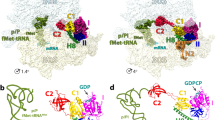
Abstract
Translation termination involves release factors RF1, RF2 and the GTPase RF3 that recycles RF1 and RF2 from the ribosome. RF3 dissociates from the ribosome in the GDP-bound form and must then exchange GDP for GTP. The 70S ribosome termination complex (70S-TC) accelerates GDP exchange in RF3, suggesting that the 70S-TC can function as the guanine nucleotide exchange factor for RF3. Here, we use cryogenic-electron microscopy to elucidate the mechanism of GDP dissociation from RF3 catalyzed by the Escherichia coli 70S-TC. The non-rotated ribosome bound to RF1 remodels RF3 and induces a peptide flip in the phosphate-binding loop, efficiently ejecting GDP. Binding of GTP allows RF3 to dock at the GTPase center, promoting the dissociation of RF1 from the ribosome. The structures recapitulate the functional cycle of RF3 on the ribosome and uncover the mechanism by which the 70S-TC allosterically dismantles the phosphate-binding groove in RF3, a previously overlooked function of the ribosome.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The atomic coordinates have been deposited in the RCSB Protein Data Bank (PDB) under accession codes 8FZE (structure I-A; 70S–RF1), 8FZD (composite I-B; 70S–apo-RF3–RF1), 8FZF (composite I-C; 70S–RF3–ppGpp), 8FZG (composite II-A; 70S–RF3–GDPCP–RF1), 8FZI (composite II-B; 70S–RF3–GDPCP, non-activated conformation), 8FZJ (composite II-C; 70S–RF3–GDPCP, GTP-activated conformation) and 8FZH (structure II-D; 70S–RF1). The cryo-EM maps have been deposited in the Electron Microscopy Data Bank (EMDB) under accession codes EMD-29621 (structure I-A; 70S–RF1), EMD-29618 (structure I-B; 70S–apo-RF3–RF1 initial map), EMD-29619 (I-B: apo-RF3; from focused refinement), EMD-29620 (structure I-B; 70S–apo-RF3–RF1 composite), EMD-29622 (structure I-C; 70S–RF3–ppGpp initial map), EMD-29623 (I-C: RF3–ppGpp; from focused refinement), EMD-29624 (structure I-C; 70S–RF3–ppGpp composite), EMD-29625 (structure II-A; 70S–RF3–GDPCP–RF1 initial map), EMD-29626 (II-A: RF3–GDPCP; from focused refinement), EMD-29627 (structure II-A; 70S–RF3–GDPCP–RF1 composite), EMD-29629 (structure II-B; 70S–RF3–GDPCP initial map, non-activated conformation), EMD-29630 (II-B: RF3–GDPCP; from focused refinement, non-activated conformation), EMD-29631 (structure II-B; 70S–RF3–GDPCP composite, non-activated conformation), EMD-29632 (structure II-C; 70S–RF3–GDPCP initial map, GTP-activated conformation), EMD-29633 (II-C: RF3–GDPCP; from focused refinement, GTP-activated conformation), EMD-29634 (structure II-C; 70S–RF3–GDPCP composite, GTP-activated conformation), and EMD-29628 (structure II-D; 70S–RF1).
References
Youngman, E. M., McDonald, M. E. & Green, R. Peptide release on the ribosome: mechanism and implications for translational control. Annu. Rev. Microbiol. 62, 353–373 (2008).
Scolnick, E., Tompkins, R., Caskey, T. & Nirenberg, M. Release factors differing in specificity for terminator codons. Proc. Natl Acad. Sci. USA 61, 768–774 (1968).
Graf, M. et al. Visualization of translation termination intermediates trapped by the Apidaecin 137 peptide during RF3-mediated recycling of RF1. Nat. Commun. 9, 3053 (2018).
Peske, F., Kuhlenkoetter, S., Rodnina, M. V. & Wintermeyer, W. Timing of GTP binding and hydrolysis by translation termination factor RF3. Nucl. Acids Res. 42, 1812–1820 (2014).
Koutmou, K. S., McDonald, M. E., Brunelle, J. L. & Green, R. RF3:GTP promotes rapid dissociation of the class 1 termination factor. RNA 20, 609–620 (2014).
