Abstract
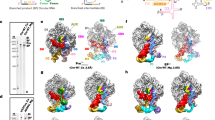
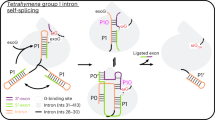
Circularly permuted group II introns (CP introns) consist of rearranged structural domains separated by two tethered exons, generating branched introns and circular exons via back-splicing. Structural and mechanistic understanding of circular RNA (circRNA) generation by CP introns remains elusive. We resolve cryo-electron microscopy structures of a natural CP intron in different states during back-splicing at a resolution of 2.5–2.9 Å. Domain 6 (D6) undergoes a conformational change of 65° after branching, to facilitate 3′-exon recognition and circularization. Previously unseen tertiary interactions compact the catalytic triad and D6 for splicing without protein, whereas a metal ion, M35, is observed to stabilize the 5′-exon during splicing. While these unique features were not observed in canonical group II introns and spliceosomes, they are common in CP introns, as demonstrated by the cryo-EM structure of another CP intron discovered by comparative genomics analysis. Our results elucidate the mechanism of CP intron back-splicing dynamics, with potential applications in circRNA research and therapeutics.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The cryo-EM maps and associated atomic coordinate models of Comamonas testosteroni KF-1 CP group II intron pre-1S, 1S, 2S and post-2S, and Paracandidimonas lactea strain Q2-2 CP group II intron 2S have been deposited in the wwPDB OneDep System under EMD accession codes EMD-38649, EMD-38647, EMD-38648, EMD-38646 and EMD-60833 and PDB codes 8XTS, 8XTQ, 8XTR, 8XTP and 9IS7, respectively. The NCBI nucleotide dataset (https://ftp.ncbi.nlm.nih.gov/blast/db/FASTA/nt.gz) was used for comparative analysis. Full-length WT and mutated Comamonas testosteroni KF-1 sequences were used according to NCBI (GenBank: NZ_AAUJ02000001.1). Full-length WT Paracandidimonas lactea strain Q2-2 sequence was used according to NCBI (GenBank: NZ_JAJJOZ000000000.1). Raw data for sequence and structure conservation analyses are included in Supplementary Data 1. All other data are available from the authors on reasonable request. Source data are provided with this paper.
References
Pyle, A. M. Group II intron self-splicing. Annu. Rev. Biophys. 45, 183–205 (2016).
Lambowitz, A. M. & Zimmerly, S. Group II introns: mobile ribozymes that invade DNA. Cold Spring Harb. Perspect. Biol. 3, a003616 (2011).
Pyle, A. & Lambowitz, A. in The RNA World 3rd edn (eds Gesteland, R. F. et al.) 469–505 (Cold Spring Harbor Laboratory, 2006).
Zimmerly, S. & Semper, C. Evolution of group II introns. Mob. DNA 6, 7 (2015).
Lambowitz, A. M. & Belfort, M. in Mobile DNA III (eds Chandler, M. et al.) 1209–1236 (ASM, 2015).
Galej, W. P., Toor, N., Newman, A. J. & Nagai, K. Molecular mechanism and evolution of nuclear pre-mRNA and group II intron splicing: insights from cryo-electron microscopy structures. Chem. Rev. 118, 4156–4176 (2018).
Wilkinson, M. E., Charenton, C. & Nagai, K. RNA splicing by the spliceosome. Annu. Rev. Biochem. 89, 359–388 (2020).
Xu, L., Liu, T., Chung, K. & Pyle, A. M. Structural insights into intron catalysis and dynamics during splicing. Nature 624, 682–688 (2023).
Haack, D. B. et al. Cryo-EM structures of a group II intron reverse splicing into DNA. Cell 178, 612–623.e12 (2019).
Pan, T. & Uhlenbeck, O. C. Circularly permuted DNA, RNA and proteins—a review. Gene 125, 111–114 (1993).
Grishin, N. V. Fold change in evolution of protein structures. J. Struct. Biol. 134, 167–185 (2001).
Bliven, S. & Prlić, A. Circular permutation in proteins. PLoS Comput. Biol. 8, e1002445 (2012).
Ford, E. & Ares, M. Synthesis of circular RNA in bacteria and yeast using RNA cyclase ribozymes derived from a group I intron of phage T4. Proc. Natl Acad. Sci. USA 91, 3117–3121 (1994).
