Abstract
Viral infection perturbs host cells and can be used to uncover regulatory mechanisms controlling cellular responses and susceptibility to infections. Using cell biological, biochemical, and genetic tools, we reveal that influenza A virus (IAV) infection induces global transcriptional defects at the 3′ ends of active host genes and RNA polymerase II (RNAPII) run-through into extragenic regions. Deregulated RNAPII leads to expression of aberrant RNAs (3′ extensions and host-gene fusions) that ultimately cause global transcriptional downregulation of physiological transcripts, an effect influencing antiviral response and virulence. This phenomenon occurs with multiple strains of IAV, is dependent on influenza NS1 protein, and can be modulated by SUMOylation of an intrinsically disordered region (IDR) of NS1 expressed by the 1918 pandemic IAV strain. Our data identify a strategy used by IAV to suppress host gene expression and indicate that polymorphisms in IDRs of viral proteins can affect the outcome of an infection.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
RNA-seq data associated with this study are available through the Gene Expression Omnibus (GEO) data repository, accession number GSE103604. Source data for Fig. 2a,c and 3h,j are available in Supplementary Dataset 2. Data underlying the analysis in Fig. 4f are available in Supplementary Dataset 3. All other data are available upon reasonable request.
References
Marazzi, I. et al. Suppression of the antiviral response by an influenza histone mimic. Nature 483, 428–433 (2012).
Rialdi, A. et al. The RNA exosome syncs IAV-RNAPII transcription to promote viral ribogenesis and infectivity. Cell 169, 679–692.e14 (2017).
Ayllon, J. & García-Sastre, A. The NS1 protein: a multitasking virulence factor. Curr. Top. Microbiol. Immunol. 386, 73–107 (2015).
Gack, M. U. et al. Influenza A virus NS1 targets the ubiquitin ligase TRIM25 to evade recognition by the host viral RNA sensor RIG-I. Cell. Host. Microbe. 5, 439–449 (2009).
Li, S., Min, J. Y., Krug, R. M. & Sen, G. C. Binding of the influenza A virus NS1 protein to PKR mediates the inhibition of its activation by either PACT or double-stranded RNA. Virology 349, 13–21 (2006).
García-Sastre, A. et al. Influenza A virus lacking the NS1 gene replicates in interferon-deficient systems. Virology 252, 324–330 (1998).
Donelan, N. R., Basler, C. F. & García-Sastre, A. A recombinant influenza A virus expressing an RNA-binding-defective NS1 protein induces high levels of beta interferon and is attenuated in mice. J. Virol. 77, 13257–13266 (2003).
Jackson, D., Hossain, M. J., Hickman, D., Perez, D. R. & Lamb, R. A. A new influenza virus virulence determinant: the NS1 protein four C-terminal residues modulate pathogenicity. Proc. Natl Acad. Sci. USA 105, 4381–4386 (2008).
Carrillo, B. et al. The influenza A virus protein NS1 displays structural polymorphism. J. Virol. 88, 4113–4122 (2014).
Hale, B. G. Conformational plasticity of the influenza A virus NS1 protein. J. Gen. Virol. 95, 2099–2105 (2014).
Davey, N. E. et al. Attributes of short linear motifs. Mol. Biosyst. 8, 268–281 (2012).
Gitlin, L., Hagai, T., LaBarbera, A., Solovey, M. & Andino, R. Rapid evolution of virus sequences in intrinsically disordered protein regions. PLoS. Pathog. 10, e1004529 (2014).
Taubenberger, J. K. & Morens, D. M. The pathology of influenza virus infections. Annu. Rev. Pathol. 3, 499–522 (2008).
Taubenberger, J. K. & Kash, J. C. Insights on influenza pathogenesis from the grave. Virus. Res. 162, 2–7 (2011).
Taubenberger, J. K. et al. Reconstruction of the 1918 influenza virus: unexpected rewards from the past. mBio 3, e00201–e00212 (2012).
Hale, B. G., Randall, R. E., Ortín, J. & Jackson, D. The multifunctional NS1 protein of influenza A viruses. J. Gen. Virol. 89, 2359–2376 (2008).
Krug, R. M. Functions of the influenza A virus NS1 protein in antiviral defense. Curr. Opin. Virol. 12, 1–6 (2015).
Obenauer, J. C. et al. Large-scale sequence analysis of avian influenza isolates. Science 311, 1576–1580 (2006).
