Abstract
Vancomycin-resistant Enterococcus faecium (VRE) is a major cause of nosocomial infections, particularly endocarditis and sepsis. With the diminishing effectiveness of antibiotics against VRE, new antimicrobial agents are urgently needed. Our previous research demonstrated the crucial role of Na+-transporting V-ATPase in Enterococcus hirae for growth under alkaline conditions. In this study, we identified a compound, V-161, from 70,600 compounds, which markedly inhibits E. hirae V-ATPase activity. V-161 not only inhibits VRE growth in alkaline conditions but also significantly suppresses VRE colonization in the mouse small intestine. Furthermore, we unveiled the high-resolution structure of the membrane VO part due to V-161 binding. V-161 binds to the interface of the c-ring and a-subunit, constituting the Na+ transport pathway in the membrane, thereby halting its rotation. This structural insight presents potential avenues for developing therapeutic agents for VRE treatment and elucidates the Na+ transport pathway and mechanism.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Cryo-EM maps are deposited in the Electron Microscopy Data Bank (EMDB) under accession code EMD-37440 for maps of the VO part of EhV-ATPase from focused refinement. Atomic models are deposited in the Protein Data Bank (PDB) under accession code PDB 8WCI. Source data are provided with this paper.
References
O’Neill, J. Antimicrobial resistance: tackling a crisis for the health and wealth of nations https://amr-review.org/Publications.html (Review on Antimicrobial Resistance, 2014).
Willyard, A. The drug-resistant bacteria that pose the greatest health threats. Nature 543, 15 (2017).
Murray, B. E. Vancomycin-resistant enterococcal infections. N. Engl. J. Med. 342, 710–721 (2000).
Arias, C. A., Contreras, G. A. & Murray, B. E. Management of multidrug-resistant enterococcal infections. Clin. Nicrobiol. Infect. 16, 555–562 (2010).
Kakinuma, Y., Yamato, I. & Murata, T. Structure and function of vacuolar Na+-translocating ATPase in Enterococcus hirae. J. Bioenerg. Biomembr. 31, 7–14 (1999).
Murata, T., Yamao, I., Igarashi, K. & Kakinuma, Y. Intracellular Na+ regulates transcription of the ntp operon encoding a vacuolar-type Na+-translocating ATPase in Enterococcus hirae. J. Biol. Chem. 271, 23661–23666 (1996).
Murata, T., Takase, K., Yamato, I., Igarashi, K. & Kakinuma, Y. The ntpJ gene in the Enterococcus hirae ntp operon encodes a component of KtrII potassium transport system functionally independent of vacuolar Na+-ATPase. J. Biol. Chem. 271, 10042–10047 (1996).
Shimizu, K., Seiki, I., Goto, Y. & Murata, T. Measurement of the intestinal pH in mice under various conditions reveals alkalization induced by antibiotics. Antibiotics 10, 180 (2021).
Saijo, S. et al. Crystal structure of the central axis DF complex of the prokaryotic V-ATPase. Proc. Natl Acad. Sci. USA 108, 19955–19960 (2011).
Arai, S. et al. Rotation mechanism of Enterococcus hirae V1-ATPase based on asymmetric crystal structures. Nature 493, 703–707 (2013).
Suzuki, K. et al. Crystal structures of the ATP-binding and ADP-release dwells of the V1 rotary motor. Nat. Commun. 7, 13235 (2016).
Maruyama, S. et al. Metastable asymmetrical structure of a shaftless V1 motor. Sci. Adv. 5, eaau8149 (2019).
Minagawa, Y. et al. Basic properties of rotary dynamics of the molecular motor Enterococcus hirae V1-ATPase. J. Biol. Chem. 288, 32700–32707 (2013).
Iida, T. et al. Single-molecule analysis reveals rotational substeps and chemo-mechanical coupling scheme of Enterococcus hirae V1-ATPase. J. Biol. Chem. 294, 17017–17030 (2019).