Jarrell, K. A. Inverse splicing of a group II intron. Proc. Natl Acad. Sci. USA 90, 8624–8627 (1993).
Liu, C.-X. & Chen, L.-L. Circular RNAs: characterization, cellular roles, and applications. Cell 185, 2016–2034 (2022).
Qu, L. et al. Circular RNA vaccines against SARS-CoV-2 and emerging variants. Cell 185, 1728–1744.e16 (2022).
Roth, A., Weinberg, Z., Vanderschuren, K., Murdock, M. H. & Breaker, R. R. Natural circularly permuted group II introns in bacteria produce RNA circles. iScience 24, 103431 (2021).
Chen, C. et al. A flexible, efficient, and scalable platform to produce circular RNAs as new therapeutics. Preprint at bioRxiv https://doi.org/10.1101/2022.05.31.494115 (2022).
Ma, H., Jia, X., Zhang, K. & Su, Z. Cryo-EM advances in RNA structure determination. Signal Transduct. Target. Ther. 7, 58 (2022).
Luo, B. et al. Cryo-EM reveals dynamics of Tetrahymena group I intron self-splicing. Nat. Catal. 6, 298–309 (2023).
Li, S., Palo, M. Z., Zhang, X., Pintilie, G. & Zhang, K. Snapshots of the second-step self-splicing of Tetrahymena ribozyme revealed by cryo-EM. Nat. Commun. 14, 1294 (2023).
Zhang, X., Li, S., Pintilie, G., Palo, M. Z. & Zhang, K. Snapshots of the first-step self-splicing of Tetrahymena ribozyme revealed by cryo-EM. Nucleic Acids Res. 51, 1317–1325 (2023).
Steitz, T. A. & Steitz, J. A. A general two-metal-ion mechanism for catalytic RNA. Proc. Natl Acad. Sci. USA 90, 6498–6502 (1993).
Heilman-Miller, S. L. & Woodson, S. A. Effect of transcription on folding of the Tetrahymena ribozyme. RNA 9, 722–733 (2003).
Toor, N., Keating, K. S., Taylor, S. D. & Pyle, A. M. Crystal structure of a self-spliced group II intron. Science 320, 77–82 (2008).
Haack, D. B., Rudolfs, B., Zhang, C., Lyumkis, D. & Toor, N. Structural basis of branching during RNA splicing. Nat. Struct. Mol. Biol. 31, 179–189 (2024).
Erat, M. C. & Sigel, R. K. O. Divalent metal ions tune the self-splicing reaction of the yeast mitochondrial group II intron Sc.ai5γ. J. Biol. Inorg. Chem. 13, 1025–1036 (2008).
Marcia, M. & Pyle, A. M. Visualizing group II intron catalysis through the stages of splicing. Cell 151, 497–507 (2012).
Nguyen, T. H. D. et al. CryoEM structures of two spliceosomal complexes: starter and dessert at the spliceosome feast. Curr. Opin. Struct. Biol. 36, 48–57 (2016).
Boudvillain, M., de Lencastre, A. & Pyle, A. M. A tertiary interaction that links active-site domains to the 5′ splice site of a group II intron. Nature 406, 315–318 (2000).
Costa, M. & Michel, F. Frequent use of the same tertiary motif by self‐folding RNAs. EMBO J. 14, 1276–1285 (1995).
Boudvillain, M. & Marie Pyle, A. Defining functional groups, core structural features and inter‐domain tertiary contacts essential for group II intron self‐splicing: a NAIM analysis. EMBO J. 17, 7091–7104 (1998).
Fedorova, O. & Pyle, A. M. Linking the group II intron catalytic domains: tertiary contacts and structural features of domain 3. EMBO J. 24, 3906–3916 (2005).
Robart, A. R., Chan, R. T., Peters, J. K., Rajashankar, K. R. & Toor, N. Crystal structure of a eukaryotic group II intron lariat. Nature 514, 193–197 (2014).
Qu, G. et al. Structure of a group II intron in complex with its reverse transcriptase. Nat. Struct. Mol. Biol. 23, 549–557 (2016).
Jacquier, A. & Michel, F. Base-pairing interactions involving the 5′ and 3′-terminal nucleotides of group II self-splicing introns. J. Mol. Biol. 213, 437–447 (1990).