Zhou, J., Lancaster, L., Trakhanov, S. & Noller, H. F. Crystal structure of release factor RF3 trapped in the GTP state on a rotated conformation of the ribosome. RNA 18, 230–240 (2012).
Jin, H., Kelley, A. C. & Ramakrishnan, V. Crystal structure of the hybrid state of ribosome in complex with the guanosine triphosphatase release factor 3. Proc. Natl Acad. Sci. USA 108, 15798–15803 (2011).
Klaholz, B. P., Myasnikov, A. G. & Van Heel, M. Visualization of release factor 3 on the ribosome during termination of protein synthesis. Nature 427, 862–865 (2004).
Zavialov, A. V. & Ehrenberg, M. Peptidyl-tRNA regulates the GTPase activity of translation factors. Cell 114, 113–122 (2003).
Gao, H. et al. RF3 induces ribosomal conformational changes responsible for dissociation of class I release factors. Cell 129, 929–941 (2007).
Freistroffer, D. V., Pavlov, M. Y., MacDougall, J., Buckingham, R. H. & Ehrenberg, M. Release factor RF3 in E. coli accelerates the dissociation of release factors RF1 and RF2 from the ribosome in a GTP-dependent manner. EMBO J. 16, 4126–4133 (1997).
Pallesen, J. et al. Cryo-EM visualization of the ribosome in termination complex with apo-RF3 and RF1. eLife 2, e00411 (2013).
Zavialov, A. V., Buckingham, R. H. & Ehrenberg, M. A posttermination ribosomal complex is the guanine nucleotide exchange factor for peptide release factor RF3. Cell 107, 115–124 (2001).
Adio, S. et al. Dynamics of ribosomes and release factors during translation termination in E. coli. Elife 7, e34252 (2018).
Prabhakar, A. et al. Dynamics of release factor recycling during translation termination in bacteria. Nucl. Acids Res. 51, 5774–5790 (2023).
Buckstein, M. H., He, J. & Rubin, H. Characterization of nucleotide pools as a function of physiological state in Escherichia coli. J. Bacteriol. 190, 718–726 (2008).
Hauryliuk, V. et al. Thermodynamics of GTP and GDP binding to bacterial initiation factor 2 suggests two types of structural transitions. J. Mol. Biol. 394, 621–626 (2009).
Wilden, B., Savelsbergh, A., Rodnina, M. V. & Wintermeyer, W. Role and timing of GTP binding and hydrolysis during EF-G-dependent tRNA translocation on the ribosome. Proc. Natl Acad. Sci. USA 103, 13670–13675 (2006).
Gromadski, K. B., Wieden, H. J. & Rodnina, M. V. Kinetic mechanism of elongation factor Ts-catalyzed nucleotide exchange in elongation factor Tu. Biochemistry 41, 162–169 (2002).
Shi, X. & Joseph, S. Mechanism of translation termination: RF1 dissociation follows dissociation of RF3 from the ribosome. Biochemistry 55, 6344–6354 (2016).
Zavialov, A. V., Mora, L., Buckingham, R. H. & Ehrenberg, M. Release of peptide promoted by the GGQ motif of class 1 release factors regulates the GTPase activity of RF3. Mol. Cell 10, 789–798 (2002).
Wang, Y., Jiang, Y., Meyering-Voss, M., Sprinzl, M. & Sigler, P. B. Crystal structure of the EF-Tu.EF-Ts complex from Thermus thermophilus. Nat. Struct. Biol. 4, 650–656 (1997).
Kawashima, T., Berthet-Colominas, C., Wulff, M., Cusack, S. & Leberman, R. The structure of the Escherichia coli EF-Tu.EF-Ts complex at 2.5 A resolution. Nature 379, 511–518 (1996).
Sternberg, S. H., Fei, J., Prywes, N., McGrath, K. A. & Gonzalez, R. L. Jr. Translation factors direct intrinsic ribosome dynamics during translation termination and ribosome recycling. Nat. Struct. Mol. Biol. 16, 861–868 (2009).
Ge, X., Mandava, C. S., Lind, C., Aqvist, J. & Sanyal, S. Complementary charge-based interaction between the ribosomal-stalk protein L7/12 and IF2 is the key to rapid subunit association. Proc. Natl Acad. Sci. USA 115, 4649–4654 (2018).