Neumann, G., Whitt, M. A. & Kawaoka, Y. A decade after the generation of a negative-sense RNA virus from cloned cDNA: what have we learned? J. Gen. Virol. 83, 2635–2662 (2002).
Van Roey, K. et al. Short linear motifs: ubiquitous and functionally diverse protein interaction modules directing cell regulation. Chem. Rev. 114, 6733–6778 (2014).
Zhu, Q. et al. BRCA1 tumour suppression occurs via heterochromatin-mediated silencing. Nature 477, 179–184 (2011).
Manicassamy, B. et al. Analysis of in vivo dynamics of influenza virus infection in mice using a GFP reporter virus. Proc. Natl Acad. Sci. USA 107, 11531–11536 (2010).
Geiss, G. K. et al. Cellular transcriptional profiling in influenza A virus-infected lung epithelial cells: the role of the nonstructural NS1 protein in the evasion of the host innate defense and its potential contribution to pandemic influenza. Proc. Natl Acad. Sci. USA 99, 10736–10741 (2002).
Hatada, E. & Fukuda, R. Binding of influenza A virus NS1 protein to dsRNA in vitro. J. Gen. Virol. 73, 3325–3329 (1992).
Nemeroff, M. E., Qian, X. Y. & Krug, R. M. The influenza virus NS1 protein forms multimers in vitro and in vivo. Virology 212, 422–428 (1995).
Bornholdt, Z. A. & Prasad, B. V. X-ray structure of NS1 from a highly pathogenic H5N1 influenza virus. Nature 456, 985–988 (2008).
Molliex, A. et al. Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell 163, 123–133 (2015).
Lin, Y., Protter, D. S., Rosen, M. K. & Parker, R. Formation and maturation of phase-separated liquid droplets by RNA-binding proteins. Mol. Cell 60, 208–219 (2015).
Kwon, I. et al. Phosphorylation-regulated binding of RNA polymerase II to fibrous polymers of low-complexity domains. Cell 155, 1049–1060 (2013).
Banani, S. F. et al. Compositional control of phase-separated cellular bodies. Cell 166, 651–663 (2016).
Kato, M. et al. Cell-free formation of RNA granules: low complexity sequence domains form dynamic fibers within hydrogels. Cell 149, 753–767 (2012).
Harlen, K. M. & Churchman, L. S. The code and beyond: transcription regulation by the RNA polymerase II carboxy-terminal domain. Nat. Rev. Mol. Cell Biol. 18, 263–273 (2017).
Heyn, P., Salmonowicz, H., Rodenfels, J. & Neugebauer, K. M. Activation of transcription enforces the formation of distinct nuclear bodies in zebrafish embryos. RNA. Biol. 14, 752–760 (2017).
Hnisz, D., Shrinivas, K., Young, R. A., Chakraborty, A. K. & Sharp, P. A. A phase separation model for transcriptional control. Cell 169, 13–23 (2017).
Lu, H. et al. Phase-separation mechanism for C-terminal hyperphosphorylation of RNA polymerase II. Nature 558, 318–323 (2018).
Richard, P. & Manley, J. L. Transcription termination by nuclear RNA polymerases. Genes Dev. 23, 1247–1269 (2009).
Schlackow, M. et al. Distinctive patterns of transcription and RNA processing for human lincRNAs. Mol. Cell 65, 25–38 (2017).
Martinson, H. G. An active role for splicing in 3′-end formation. Wiley Interdiscip. Rev. RNA 2, 459–470 (2011).
Misra, A., Ou, J., Zhu, L. J. & Green, M. R. Global promotion of alternative internal exon usage by mRNA 3′ end formation factors. Mol. Cell 58, 819–831 (2015).
Misra, A. & Green, M. R. From polyadenylation to splicing: dual role for mRNA 3′ end formation factors. RNA. Biol. 13, 259–264 (2016).
Tseng, C. K. et al. Human telomerase RNA processing and quality control. Cell Rep. 13, 2232–2243 (2015).
Orphanides, G. & Reinberg, D. A unified theory of gene expression. Cell 108, 439–451 (2002).
Perales, R. & Bentley, D. “Cotranscriptionality”: the transcription elongation complex as a nexus for nuclear transactions. Mol. Cell 36, 178–191 (2009).
Mapendano, C. K., Lykke-Andersen, S., Kjems, J., Bertrand, E. & Jensen, T. H. Crosstalk between mRNA 3′ end processing and transcription initiation. Mol. Cell 40, 410–422 (2010).