Isaka, Y. et al. Rotation mechanism of molecular motor V1-ATPase studied by multiscale molecular dynamics simulation. Biophys. J. 112, 911–920 (2017).
Shekhar, M. et al. Revealing a hidden intermediate of rotatory catalysis with X-ray crystallography and molecular simulations. ACS Cent. Sci. 8, 915–925 (2022).
Murata, T., Igarashi, K., Kakinuma, Y. & Yamato, I. Na+ binding of V-type Na+-ATPase in Enterococcus hirae. J. Biol. Chem. 275, 13415–13419 (2000).
Murata, T., Takase, K., Yamato, I., Igarashi, K. & Kakinuma, Y. Purification and reconstitution of Na+-translocating vacuolar ATPase from Enterococcus hirae. J. Biol. Chem. 272, 24885–24890 (1997).
Murata, T., Takase, K., Yamato, I., Igarashi, K. & Kakinuma, Y. Properties of the VOV1 Na+-ATPase from Enterococcus hirae and its VO moiety. J. Biochem. 125, 414–421 (1999).
Murata, T., Yamato, I., Kakinuma, Y., Leslie, A. G. & Walker, J. E. Structure of the rotor of the V-Type Na+-ATPase from Enterococcus hirae. Science 308, 654–659 (2005).
Mizutani, K. et al. Structure of the rotor ring modified with N,N′-dicyclohexylcarbodiimide of the Na+-transporting vacuolar ATPase. Proc. Natl Acad. Sci. USA 108, 13474–13479 (2011).
Murata, T. et al. Ion binding and selectivity of the rotor ring of the Na+-transporting V-ATPase. Proc. Natl Acad. Sci. USA 105, 8607–8612 (2008).
Zhou, M. et al. Mass spectrometry of intact V-type ATPases reveals bound lipids and the effects of nucleotide binding. Science 334, 380–385 (2011).
Murata, T., Yamato, I. & Kakinuma, Y. Structure and mechanism of vacuolar Na+-translocating ATPase from Enterococcus hirae. J. Bioenerg. Biomembr. 37, 411–413 (2005).
Murata, T., Kawano, M., Igarashi, K., Yamato, I. & Kakinuma, Y. Catalytic properties of Na+-translocating V-ATPase in Enterococcus hirae.Biochim. Biophys. Acta 1505, 75–81 (2001).
Guo, H., Bueler, S. A. & Rubinstein, J. L. Atomic model for the dimeric FO region of mitochondrial ATP synthase. Science 358, 936–940 (2017).
Srivastava, A. P. et al. High-resolution cryo-EM analysis of the yeast ATP synthase in a lipid membrane. Science 360, eaas9699 (2018).
Murphy, B. J. et al. Rotary substates of mitochondrial ATP synthase reveal the basis of flexible F1–FO coupling. Science 364, eaaw9128 (2019).
Spikes, T. E., Montgomery, M. G. & Walker, J. E. Structure of the dimeric ATP synthase from bovine mitochondria. Proc. Natl Acad. Sci. USA 117, 23519–23526 (2020).
Demmer, J. K. et al. Structure of ATP synthase from ESKAPE pathogen Acinetobacter baumannii. Sci. Adv. 8, eabl5966 (2022).
Zhou, L. & Sazanov, L. A. Structure and conformational plasticity of the intact Thermus thermophilus V/A-type ATPase. Science 365, eaaw9144 (2019).
Zhao, J., Benlekbir, S. & Rubinstein, J. L. Electron cryomicroscopy observation of rotational states in a eukaryotic V-ATPase. Nature 521, 241–245 (2015).
Mazhab-Jafari, M. T. et al. Atomic model for the membrane-embedded VO motor of a eukaryotic V-ATPase. Nature 539, 118–122 (2016).
Roh, S. H. et al. The 3.5-Å cryoEM structure of nanodisc-reconstituted yeast vacuolar ATPase VO proton channel. Mol. Cell 69, 993–1004.e3 (2018).
Abbas, Y. M., Wu, D., Bueler, S. A., Robinson, C. V. & Rubinstein, J. L. Structure of V-ATPase from the mammalian brain. Science 367, 1240–1246 (2020).