Liu, N. et al. Exon and protein positioning in a pre-catalytic group II intron RNP primed for splicing. Nucleic Acids Res. 48, 11185–11198 (2020).
Chung, K. et al. Structures of a mobile intron retroelement poised to attack its structured DNA target. Science 378, 627–634 (2022).
Costa, M., Walbott, H., Monachello, D., Westhof, E. & Michel, F. Crystal structures of a group II intron lariat primed for reverse splicing. Science 354, aaf9258 (2016).
Plangger, R. et al. Branch site bulge conformations in domain 6 determine functional sugar puckers in group II intron splicing. Nucleic Acids Res. 47, 11430–11440 (2019).
Bertram, K. et al. Structural insights into the roles of metazoan-specific splicing factors in the human step 1 spliceosome. Mol. Cell 80, 127–139.e6 (2020).
Wilkinson, M. E., Fica, S. M., Galej, W. P. & Nagai, K. Structural basis for conformational equilibrium of the catalytic spliceosome. Mol. Cell 81, 1439–1452.e39 (2021).
Jacquier, A. & Michel, F. Multiple exon-binding sites in class II self-splicing introns. Cell 50, 17–29 (1987).
Costa, M., Michel, F. & Westhof, E. A three‐dimensional perspective on exon binding by a group II self‐splicing intron. EMBO J. 19, 5007–5018 (2000).
Weinberg, Z. et al. Detection of 224 candidate structured RNAs by comparative analysis of specific subsets of intergenic regions. Nucleic Acids Res. 45, 10811–10823 (2017).
Costa, M., Dème, E., Jacquier, A. & Michel, F. Multiple tertiary interactions involving domain II of group II self-splicing introns. J. Mol. Biol. 267, 520–536 (1997).
Harris-Kerr, C. L., Zhang, M. & Peebles, C. L. The phylogenetically predicted base-pairing interaction between alpha and alpha′ is required for group II splicing in vitro. Proc. Natl Acad. Sci. USA 90, 10658–10662 (1993).
Fedorova, O., Mitros, T. & Pyle, A. M. Domains 2 and 3 interact to form critical elements of the group II intron active site. J. Mol. Biol. 330, 197–209 (2003).
Mikheeva, S., Murray, H. L., Zhou, H., Turczyk, B. M. & Jarrell, K. A. Deletion of a conserved dinucleotide inhibits the second step of group II intron splicing. RNA 6, 1509–1515 (2000).
de Lencastre, A. & Pyle, A. M. Three essential and conserved regions of the group II intron are proximal to the 5′-splice site. RNA 14, 11–24 (2008).
Lambert, D., Leipply, D., Shiman, R. & Draper, D. E. The influence of monovalent cation size on the stability of RNA tertiary structures. J. Mol. Biol. 390, 791–804 (2009).
Woodson, S. A. Metal ions and RNA folding: a highly charged topic with a dynamic future. Curr. Opin. Chem. Biol. 9, 104–109 (2005).
Gordon, P. M. & Piccirilli, J. A. Metal ion coordination by the AGC triad in domain 5 contributes to group II intron catalysis. Nat. Struct. Biol. 8, 893–898 (2001).
Sontheimer, E. J., Gordon, P. M. & Piccirilli, J. A. Metal ion catalysis during group II intron self-splicing: parallels with the spliceosome. Genes Dev. 13, 1729–1741 (1999).
Marcia, M. & Pyle, A. M. Principles of ion recognition in RNA: insights from the group II intron structures. RNA 20, 516–527 (2014).
Su, L. J., Waldsich, C. & Pyle, A. M. An obligate intermediate along the slow folding pathway of a group II intron ribozyme. Nucleic Acids Res. 33, 6674–6687 (2005).
Pyle, A. M., Fedorova, O. & Waldsich, C. Folding of group II introns: a model system for large, multidomain RNAs? Trends Biochem. Sci. 32, 138–145 (2007).
Cate, J. H. et al. Crystal structure of a group I ribozyme domain: principles of RNA packing. Science 273, 1678–1685 (1996).
Roth, A. et al. A widespread self-cleaving ribozyme class is revealed by bioinformatics. Nat. Chem. Biol. 10, 56–60 (2014).