Kihira, K. et al. Crystal structure analysis of the translation factor RF3 (release factor 3). FEBS Lett. 586, 3705–3709 (2012).
Polekhina, G. et al. Helix unwinding in the effector region of elongation factor EF-Tu-GDP. Structure 4, 1141–1151 (1996).
Sahu, B., Khade, P. K. & Joseph, S. Highly conserved base A55 of 16S ribosomal RNA is important for the elongation cycle of protein synthesis. Biochemistry 52, 6695–6701 (2013).
Diaconu, M. et al. Structural basis for the function of the ribosomal L7/12 stalk in factor binding and GTPase activation. Cell 121, 991–1004 (2005).
Kothe, U., Wieden, H. J., Mohr, D. & Rodnina, M. V. Interaction of helix D of elongation factor Tu with helices 4 and 5 of protein L7/12 on the ribosome. J. Mol. Biol. 336, 1011–1021 (2004).
Carlson, M. A. et al. Ribosomal protein L7/L12 is required for GTPase translation factors EF-G, RF3, and IF2 to bind in their GTP state to 70S ribosomes. FEBS J. 284, 1631–1643 (2017).
Mohr, D., Wintermeyer, W. & Rodnina, M. V. GTPase activation of elongation factors Tu and G on the ribosome. Biochemistry 41, 12520–12528 (2002).
Petrychenko, V. et al. Structural mechanism of GTPase-powered ribosome-tRNA movement. Nat. Commun. 12, 5933 (2021).
Carbone, C. E. et al. Time-resolved cryo-EM visualizes ribosomal translocation with EF-G and GTP. Nat. Commun. 12, 7236 (2021).
Voorhees, R. M., Schmeing, T. M., Kelley, A. C. & Ramakrishnan, V. The mechanism for activation of GTP hydrolysis on the ribosome. Science 330, 835–838 (2010).
Loveland, A. B., Demo, G., Grigorieff, N. & Korostelev, A. A. Ensemble cryo-EM elucidates the mechanism of translation fidelity. Nature 546, 113–117 (2017).
Loveland, A. B., Demo, G. & Korostelev, A. A. Cryo-EM of elongating ribosome with EF-Tu*GTP elucidates tRNA proofreading. Nature 584, 640–645 (2020).
Aqvist, J. & Kamerlin, S. C. The conformation of a catalytic loop is central to GTPase activity on the ribosome. Biochemistry 54, 546–556 (2015).
Maracci, C., Peske, F., Dannies, E., Pohl, C. & Rodnina, M. V. Ribosome-induced tuning of GTP hydrolysis by a translational GTPase. Proc. Natl Acad. Sci. USA 111, 14418–14423 (2014).
McDonald, M. E. & Green, R. Another burst of smoke: atomic resolution structures of RF3 bound to the ribosome. RNA 18, 605–609 (2012).
Agirrezabala, X. et al. Ribosome rearrangements at the onset of translational bypassing. Sci. Adv. 3, e1700147 (2017).
Zhang, J. et al. Mechanisms of ribosome stalling by SecM at multiple elongation steps. eLife 4, e09684 (2015).
Klimova, M. et al. EF-G-induced ribosome sliding along the noncoding mRNA. Sci. Adv. 5, eaaw9049 (2019).
Hassan, A. et al. Ratchet, swivel, tilt and roll: a complete description of subunit rotation in the ribosome. Nucl. Acids Res. 51, 919–934 (2022).
Behrmann, E. et al. Structural snapshots of actively translating human ribosomes. Cell 161, 845–857 (2015).
Budkevich, T. V. et al. Regulation of the mammalian elongation cycle by subunit rolling: a eukaryotic-specific ribosome rearrangement. Cell 158, 121–131 (2014).
Tourigny, D. S., Fernandez, I. S., Kelley, A. C. & Ramakrishnan, V. Elongation factor G bound to the ribosome in an intermediate state of translocation. Science 340, 1235490 (2013).
Pulk, A. & Cate, J. H. Control of ribosomal subunit rotation by elongation factor G. Science 340, 1235970 (2013).
Basu, R. S., Sherman, M. B. & Gagnon, M. G. Compact IF2 allows initiator tRNA accommodation into the P site and gates the ribosome to elongation. Nat. Commun. 13, 3388 (2022).