Burton-Smith, R. N. et al. Six states of Enterococcus hirae V-type ATPase reveals non-uniform rotor rotation during turnover. Commun. Biol. 6, 755 (2023).
Kawano, M., Igarashi, K., Yamato, I. & Kakinuma, Y. Arginine residue at position 573 in Enterococcus hirae vacuolar-type ATPase NtpI subunit plays a crucial role in Na+ translocation. J. Biol. Chem. 277, 24405–24410 (2002).
Savall, B. M., Fontimayor, J. R. & Edwards, J. P. Selective phenol alkylation for an improved synthesis of 2-arylbenzimidazole H4 receptor ligands. Tetrahedron Lett. 50, 2490–2492 (2009).
Ueno, H. et al. Torque generation of Enterococcus hirae V-ATPase. J. Biol. Chem. 289, 31212–31223 (2014).
Otomo, A. et al. Direct observation of stepping rotation of V-ATPase reveals rigid component in coupling between VO and V1 motors. Proc. Natl Acad. Sci. USA 119, e2210204119 (2022).
Fukuda, S. et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 469, 543–547 (2011).
Theriot, C. M. et al. Cefoperazone-treated mice as an experimental platform to assess differential virulence of Clostridium difficile strains. Gut Microbes 2, 326–334 (2011).
Sobti, M. et al. Cryo-EM structures provide insight into how E. coli F1FO ATP synthase accommodates symmetry mismatch. Nat. Commun. 11, 2615 (2020).
Roh, S. H. et al. Cryo-EM and MD infer water-mediated proton transport and autoinhibition mechanisms of VO complex. Sci. Adv. 6, eabb9605 (2020).
Kakinuma, Y. & Igarashi, K. Purification and characterization of the catalytic moiety of vacuolar-type Na+-ATPase from Enterococcus hirae. J. Biochem. 116, 1302–1308 (1994).
Mastronarde, D. N. SerialEM: a program for automated tilt series acquisition on Tecnai microscopes using prediction of specimen position. Microsc. Microanal. 9, 1182–1183 (2003).
Zivanov, J., Nakane, T. & Scheres, S. H. W. Estimation of high-order aberrations and anisotropic magnification from cryo-EM data sets in RELION-3.1. IUCrJ 7, 253–267 (2020).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Rohou, A. & Grigorieff, N. CTFFIND4: fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Zivanov, J., Nakane, T. & Sheres, S. H. W. A Bayesian approach to beam-induced motion correction in cryo-EM single-particle analysis. IUCrJ 6, 5–17 (2019).
Chen, S. et al. High-resolution noise substitution to measure overfitting and validate resolution in 3D structure determination by single particle electron cryomicroscopy. Ultramicroscopy 135, 24–35 (2013).
Waterhouse, A. et al. Swiss-model: homology modelling of protein structures and complexes. Nucleic Acids Res. 46, W296–W303 (2018).
Kishikawa, J. I. et al. Mechanical inhibition of isolated VO from V/A-ATPase for proton conductance. eLife 9, e56862 (2020).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Liebschner, D. et al. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr. D Struct. Biol. 75, 861–877 (2019).
Schüttelkopf, A. W. & van Aalten, D. M. PRODRG: a tool for high-throughput crystallography of protein–ligand complexes. Acta Crystallogr D Biol. Crystallogr 60, 1355–1363 (2004).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Williams, C. J. et al. MolProbity: more and better reference data for improved all-atom structure validation. Protein Sci. 27, 293–315 (2018).
Lovell, S. C. et al. Structure validation by Cα geometry: ϕ, ψ and Cβ deviation. Proteins 50, 437–450 (2003).
Dolinsky, T. J., Nielsen, J. E., McCammon, J. A. & Baker, N. A. PDB2PQR: an automated pipeline for the setup, execution, and analysis of Poisson–Boltzmann electrostatics calculations. Nucleic Acids Res. 32, W665–W667 (2004).
