cells comparing fluorescent streptavidin signal before and after reduction with DTT. d, Schematic of a pSABER experiment using biotin-ss-tyramide in which reduction occurred before fluorescent streptavidin staining to assess reduction efficiency. e, Images of DNA pSABER targeting telomeres in HCT-116 CTCF-AID human colorectal cancer cells showing reduction before staining (left) and reduction after staining (right), highlighting the effect bound fluorescent streptavidin imposes on reduction efficiency. Scale bars, 10 µm. Each experiment was repeated ≥3 times and yielded similar results.
Extended Data Fig. 6 Combinatorial labeling with fluorescent pSABER.
a, Schematic of a pSABER experiment depicting two rounds of labeling with different fluorescent tyramides. One target has a combination of hairpins, recruiting multiple HRP oligos throughout the experiment instead of one. b, Images of DNA pSABER targeting four 200-kb spots on chromosome X in a male human metaphase spread. The white box in the left image indicates the zoomed in field of view. The red arrowhead marks spot 17, which was labeled with two colors. Scale bar, 20 µm. Each experiment was repeated ≥3 times and yielded similar results.
Supplementary information
Supplementary Data 1
Sequences of oligonucleotides used in the study.
Source data
Source Data Fig. 4
Source numerical data plotted in Fig. 4b,d,f.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Attar, S., Browning, V.E., Krebs, M. et al. Efficient and highly amplified imaging of nucleic acid targets in cellular and histopathological samples with pSABER. Nat Methods 22, 156–165 (2025). https://doi.org/10.1038/s41592-024-02512-2
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41592-024-02512-2