Sprink, T. et al. Structures of ribosome-bound initiation factor 2 reveal the mechanism of subunit association. Sci. Adv. 2, e1501502 (2016).
Sprang, S. R. & Coleman, D. E. Invasion of the nucleotide snatchers: structural insights into the mechanism of G protein GEFs. Cell 95, 155–158 (1998).
Bourne, H. R., Sanders, D. A. & McCormick, F. The GTPase superfamily: conserved structure and molecular mechanism. Nature 349, 117–127 (1991).
Wieden, H. J., Gromadski, K., Rodnin, D. & Rodnina, M. V. Mechanism of elongation factor (EF)-Ts-catalyzed nucleotide exchange in EF-Tu. Contribution of contacts at the guanine base. J. Biol. Chem. 277, 6032–6036 (2002).
Hwang, Y. W. & Miller, D. L. A study of the kinetic mechanism of elongation factor Ts. J. Biol. Chem. 260, 11498–11502 (1985).
Chau, V., Romero, G. & Biltonen, R. L. Kinetic studies on the interactions of Escherichia coli K12 elongation factor Tu with GDP and elongation factor Ts. J. Biol. Chem. 256, 5591–5596 (1981).
Lucas-Lenard, J. & Lipmann, F. Separation of three microbial amino acid polymerization factors. Proc. Natl Acad. Sci. USA 55, 1562–1566 (1966).
Mora, L., Zavialov, A., Ehrenberg, M. & Buckingham, R. H. Stop codon recognition and interactions with peptide release factor RF3 of truncated and chimeric RF1 and RF2 from Escherichia coli. Mol. Microbiol. 50, 1467–1476 (2003).
Margus, T., Remm, M. & Tenson, T. Phylogenetic distribution of translational GTPases in bacteria. BMC Genomics 8, 15 (2007).
Zaher, H. S. & Green, R. Quality control by the ribosome following peptide bond formation. Nature 457, 161–166 (2009).
Petropoulos, A. D., McDonald, M. E., Green, R. & Zaher, H. S. Distinct roles for release factor 1 and release factor 2 in translational quality control. J. Biol. Chem. 289, 17589–17596 (2014).
Zaher, H. S. & Green, R. A primary role for release factor 3 in quality control during translation elongation in Escherichia coli. Cell 147, 396–408 (2011).
Zhao, L. et al. Bacterial RF3 senses chaperone function in co-translational folding. Mol. Cell 81, 2914–2928 (2021).
Hoffmann, F. & Rinas, U. Stress induced by recombinant protein production in Escherichia coli. Adv. Biochem. Eng. Biotechnol. 89, 73–92 (2004).
Schmidt, A. et al. The quantitative and condition-dependent Escherichia coli proteome. Nat. Biotechnol. 34, 104–110 (2016).
Florin, T. et al. An antimicrobial peptide that inhibits translation by trapping release factors on the ribosome. Nat. Struct. Mol. Biol. 24, 752–757 (2017).
Milon, P. et al. The nucleotide-binding site of bacterial translation initiation factor 2 (IF2) as a metabolic sensor. Proc. Natl Acad. Sci. USA 103, 13962–13967 (2006).
Hamel, E. & Cashel, M. Guanine nucleotides in protein synthesis. Utilization of pppGpp and dGTP by initiation factor 2 and elongation factor Tu. Arch. Biochem. Biophys. 162, 293–300 (1974).
Rojas, A. M., Ehrenberg, M., Andersson, S. G. & Kurland, C. G. ppGpp inhibition of elongation factors Tu, G and Ts during polypeptide synthesis. Mol. Gen. Genet 197, 36–45 (1984).
Corrigan, R. M., Bellows, L. E., Wood, A. & Grundling, A. ppGpp negatively impacts ribosome assembly affecting growth and antimicrobial tolerance in Gram-positive bacteria. Proc. Natl Acad. Sci. USA 113, E1710–E1719 (2016).
Pausch, P. et al. Structural basis for (p)ppGpp-mediated inhibition of the GTPase RbgA. J. Biol. Chem. 293, 19699–19709 (2018).
Pedro, L., Cross, M., Hofmann, A., Mak, T. & Quinn, R. J. Development of an HPLC-based guanosine monophosphate kinase assay and application to Plasmodium vivax guanylate kinase. Anal. Biochem. 575, 63–69 (2019).