Søndergaard, C. R., Olsson, M. H. M., Rostkowski, M. & Jensen, J. H. Improved treatment of ligands and coupling effects in empirical calculation and rationalization of pKa values. J. Chem. Theory Comput. 7, 2284–2295 (2011).
Holst, M. & Saied, F. Multigrid solution of the Poisson–Boltzmann equation. J. Comput. Chem. 14, 105–113 (1993).
Jurrus, E. et al. Improvements to the APBS biomolecular solvation software suite. Protein Sci. 27, 112–128 (2018).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Schrödinger, L. & DeLano, W. The PyMOL Molecular Graphics System https://www.pymol.org/pymol (Schrödinger, 2020).
Laskowski, R. A. & Swindells, M. B. LigPlot+: multiple ligand–protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 51, 2778–2786 (2011).
Case, D. A. et al. Amber 2021 https://ambermd.org (University of California, 2021).
Tian, C. et al. ff19SB: amino-acid-specific protein backbone parameters trained against quantum mechanics energy surfaces in solution. J. Chem. Theory Comput. 16, 528–552 (2020).
Wang, L.-P., Martinez, T. J. & Pande, V. S. Building force fields—an automatic, systematic, and reproducible approach. J. Phys. Chem. Lett. 5, 1885–1891 (2014).
Ryckaert, J.-P., Ciccotti, G. & Berendsen, H. J. C. Numerical integration of the Cartesian equations of motion of a system with constraints: molecular dynamics of n-alkanes. J. Comput. Phys. 23, 327–341 (1977).
Allen, M. P. & Tildesley, D. J. Computer Simulation of Liquids (Oxford University Press, 1987).
Chow, K. H. & Ferguson, D. M. Isothermal-isobaric molecular dynamics simulations with Monte Carlo volume sampling. Comput. Phys. Commun. 91, 283–289 (1995).
Acknowledgements
This paper is dedicated to the late Y. Kakinuma, who passed away at the age of 64 in January 2016, in memory of his important contributions to the research of EhV-ATPase. We thank the staff at the KEK Structure Biology Research Center and the University of Tokyo for assistance with cryo-EM operation. We also thank K. Mizutani, T. Ajiro and I. Seiki for their technical support with the in vitro experiments. This work was supported by Grant-in-Aid for Scientific Research, from Japan Society for the Promotion of Science (JSPS) under grant numbers 18H05425 (T. Murata), 21H02409 (T. Murata), 24H00550 (T. Murata), 23H02427 (T. Moriya), 18H05424 (R.I.), 21H02454 (R.I.), 21K15060 (A.O.), 18H05426 (M.I.), 21K06108 (S.Y.), 19H03465 (Y.G.) and 23K18285 (Y.G.); by the Research Support Project for Life Science and Drug Discovery (Basis for Supporting Innovative Drug Discovery and Life Science Research (BINDS)) from Japan Agency for Medical Research and Development (AMED) under grant numbers JP23ama121001 (T.S.), JP23ama121002 (M.K.), JP23ama121007 (S.I.), JP23ama121013 (T. Murata), JP23ama121023 (M.I.), JP23ama121053 (H.K.), 21fk0108092h0003 (T. Murata), 23fk0108604h0903 (H.T.) and 23fk0108665h0301 (H.T.) and JP223fa627003 (Y.G.); by Research Program on ensuring Food Safety from Japanese Ministry of Health, Labour and Welfare under grant numbers 21KA1004 (H.T.); by Ministry of Education, Culture, Sports, Science and Technology (MEXT) under grant numbers 22K07067 (H.T.), 22K16368 (Y.H.) and 22K07052 (J.K.); by the grant of OML Project by the National Institutes of Natural Sciences (NINS program No. OML022301); by JST FOREST Program under grant number JPMJFR225D (Y.G.); and by IAAR Research Support Program and Initiative for Realizing Diversity in the Research Environment (T. Murata, K. Suzuki), Chiba University, Japan.