Gu, B., Eick, D. & Bensaude, O. CTD serine-2 plays a critical role in splicing and termination factor recruitment to RNA polymerase II in vivo. Nucleic Acids Res. 41, 1591–1603 (2013).
Niwa, M., Rose, S. D. & Berget, S. M. In vitro polyadenylation is stimulated by the presence of an upstream intron. Genes Dev. 4, 1552–1559 (1990).
Dye, M. J. & Proudfoot, N. J. Terminal exon definition occurs cotranscriptionally and promotes termination of RNA polymerase II. Mol. Cell 3, 371–378 (1999).
Bauer, D. L. V. et al. Influenza virus mounts a two-pronged attack on host RNA polymerase II transcription. Cell Rep. 23, 2119–2129.e3 (2018).
Heinz, S. et al. Transcriptional elongation can affect 3D genome architecture. Cell (in the press).
Kuo, R. L. & Krug, R. M. Influenza a virus polymerase is an integral component of the CPSF30-NS1A protein complex in infected cells. J. Virol. 83, 1611–1616 (2009).
Pichlmair, A. et al. Viral immune modulators perturb the human molecular network by common and unique strategies. Nature 487, 486–490 (2012).
Rozenblatt-Rosen, O. et al. Interpreting cancer genomes using systematic host network perturbations by tumour virus proteins. Nature 487, 491–495 (2012).
Marazzi, I. & Garcia-Sastre, A. Interference of viral effector proteins with chromatin, transcription, and the epigenome. Curr. Opin. Microbiol. 26, 123–129 (2015).
Heaton, N. S. et al. Targeting viral proteostasis limits influenza virus, HIV, and dengue virus infection. Immunity 44, 46–58 (2016).
Marazzi, I., Greenbaum, B. D., Low, D. H. P. & Guccione, E. Chromatin dependencies in cancer and inflammation. Nat. Rev. Mol. Cell Biol. 19, 245–261 (2018).
Maldonado, E., Cabrejos, M. E., Banks, L. & Allende, J. E. Human papillomavirus-16 E7 protein inhibits the DNA interaction of the TATA binding transcription factor. J. Cell. Biochem. 85, 663–669 (2002).
Dasgupta, A. Targeting TFIIH to inhibit host cell transcription by Rift Valley Fever Virus. Mol. Cell 13, 456–458 (2004).
Di Valentin, E. et al. Varicella-zoster virus IE63 protein represses the basal transcription machinery by disorganizing the pre-initiation complex. Biol. Chem. 386, 255–267 (2005).
Kundu, P., Raychaudhuri, S., Tsai, W. & Dasgupta, A. Shutoff of RNA polymerase II transcription by poliovirus involves 3C protease-mediated cleavage of the TATA-binding protein at an alternative site: incomplete shutoff of transcription interferes with efficient viral replication. J. Virol. 79, 9702–9713 (2005).
Fraser, K. A. & Rice, S. A. Herpes simplex virus immediate-early protein ICP22 triggers loss of serine 2-phosphorylated RNA polymerase II. J. Virol. 81, 5091–5101 (2007).
Geng, F., Wenzel, S. & Tansey, W. P. Ubiquitin and proteasomes in transcription. Annu. Rev. Biochem. 81, 177–201 (2012).
Hagai, T., Azia, A., Babu, M. M. & Andino, R. Use of host-like peptide motifs in viral proteins is a prevalent strategy in host-virus interactions. Cell Rep. 7, 1729–1739 (2014).
Daugherty, M. D. & Malik, H. S. Rules of engagement: molecular insights from host-virus arms races. Annu. Rev. Genet. 46, 677–700 (2012).
Garamszegi, S., Franzosa, E. A. & Xia, Y. Signatures of pleiotropy, economy and convergent evolution in a domain-resolved map of human-virus protein-protein interaction networks. PLoS. Pathog. 9, e1003778 (2013).
Dyson, H. J. & Wright, P. E. Intrinsically unstructured proteins and their functions. Nat. Rev. Mol. Cell Biol. 6, 197–208 (2005).
Uversky, V. N. & Dunker, A. K. Understanding protein non-folding. Biochim. Biophys. Acta 1804, 1231–1264 (2010).
Babu, M. M., Kriwacki, R. W. & Pappu, R. V. Versatility from protein disorder. Science 337, 1460–1461 (2012).