Zhou, D., Tanzawa, T., Lin, J. & Gagnon, M. G. Structural basis for ribosome recycling by RRF and tRNA. Nat. Struct. Mol. Biol. 27, 25–32 (2020).
Gagnon, M. G., Lin, J. & Steitz, T. A. Elongation factor 4 remodels the A-site tRNA on the ribosome. Proc. Natl Acad. Sci. USA 113, 4994–4999 (2016).
Lin, J., Gagnon, M. G., Bulkley, D. & Steitz, T. A. Conformational changes of elongation factor G on the ribosome during tRNA translocation. Cell 160, 219–227 (2015).
Gagnon, M. G., Lin, J., Bulkley, D. & Steitz, T. A. Crystal structure of elongation factor 4 bound to a clockwise ratcheted ribosome. Science 345, 684–687 (2014).
Junemann, R. et al. In vivo deuteration of transfer RNAs: overexpression and large-scale purification of deuterated specific tRNAs. Nucl. Acids Res. 24, 907–913 (1996).
Peske, F., Kuhlenkoetter, S., Rodnina, M. V. & Wintermeyer, W. Timing of GTP binding and hydrolysis by translation termination factor RF3. Nucleic Acids Res. 42, 1812–1820 (2013).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Watson, Z. L. et al. Structure of the bacterial ribosome at 2 A resolution. Elife 9, e60482 (2020).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Svetlov, M. S. et al. Structure of Erm-modified 70S ribosome reveals the mechanism of macrolide resistance. Nat. Chem. Biol. 17, 412–420 (2021).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Liebschner, D. et al. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr. D Struct. Biol. 75, 861–877 (2019).
Williams, C. J. et al. MolProbity: more and better reference data for improved all-atom structure validation. Protein Sci. 27, 293–315 (2018).
Afonine, P. V. et al. New tools for the analysis and validation of cryo-EM maps and atomic models. Acta Crystallogr. D Struct. Biol. 74, 814–840 (2018).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Cardone, G., Heymann, J. B. & Steven, A. C. One number does not fit all: mapping local variations in resolution in cryo-EM reconstructions. J. Struct. Biol. 184, 226–236 (2013).
Acknowledgements
We thank the members of the Gagnon and Lin laboratories for critical reading of the manuscript and suggestions. We are thankful to M. Sherman for help with cryo-EM data acquisition, the Sealy Center for Structural Biology and Molecular Biophysics at the University of Texas Medical Branch for providing critical infrastructure and expertise, and K.-Y. (Clem) Wong and J. Perkyns for computational support. We also thank the Cryo-Electron Microscopy at the Multiscale Research Institute of Complex Systems and the School of Life Sciences at Fudan University. This work was supported by grants from the National Key R&D Program of China (2017YFA0504602 to J.L.), the National Natural Science Foundation of China (no. 31770784 to J.L.), the Innovation Program of Shanghai Municipal Education Commission (2021-01-07-00-07-E00074 to J.L.), the Starry Night Science Fund at Shanghai Institute for Advanced Study of Zhejiang University (SN-ZJU-SIAS-009 to J.L.), the National Institutes of Health (R01GM136936 to M.G.G.), the Welch Foundation (H-2032-20230405 to M.G.G.), startup funds from the University of Texas Medical Branch (to M.G.G.), a National Center for Research Resources NIH grant P41RR001081 (to the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco) for developing UCSF Chimera, and NIH grant R01GM129325 (to the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco) and the Office of Cyber Infrastructure and Computational Biology, National Institute of Allergy and Infectious Diseases for developing UCSF ChimeraX.
Author information
Authors and Affiliations
Contributions
J.L. and M.G.G. designed the project; L.L. purified 70S ribosomes, generated wild-type and mutants RF3 and performed ribosome binding assays; M.Y.R. generated wild-type and mutant RF1, purified tRNAPhe, performed stopped-flow experiments, prepared the samples for structure determination and collected the cryo-EM data. M.Y.R. and M.G.G. processed the cryo-EM data and built the models. J.L. and M.G.G. wrote the manuscript and made the figures with inputs from all authors. All authors reviewed, commented on and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Sara Osman, in collaboration with the Nature Structural & Molecular Biology team. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Cryo-EM data processing and particle classification workflow (dataset I with RF3-GDP and no exogenous nucleotide).