Author information
Authors and Affiliations
Contributions
T. Murata conceived the project. K. Suzuki, A.O., K. Shimizu, S.M., F.L.I. and T. Murata prepared the protein samples. K. Shimizu, F.L.I., Y.S. and S.I. screened the inhibitors. K.M. synthesized the inhibitor. A.O. and R.I. performed single-molecule rotation experiments. Y.H., J.K. and H.T. developed deficient strains. K. Suzuki, A.O., K. Shimizu, S.A., S.M., F.L.I. and Y.S. performed the in vitro experiments. Y.G., K. Shimizu, S.A. and S.O. performed in vivo experiments. K. Suzuki, S.M., N.A., M.K., T.S. and T. Moriya performed cryo-EM data collection and processing. K. Suzuki built and refined the atomic models. S.Y. and M.I. performed MD simulations. K. Suzuki, Y.G. A.O., K.M., S.Y., R.I., T. Moriya and T. Murata analyzed the results, prepared the figures and wrote the paper. All the authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks Alastair Stewart, Stephan Wilkens and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Katarzyna Ciazynska and Dimitris Typas, in collaboration with the Nature Structural & Molecular Biology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Previous structural analyses of Na+-transporting V-ATPase (EhV-ATPase) from Enterococcus hirae.
(a) Mechanistic model of EhV-ATPase. (b) Crystal structure (PDB ID: 3VR4) of the EhV1-ATPase (A3B3DF complex)10. (c) Crystal structure (PDB ID: 2BL2) of the Na+-bound c-ring20. The right panel displays a magnified view of the Na+-binding site. (d) Local resolution assessment of the reconstructed map from a previously reported Cryo-EM structure of EhV-ATPase36.
Extended Data Fig. 2 Na+-transporting V-ATPase is required for VRE growth under alkaline conditions and for colonization in the small intestine in Mice.
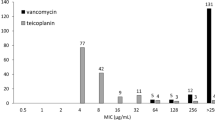
(a) The ntpI gene cording the a-subunit was replaced with the cat gene to generate the a-subunit-deficient VRE (ΔEfV). Refer to Supplementary Methods. (b) Susceptibility of ΔEfV to the antibiotics was comparable to the WT VRE. (c) Time-course growth curves of WT and ΔEfV under varying pH conditions. (d) Growth comparison of WT and ΔEfV at 12 h, as indicated by the red line in (c). (c–d) All data are presented as mean ± standard error of the mean from three independent experiments. P values were determined using a two-tailed t-test. (e) The number of VRE in the intestinal contents and faeces of WT or ΔEfV-infected mice (n = 5 per group). P values, determined using a two-tailed Mann-Whitney U test, are shown in parentheses. L.o.D., limit of detection. Statistical significance is denoted as *P < 0.05, **P < 0.01. NS indicates no significant difference.
Extended Data Fig. 3 Cryo-EM data processing and analysis focusing on the VO part.
(a) Workflow diagram illustrating the process to derive the final VO part Cryo-EM map. 3,264 micrographs were collected and processed for the cryo-EM reconstruction. (b) Fourier shell correlation (FSC) curves for the reconstructed Cryo-EM half-set maps and between the atomic model and full-set map. (c) Angular distribution plot. (d) Local resolution of the reconstructed map. The right panel provides a detailed view of the V-161 binding site at the interface of the c-ring and the a-subunit.
Extended Data Fig. 4 Example model-in-map fit for various helices, cardiolipin, and V-161 map regions.
(a) Overall structure of the VO part showing positions (b–k) of helices. (b–n) Model-in-map fits for: (b,c) Na+-bound c-subunit, (d,e) Na+-unbound c-subunit situated at the centre region of the a-subunit boundary (black), (f–k) C-terminal domain of a-subunit, (l) Cardiolipin, and (m,n) V-161. The surface structures of the Cryo-EM densities are rendered at 5.0 sigma (b–m) and 9.0 sigma (n) are shown, respectively.
Extended Data Fig. 5 Na+-binding sites in the VO part.