Tompa, P., Davey, N. E., Gibson, T. J. & Babu, M. M. A million peptide motifs for the molecular biologist. Mol. Cell 55, 161–169 (2014).
Coletta, A. et al. Low-complexity regions within protein sequences have position-dependent roles. BMC Syst. Biol. 4, 43 (2010).
Beltrao, P. et al. Systematic functional prioritization of protein posttranslational modifications. Cell 150, 413–425 (2012).
Holt, L. J. et al. Global analysis of Cdk1 substrate phosphorylation sites provides insights into evolution. Science 325, 1682–1686 (2009).
Mosca, R., Pache, R. A. & Aloy, P. The role of structural disorder in the rewiring of protein interactions through evolution. Mol. Cell. Proteomics. 11, 014969 (2012).
Schrauwen, E. J. & Fouchier, R. A. Host adaptation and transmission of influenza A viruses in mammals. Emerg. Microbes Infect. 3, e9 (2014).
Miller, M. S. & Palese, P. Peering into the crystal ball: influenza pandemics and vaccine efficacy. Cell 157, 294–299 (2014).
Taft, A. S. et al. Identification of mammalian-adapting mutations in the polymerase complex of an avian H5N1 influenza virus. Nat. Commun. 6, 7491 (2015).
Eswar, N., Eramian, D., Webb, B., Shen, M. Y. & Sali, A. Protein structure modeling with MODELLER. Methods Mol. Biol. 426, 145–159 (2008).
Penkert, R. R., DiVittorio, H. M. & Prehoda, K. E. Internal recognition through PDZ domain plasticity in the Par-6–Pals1 complex. Nat. Struct. Mol. Biol. 11, 1122–1127 (2004).
London, N., Raveh, B., Cohen, E., Fathi, G. & Schueler-Furman, O. Rosetta FlexPepDock web server: high resolution modeling of peptide-protein interactions. Nucleic Acids Res. 39, W249–W253 (2011).
Reverter, D. & Lima, C. D. A basis for SUMO protease specificity provided by analysis of human Senp2 and a Senp2-SUMO complex. Structure 12, 1519–1531 (2004).
Halfmann, R. & Lindquist, S. Screening for amyloid aggregation by semi-denaturing detergent-agarose gel electrophoresis. J. Vis. Exp. 17, e838 (2008).
Shah, N. B. & Duncan, T. M. Bio-layer interferometry for measuring kinetics of protein-protein interactions and allosteric ligand effects. J. Vis. Exp. 84, e51383 (2014).
Martínez-Sobrido, L. & García-Sastre, A. Generation of recombinant influenza virus from plasmid DNA. J. Vis. Exp. 42, 2057 (2010).
Cox, J. & Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26, 1367–1372 (2008).
Cox, J. et al. Andromeda: a peptide search engine integrated into the MaxQuant environment. J. Proteome. Res. 10, 1794–1805 (2011).
The UniProt Consortium. UniProt: the universal protein knowledgebase. Nucleic Acids Res. 45, D158–D169 (2017).
Choi, M. et al. MSstats: an R package for statistical analysis of quantitative mass spectrometry-based proteomic experiments. Bioinformatics 30, 2524–2526 (2014).
Mi, H. et al. PANTHER version 11: expanded annotation data from Gene Ontology and Reactome pathways, and data analysis tool enhancements. Nucleic Acids Res. 45, D183–D189 (2017).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 17, 10–12 (2011).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014).
Ritchie, M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 (2015).
Li, B. & Dewey, C. N. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 12, 323 (2011).
Law, C. W., Chen, Y., Shi, W. & Smyth, G. K. voom: precision weights unlock linear model analysis tools for RNA-seq read counts. Genome. Biol. 15, R29 (2014).
Rahl, P. B. et al. c-Myc regulates transcriptional pause release. Cell 141, 432–445 (2010).
Rhoads, A. & Au, K. F. PacBio sequencing and its applications. Genomics. Proteomics. Bioinformatics. 13, 278–289 (2015).
Acknowledgements
We thank all members of the laboratories of I.M. and A.G.-S., and J. Bloom and A. Kornblihtt for valuable discussions and suggestions on the manuscript. We thank the Medicinal Chemistry Core, Integrated Screening Core, Microscopy CoRE,, and Global Health and Emerging Pathogens Institute (GHEPI) at the Icahn School of Medicine at Mount Sinai. H.v.B., I.M., and A.G.-S. are partially supported by HHSN272201400008C–Center for Research on Influenza Pathogenesis (CRIP), a NIAID-funded Center of Excellence for Influenza Research and Surveillance (CEIRS). I.M. is supported in part by the Department of Defense W911NF-14-1-0353. I.M. and H.v.B. are supported by NIH grant 1R01AN3663134. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention/the Agency for Toxic Substances and Disease Registry. This work was supported in part through the computational resources and staff expertise provided by Scientific Computing at the Icahn School of Medicine at Mount Sinai.