Weinberg, C. E., Weinberg, Z. & Hammann, C. Novel ribozymes: discovery, catalytic mechanisms, and the quest to understand biological function. Nucleic Acids Res. 47, 9480–9494 (2019).
Jimenez, R. M., Polanco, J. A. & Lupták, A. Chemistry and biology of self-cleaving ribozymes. Trends Biochem. Sci. 40, 648–661 (2015).
Chi, S. I., Dahl, M., Emblem, Å. & Johansen, S. D. Giant group I intron in a mitochondrial genome is removed by RNA back-splicing. BMC Mol. Biol. 20, 16 (2019).
Birkedal, U., Beckert, B., Wilson, D. N. & Nielsen, H. The 23S ribosomal RNA from Pyrococcus furiosus is circularly permuted. Front. Microbiol. 11, 582022 (2020).
Soma, A. Circularly permuted tRNA genes: their expression and implications for their physiological relevance and development. Front. Genet. 5, 63 (2014).
Hammann, C., Luptak, A., Perreault, J. & de la Peña, M. The ubiquitous hammerhead ribozyme. RNA 18, 871–885 (2012).
Chen, L.-L. The expanding regulatory mechanisms and cellular functions of circular RNAs. Nat. Rev. Mol. Cell Biol. 21, 475–490 (2020).
Xiao, M.-S., Ai, Y. & Wilusz, J. E. Biogenesis and functions of circular RNAs come into focus. Trends Cell Biol. 30, 226–240 (2020).
Chen, C.-Y. & Sarnow, P. Initiation of protein synthesis by the eukaryotic translational apparatus on circular RNAs. Science 268, 415–417 (1995).
Pamudurti, N. R. et al. Translation of CircRNAs. Mol. Cell 66, 9–21.e7 (2017).
Chen, C.-K. et al. Structured elements drive extensive circular RNA translation. Mol. Cell 81, 4300–4318.e13 (2021).
Yang, Y. et al. Extensive translation of circular RNAs driven by N6-methyladenosine. Cell Res. 27, 626–641 (2017).
Litke, J. L. & Jaffrey, S. R. Highly efficient expression of circular RNA aptamers in cells using autocatalytic transcripts. Nat. Biotechnol. 37, 667–675 (2019).
Gray, M. W., Lukeš, J., Archibald, J. M., Keeling, P. J. & Doolittle, W. F. Irremediable complexity? Science 330, 920–921 (2010).
Zhou, B. et al. Coevolution of RNA and protein subunits in RNase P and RNase MRP, two RNA processing enzymes. J. Biol. Chem. 300, 105729 (2024).
Deng, P. et al. Structural RNA components supervise the sequential DNA cleavage in R2 retrotransposon. Cell 186, 2865–2879.e20 (2023).
Chen, X. et al. RNA sample optimization for cryo-EM analysis. Nat. Protoc. https://doi.org/10.1038/s41596-024-01072-1 (2024).
Kao, C., Zheng, M. & Rüdisser, S. A simple and efficient method to reduce nontemplated nucleotide addition at the 3 terminus of RNAs transcribed by T7 RNA polymerase. RNA 5, 1268–1272 (1999).
Inoue, T., Sullivan, F. X. & Cech, T. R. New reactions of the ribosomal RNA precursor of Tetrahymena and the mechanism of self-splicing. J. Mol. Biol. 189, 143–165 (1986).
Punjani, A. et al. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Meng, E. C. et al. UCSF ChimeraX: tools for structure building and analysis. Protein Sci. 32, e4792 (2023).
Ma, H. et al. Auto-DRRAFTER: automated RNA modeling based on cryo-EM density. Methods Mol. Biol. 2568, 193–211 (2023).
Wang, X. et al. CryoREAD: de novo structure modeling for nucleic acids in cryo-EM maps using deep learning. Nat. Methods 20, 1739–1747 (2023).
Emsley, P. et al. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Liebschner, D. et al. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr. D Struct. Biol. 75, 861–877 (2019).
Williams, C. J. et al. MolProbity: more and better reference data for improved all-atom structure validation. Protein Sci. 27, 293–315 (2018).
Pintilie, G. et al. Measurement of atom resolvability in cryo-EM maps with Q-scores. Nat. Methods 17, 328–334 (2020).