Scheme of the data processing workflow. All steps were performed in cryoSPARC 3.3.278. 10,284 micrographs were collected, of which 8,923 were selected for further processing. After two rounds of reference-free 2D classification, the selected particles were used to generate ab-initio volumes. Particles from the 3D volumes that appeared to be the 70S ribosome based on their shape and size were separated according to their ratcheted state using heterogeneous refinement, yielding three main classes of particles (class averages I, II, and III). Among the non-ratcheted 326,675 particles (class average I), focused 3D variability analysis around RF3 and RF1 was utilized to separate 80,766 particles for containing solid density for RF3 and RF1 or for RF1 only (127,567 particles). The process discarded 40,336 particles containing non-rotated ribosomes and 12,965 particles of rotated ribosomes, both of which were not bound to any factor. The remaining 65,041 particles yielded highly noisy reconstructions and were not further analyzed. Non-uniform and CTF refinement of particles with RF1 and RF3, or with RF1 only, produced structures I-B and I-A, respectively. Local refinement was combined with particle subtraction to produce a higher-quality map of RF3 and RF1 in structure I-B. Similarly, the rotated ribosome particles (74,452 particles in class average II) were separated by focused 3D variability analysis around RF3, producing two defined volumes, one containing rotated ribosome with no factor bound (28,078 particles) and one with rotated ribosome bound to RF3 (33,348 particles). Non-uniform and CTF refinement of the latter volume yielded structure I-C. Local refinement combined with particle subtraction produced a higher-quality map of RF3 in structure I-C.
Extended Data Fig. 2 Local resolution estimation and Fourier Shell Correlation (FSC) validation (dataset I with RF3-GDP and no exogenous nucleotide).
a, Local resolution heat maps on slices of density for structures I-A, I-B, and I-C shown in the range of 2.5 – 6.5 Å resolution, calculated with cryoSPARC 3.3.2 implementation of BlocRes87. b, Gold-standard FSC curves of each half-map (red), using a ‘soft mask’ excluding solvent and model-map (blue), are plotted across resolution. Map and model validation was performed in PHENIX 1.19.285. For structures I-B and I-C, for which composite maps were generated from EM maps of RF3-RF1 (I-B) or RF3 (I-C) obtained after focused refinement and the 70S ribosome, FSC curves for the composite EM map and the model vs composite map fit are shown. c, Local resolution maps shown in the range of 3.0 – 7.0 Å resolution and FSC curves of half-maps (masked with a ‘soft mask’) for RF3-RF1 (I-B) and for RF3 (I-C).
Extended Data Fig. 3 Cryo-EM data processing and particle classification workflow (dataset II with GDPCP).
All steps were performed in cryoSPARC 3.3.278. 9,810 micrographs were collected, of which 9,390 were selected for further processing. After two rounds of reference-free 2D classification, the selected particles were used to generate ab-initio volumes. Particles from the 3D volumes that appeared to be the 70S ribosome based on their shape and size were separated according to their ratcheted state using heterogeneous refinement, yielding three main classes of particles (class averages I, II, and III). Among the non-ratcheted 334,113 particles (class average I), focused 3D variability analysis around RF3 and RF1 was utilized to separate 31,256 particles for containing solid density for RF3 and RF1 or for RF1 only (146,778 particles). The process discarded 59,038 particles containing non-rotated ribosomes with weak density for RF3 and RF1, 58,392 particles containing non-rotated ribosomes with no factor bound, 8,460 particles of non-rotated ribosomes with only RF3 bound, and 30,189 particles that yielded noisy reconstructions. Non-uniform and CTF refinement of particles with RF1 and RF3, or with RF1 only, produced structures II-A and II-D, respectively. Local refinement was combined with particle subtraction to produce a higher-quality map of RF3 and RF1 in structure II-A. Similarly, the rotated ribosome particles (186,545 particles in class average II) were separated by focused 3D variability analysis around RF3, producing two defined volumes (56,202 and 116,992 particles), both of which containing RF3 in a distinct binding position. Non-uniform and CTF refinement of the latter two volumes yielded structures II-B and II-C. Local refinement combined with particle subtraction produced higher-quality maps of RF3 in structures II-B and II-C.