Author information
Authors and Affiliations
Contributions
N.Z., N. Moshkina, A.R., and V.Y. performed experiments with modeling of PDZ–peptide–SUMO complexes, transfection, infection, WB, SDD-AGE, purification of recombinant proteins, BLI, pulldown assays, analysis of proteins stability, immunofluorescence, RT–PCR, chemical synthesis of B-isox, and B-isox-mediated precipitation; V.S., S.R., and B.H. performed independent validation of SUMOylation of the SUP domain; N. Mena, R.A., M.T.S.-A., J.A., and T.T. performed experiments with rescue of recombinant influenza viruses, single-cycle growth curves, plaque phenotypes, and analysis of infectious viral progeny; J.H., D.J.-M., and Y.M. performed experiments with preparation of peptides for MS, protein identification by LC–MS/MS, and statistical relative quantification of proteins and enrichment analysis; R.F., M.S., D.P., A.K., M.B., M.L.S., R.S., and H.v.B. performed directional RNA-seq, differential gene expression analysis, TR and intron/exon ratio calculation, and iso-seq analysis. S.-Y.H., D.L., and E.G. performed bioinformatics analysis on splicing and termination; B.G. performed conservation analysis; J.J., R.K.P., A.T., and N.K. provided materials and insights in experimental procedures; and A.G.-S. and I.M. supervised the project. I.M. wrote the paper, on which coauthors provided feedback.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
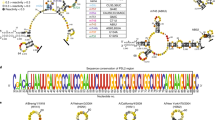
Supplementary Figure 1 NS1 C-terminal diversity in different strains of influenza virus.
a, Diagram representing the C-terminus of an NS1 protein in different Influenza strains. b, Visual representation of the conservation score among NS1 proteins averaged over 10 amino acidic residues.
Supplementary Figure 2 Control experiments.
Control experiments for Fig. 2. a, IF of ectopically expressed GFP, NS1, NS1-KR and NS1-SUMO in A549 (scale bar indicates 100um). b, Pull-down assay between recombinant NS1, NS1-KR, NS1-SUMO and PSD95. PBS served as a negative control. Coomassie staining SDS-PAGE showing purified recombinant proteins (left) and eluted complexes (right). c-e, Structural modeling of SUMOylated NS1 tail and the indicated PDZ domain containing-proteins GIPC2 (c), DVL1 (d) and PSD95 (e). f, Schematic diagram of plaque selection and analysis. All plaques selected after 24 hpi with NS1-SUMO virus indicate that the virus has reverted back to NS1 virus (WT virotype) by mutagenesis of its C-term SUMO extension. This result indicates that NS1-SUMO virus can infect but it is compromised in its ability to bud and spread from cell-to-cell.
Supplementary Figure 3 Control experiments for 1918 NS1 and NS1-SUMO viruses.
a, A549 cells were mock infected (M) or infected with NS1 and NS1-SUMO virus for 12 hours and sequentially treated with mg132 (50 nM) or cycloheximide (CHX, 50 ug/mL) for 0/4/8/24hours. Lysates were immunoblotted with anti-NS1 antibody. b, Cumulative expression levels of positive- and negative-sense viral RNAs for all segments in A549 cells at 6 or 12 hours post-infection with NS1 virus (blue), or NS1-SUMO virus (red). Normalized log2 counts of viral RNAs per million sequenced reads (CPM) are shown for two biological replicate experiments.
Supplementary Figure 4 Control experiments.