(a) Model-in-map of the VO part presented in a cross-sectional top view as in Fig. 3e. The left panel shows a magnified view of the empty Na+-binding site, as highlighted in the red box. (b–k) Model-in-maps of all Na+-binding sites, corresponding to the black boxes in (a). (l) The Na+-binding site in (b) is superimposed at all Cα atom onto those of 8 Na+ bound sites. (m) The Na+-binding site in (b) is superimposed at Cα atom onto that of the empty Na+-binding site (c) shown in black. The surface structures of the Cryo-EM densities are rendered at 5.0 sigma are shown.
Extended Data Fig. 6 Schematic representation of the interactions between the ligands and VO part.
(a–c) The interactions between the ligands (a: V-161, b: Na+, c: cardiolipin) and VO part were analysed using the Ligplot+ program67. Hydrogen bonds are shown as green dashed lines. Van der Waals contacts are represented by the spoked arcs oriented toward the ligand atoms they contact. The range of distances defining van derWaals contacts is 2.9–3.9 Å.
Extended Data Fig. 7 Comparison of V-161 binding site of EhV-ATPase with the corresponding sites of other V- and F-ATPases.
(a–d) Detailed views of the V-161 binding site of EhV-ATPase (a,b) and the corresponding site of F-ATPase (PDB ID: 6RDC) from Polytomella sp. Pringsheim (c,d), viewed as in Figs. 4i, j. (e) Sequence alignment of the c-subunit and the a-subunit from various V-ATPases (Na+-transporting types: from E. hirae and E. faecium; H+-transporting types: from rat and yeast) and F-ATPases (Na+-transporting types: from I. tartaricus and P. modestum; H+-transporting types: from bovine mitochondria (PDB ID: 6ZQM), yeast mitochondria (PDB ID: 6B8H), and Polytomella sp. Pringsheim (PDB ID: 6RDC)). Box colours adhere to the scheme in Fig. 3g. Residue numbers for E. hirae and Polytomella sp. Pringsheim are provided above and below the alignment, respectively. The residues c-T140 and a-N615 of Na+-transporting V-ATPase (shown in red boxes) are not conserved in other ATPases.
Extended Data Fig. 8 Comparison of ion transport pathways of Na+-transporting EhV-ATPase and H+-transporting V-ATPases.
The colours of the ion transport pathways are consistent with those in Fig. 4. (a–f) Side views (as in Fig. 4j) and top views (as in Fig. 4c) of the electronegative cavity leading to the periplasm of the VO parts from E. hirae (a, d), yeast (b, e; PDB ID: 6M0S), and rat (c, f; PDB ID: 6VQC). Residues conserved across both the Na+- and H+-transporting V-ATPases are highlighted in cyan boxes. The V-161 binding sites specific to EhV-ATPase and analogous sites in H+-transporting V-ATPases from yeast and rat are shown in red boxes.
Supplementary information
Supplementary Information
Supplementary Tables 1–2, Supplementary Figs. 1–3, Supplementary Methods
Supplementary Video 1
Structure of the VO part of EhV-ATPase with V-161.
Supplementary Video 2
Na+ transport pathway of EhV-ATPase.
Supplementary Video 3
Na+ transport pathway to periplasm predicted using MD simulations.
Supplementary Video 4
Proposed mechanisms of Na+ transport and inhibition in EhV-ATPase.
Supplementary Data 1
Source data for Supplementary Fig. 1.
Source data
Source Data Fig. 1
Statistical Source Data
Source Data Fig. 2
Statistical Source Data
Source Data Fig. 4
Statistical Source Data
Source Data Extended Data Fig./Table 2
Unprocessed gels related to Extended Data Fig. 2a
Source Data Extended Data Fig./Table 2
Statistical Source Data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Suzuki, K., Goto, Y., Otomo, A. et al. Na+-V-ATPase inhibitor curbs VRE growth and unveils Na+ pathway structure. Nat Struct Mol Biol 32, 450–458 (2025). https://doi.org/10.1038/s41594-024-01419-y
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41594-024-01419-y