Extended Data Fig. 4 Local resolution estimation and Fourier Shell Correlation (FSC) validation (dataset II with GDPCP).
a, Local resolution heat maps on slices of density for structures II-A, II-B, II-C, and II-D shown in the range of 2.5 – 6.5 Å resolution, calculated with cryoSPARC 3.3.2 implementation of BlocRes87. b, Gold-standard FSC curves of each half-map (red), using a ‘soft mask’ excluding solvent and model-map (blue), are plotted across resolution. Map and model validation was performed in PHENIX 1.19.285. For structures II-A, II-B, and II-C, for which composite maps were generated from EM maps of RF3-RF1 (II-A), or RF3 (II-B and II-C) obtained after focused refinement and the 70S ribosome, FSC curves for the composite EM map and the model vs composite map fit are shown. c, Local resolution maps shown in the range of 3.0 – 7.0 Å resolution and FSC curves of half-maps (masked with a ‘soft mask’) for RF3-RF1 (II-A) and RF3 (II-B and II-C).
Extended Data Fig. 5 Interface between domain III of apo-RF3 and domain I of RF1 in structure I-B.
a, In apo-RF3, the residues for which mutation to alanine reduces the rate of GDP exchange are shown with yellow spheres. These residues form the interface with RF1. Mutation of the residues shown with light blue spheres has a mild effect on the rate of GDP exchange. Note that residue E516 does not interact with RF1. b, The Cα and Cβ atoms of residue N510 contribute to the interface with RF1, while the hydrophilic groups of N510 are not interacting with RF1.
Extended Data Fig. 6 Binding of RF3 to the 70S ribosome termination complex stabilizes domain I of RF1.
a, EM density of structure I-B bound to apo-RF3 and RF1 relative to that of structure I-A bound to RF1 only. b, Clear density for ribosomal proteins uL10 and uL11 in structure I-B bound to apo-RF3 and RF1 (mesh). In structure I-A, these proteins are more flexible as seen from the lack of density (gray surface). c, EM maps of RF1 in structures I-B (mesh) and I-A (gray surface). The presence of apo-RF3 in structure I-B stabilizes domain I of RF1. d, Additional density for the C-terminal domain of ribosomal protein bL12 is observed proximal to the α2-turn-α3 in RF1 and the G’ sub-domain in RF3. e, Relative to structure I-A, the tips of helices H43-H44 of the uL11-ribosomal stalk in structure I-B move toward domain I of RF1 by more than 3 Å. The α1-turn-α2 region in domain I of RF1 forms π-π-stacking interactions with the proline-rich 310-helix in the N-terminus of protein uL11, while nucleotides A1067 (H43) and A1095 (H44) stack along helix α2 of RF1.
Extended Data Fig. 7 Relative orientation of RF3-GDPCP in structure II-A versus that from a prior study (PDB 6GWT)3 (a), and potential steric clash between RF3-GDPCP in structure II-C with RF1 (taken from structure II-A) (b).
a, The structures are aligned based on the 16S rRNA. In the previous study, RF3-GDPCP (gray) is bound to a non-rotated ribosome with RF1 trapped by the antimicrobial peptide apidaecin (PDB 6GWT, state I)3. The orientation of domain III (gray RF3) is such that the closest distance to domain I of RF1 is ~9 Å, precluding the formation of any interaction3. b, The structures are aligned based on the 23S rRNA. On the rotated ribosome, RF3-GDPCP rotates by 27° as a whole between structures II-B (gray) and II-C (colored by domain) to dock against the sarcin-ricin loop (SRL) in the 50S subunit. In the GTP-activated state (II-C), domain III of RF3-GDPCP would clash with domain I of RF1 (red arrow), suggesting that complete accommodation of RF3 on the 50S subunit only occurs following dissociation of the class-I release factor.
Extended Data Fig. 8 Relative orientation of RF3 with GDPCP or GDPNP in previous structures versus that in structure II-B.