Control experiments for Fig. 3. a, Synthetic route of B-isox. Reagents and conditions: (1) HOSu, EDC·HCl, THF, RT, overnight; (2) 6-amino-1-hexanol, THF, 5 hours; (3) Biotin, DMAP, EDC·HCl, DCM, 3 days. b, LC-HRMS of synthesized B-isox. c, Silver staining gel showing B-isox-mediated precipitation of proteins from cell lysate. d, Statistical Analyses of the B-isox precipitated proteins after infection. Statistical quantitation of the overlap between proteins precipitated by B-isox during infection compared to a set of 1286 B-isox-precipitated proteins from the McKnight’s lab (Cell, 2013, 155(5): 1049-1060), a set of 1786 RNA binding proteins from UniProt, a set of 126 literature-cited RNA granule proteins, and the entire human proteome. Fisher’s exact test was used to determine the significance of the overlapping between datasets. e, qPCR analysis of run through transcription downstream of the GAPDH and CDC25A genes in A549 cells infected with WT or NS1-GFP A/Vietnam/1203/2004/H5N1 influenza virus at MOI of 3 for 6 and 12 hours. Expression levels were calculated by using primer sets amplifying region downstream of termination sites and calculating fold enrichment comparing 6 hours and 12 hours time post infection (as mock infection has no detectable signal indicating absence of RNAPII run-through). Error bars correspond to mean ± s.e.m.
Supplementary Figure 5 Control experiments.
Control experiments related to Fig. 4. a, TR density at termination region plotted as a function of gene induction (red) or repression (green) during infection with NS1-SUMO virus at 6 hpi (left) and 12 hpi (right). b, Normalized RNA-Seq read coverage tracks showing increased 3’-end transcript levels for representative genes: CXCL1, MYC, NFKBIA, and CDC25A.
Supplementary Figure 6 TR region changes at specific gene sets and human variation in SUMO-modifying genes.
a, TR plots at termination region of active (RPKM > 1 in 50% of samples) multi-exonic (upper panels) and mono-exonic (lower panels) genes in uninfected A549 cells and 6 or 12 hpi with NS1 or NS1-SUMO virus. Data is shown for two replicates in each condition. b, Scatter plot showing the relationship between the log fold-change (logFC) in gene expression between NS1-SUMO virus and NS1 virus-infected A549 cells at 6 hpi, and the logFC in transcript levels in 5,000 bp 3’ gene-flanking regions. Genes with a significant increase or decrease in expression in NS1-SUMO vs NS1 infected A549 cells at 6 hpi are highlighted in red and green, respectively. All other genes are highlighted in grey. Solid and dotted lines correspond to the regression line and 95% confidence interval for genes with significant expression differences, with the R2 and significance shown at the top-left. c, Analysis of SUMO-modifying gene mutations using a compendium of protein-coding genetic variation in 60,706 humans from the ENCODE Consortium. The probability of being loss-of-function intolerant (intolerant of both heterozygous and homozygous of variants) is shown for all genes compared to SUMO-modifying genes.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6
Supplementary Table 1
Information of PDZ domain containing proteins used in this study
Supplementary Table 2
Cross-comparison of B-isox precipitated proteins
Supplementary Table 3
Quantitative mass-spectrometry analysis of B-isox precipitated proteins
Supplementary Table 4
RNA-seq analysis of NS1 vs NS1-SUMO viral infection
Supplementary Table 5
Human polymorphism and damaging-mutations of SUMO-modifying enzymes
Supplementary Dataset 1
Uncropped WB images for Figures 1C, 1D, 1E, 3A, and 3C
Supplementary Dataset 2
Supporting data for Figures 2A, 2C, 3H, 3I, and 3J
Supplementary Dataset 3
Supporting data for Figure 4F, containing the raw circular consensus sequencing (CCS) reads, the reads after adapter trimming (cutadapt.fa), removal of Poly(A) tails (cutadapt_polA.fa), and removal of short reads <300 nt (cutadapt_polA_gt300.fa)
Rights and permissions
About this article
Cite this article
Zhao, N., Sebastiano, V., Moshkina, N. et al. Influenza virus infection causes global RNAPII termination defects. Nat Struct Mol Biol 25, 885–893 (2018). https://doi.org/10.1038/s41594-018-0124-7
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41594-018-0124-7
This article is cited by
-
Influenza A virus NS1 suppresses nuclear speckles promoted gene expression by inhibition of transcription
npj Viruses (2025)
-
The host RNA polymerase II C-terminal domain is the anchor for replication of the influenza virus genome
Nature Communications (2024)
-
mRNA 3’UTR lengthening by alternative polyadenylation attenuates inflammatory responses and correlates with virulence of Influenza A virus
Nature Communications (2023)
-
Cut site preference allows influenza A virus PA-X to discriminate between host and viral mRNAs
Nature Microbiology (2023)
-
Avian influenza viruses suppress innate immunity by inducing trans-transcriptional readthrough via SSU72
Cellular & Molecular Immunology (2022)