The structures are aligned based on the 23S rRNA. a, The conformation of RF3-GDPCP in structure II-B on the rotated ribosome (colored by domain) is highly similar to that of RF3-GDPCP reported in a previous study with the antimicrobial peptide apidaecin (state IV; rotated ribosome) (PDB 6GXO, gray)3. b, Same as in panel (a) relative to previous crystal structures of RF3-GDPNP bound to the rotated E. coli 70S ribosome (PDB 4V85)6 or RF3-GDPCP bound to the rotated Thermus thermophilus 70S ribosome (PDB 4V8O)7. c-f, Close-up views of the sarcin-ricin loop (SRL) and domain I of RF3 from (a) and (b). The catalytic histidine 92 in switch 2 (sw2) is located 9 to 13 Å away from the non-bridging oxygen of A2662 in the SRL, and RF3 is therefore in a GTP-inactive state on the ribosome. Switch 1 (sw1) is orange.
Extended Data Fig. 9 Structure of the ribosome bound to RF3-ppGpp.
a, Relative to the non-rotated ribosome in structure II-A (light gray), the ribosome has the rotated conformation in structure I-C bound to RF3-ppGpp (beige), similar to the ribosome bound to RF3-GDPCP in structure II-B (black). The structures are aligned by the 23S rRNA. b, Close-up view of ribosome-free RF3-ppGpp (PDB 3VR1, beige)26 and RF3-GDP (PDB 3VQT ref. 26, black; PDB 2H5E ref. 10, gray) superimposed with ribosome-bound RF3-ppGpp in structure I-C (colored domains). c, Orthogonal view of (a), showing that RF3-ppGpp is bound proximal to the sarcin-ricin loop (SRL, light green) in the 50S subunit. d, Cryo-EM map of the nucleotide-binding pocket. The density of ppGpp is shown as teal mesh, and that of the Mg2+ ions is shown as black mesh. e, The catalytic histidine 92 in switch 2 (sw2) is located within interaction distance from the β-phosphate non-bridging oxygen. The δ-phosphate oxygen of ppGpp forms a Mg2+-mediated hydrogen bond with A2662 in the SRL and the ε-phosphate interacts with a Mg2+ ion.
Supplementary information
Supplementary Information
Supplementary Figs. 1 and 2, Supplementary Tables 1–4, Uncropped gels for Supplementary Fig. 1a,b,d and Supplementary References
Supplementary Data 1
List and sequences of DNA oligonucleotides used in this study.
Supplementary Data 2
Representative micrograph for apo-RF3 bound to the 70S ribosome termination complex.
Supplementary Data 3
Representative micrograph for RF3–GDPCP bound to the 70S ribosome termination complex.
Supplementary Video 1
Structural basis for the GDP-to-GTP exchange in RF3 catalyzed by the ribosomal termination complex. The animation is a morph between structures I-A, I-B, and II-A. The comparison of apo-RF3 in structure I-B with the structure of ribosome-free RF3-GDP (PDB 2H5E)10 illustrates the conformational changes in RF3 that lead to remodeling of the sw2-helix and of the phosphate-binding loop (P-loop). The flip of the carbonyl oxygen at P22 encroaches into the nucleotide-binding pocket and would collide with the β-phosphate of GDP, promoting its dissociation from RF3. Further comparison between apo-RF3 (I-B) and RF3-GDPCP in structure II-A shows the rotation of domain I of RF3 toward the 50S subunit SRL. Note the absence of interaction between RF3 and the 50S subunit in structures I-B and II-A.
Supplementary Video 2
Dissociation of RF1 catalyzed by RF3–GDPCP and activation of RF3 for GTP hydrolysis. The animation is a morph between structures II-A, II-B, and II-C. The movement of domain I in RF3 toward the SRL is facilitated by the ratchet-like rotation of the 30S subunit. In structure II-B, the catalytic histidine H92 in domain I of RF3 remains 11 Å away from the SRL, indicating that RF3–GDPCP in structure II-B is in a non-activated state. The rolling-like motion of the 30S subunit and the rotation of RF3–GDPCP in structure II-C bring domain I of RF3 proximal to the SRL. The catalytic H92 is within interaction distance from A2662 in the SRL, suggesting that RF3 is in the activated state primed for GTP hydrolysis.
Supplementary Data 4
Uncropped gels for Supplementary Fig. 1a,b,d.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, L., Rybak, M.Y., Lin, J. et al. The ribosome termination complex remodels release factor RF3 and ejects GDP. Nat Struct Mol Biol 31, 1909–1920 (2024). https://doi.org/10.1038/s41594-024-01360-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41594-024-01360-0