Nawrocki, E. P. & Eddy, S. R. Infernal 1.1: 100-fold faster RNA homology searches. Bioinformatics 29, 2933–2935 (2013).
Rivas, E., Clements, J. & Eddy, S. R. A statistical test for conserved RNA structure shows lack of evidence for structure in lncRNAs. Nat. Methods 14, 45–48 (2017).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Acknowledgements
Cryo-EM data were collected on Can Cong at SKLB West China Cryo-EM Center and processed at SKLB Duyu High Performance Computing Center in West China Hospital of Sichuan University. This work was supported by National Key Research and Development Program of China (2022YFC2303700 to Z.S., 2021YFA1301900 to H.D.), Natural Science Foundation of China (NSFC 32222040 and 32070049 to Z.S., 32300030 to Y.Y. and 81970936 to D.H.) and the 1.3.5 Project for Disciplines Excellence of West China Hospital (ZYYC21006 to Z.S.).
Author information
Authors and Affiliations
Contributions
Z.S. conceived the project. L.W. and C.Z. performed RNA preparation and gel electrophoresis. L.W., C.Z. and Y.Y. collected cryo-EM data. L.W., D.H. and Z.S. processed cryo-EM data. L.W., J.X., J.Z., S.S. and Z.S. generated RNA atomic coordinates. L.W., Z.H., Y.Y., X.C., H.D. and J.L. prepared figure illustrations. All authors contributed to the preparation of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Sara Osman, in collaboration with the Nature Structural & Molecular Biology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Cte 1 CP intron back-splicing compared to canonical group II intron splicing.
a. Schematic representation of canonical group II intron self-splicing to generate linear exon product. b. Representative denaturing gel electrophoresis of Cte 1 IVT product from three independent experiments with consistent results. c. Representative denaturing gel electrophoresis of Cte 1 Pre RNA splicing in the presence of Ca2+ at different time points from three independent experiments with consistent results. Red boxes highlight samples subjected to cryo-EM analysis.
Extended Data Fig. 2
Cryo-EM workflow for the Cte 1 RNA splicing products. Cryo-EM data collection and processing of Cte 1 RNA splicing products in the presence of Ca2+ at 5 minutes (left) and 4 hours (right).
Extended Data Fig. 3
Cryo-EM workflow for the Cte 1 IVT product. Cryo-EM data collection and processing of cotranscriptionally folded Cte 1 RNA splicing product directly after IVT.
Extended Data Fig. 4 The heteronuclear metal ion center in Cte 1 intron and comparison to canonical group II introns and spliceosome.
a-c. The cryo-EM maps and models depicting the heteronuclear metal ion center showing EBS-IBS interactions in a. pre-1S, b. 1S, c. 2S, d-f. 3′-SS interaction network in d. post-2S, e. chimeric O.i intron lariat (5J02), f. E.r. intron RNP post-2F (8T2T), and homology to g. E.r. intron RNP pre-1F (8T2S), h. E.r. intron RNP pre-2F (8T2R), i. yeast S. cerevisiae spliceosome C complex (7B9V). Black dashed lines indicate metal ion coordination, blue dashed lines indicate hydrogen bonds, arrows indicate nucleophilic attacks, and base stackings are highlighted in red.
Extended Data Fig. 5 Tertiary interactions of D5 and D6 in Cte 1 intron compared to canonical group II introns.
a. Projected secondary structure with designated tertiary interactions of Cte 1 intron based on 3D structures. b. Tertiary interactions of D5 (cyan) with D1, D3 and D4 (gray). c-h. Cte 1 (cyan and gray) tertiary interactions of c. ψ‘-ψ compared to 6ME0 (tan), d. λ‘-λ compared to 8T2T (forest green), e. ζ‘-ζ compared to 4R0D (gold), f. κ‘-κ compared to 8T2T, g. μ‘-μ compared to 8T2T, h. φ‘-φ compared to 8T2T. i-k. Cte 1 (gray) tertiary interactions of i. π-π‘ compared to 8T2T (forest green), j. η-η‘ compared to 6ME0 (tan), k. γ-γ‘ compared to 8T2T.
Extended Data Fig. 6 Local shifts of bulged A between pre-1S and 1S.
a. Secondary structure, cryo-EM density map and model of D6 showing a 2-nt bulge (G126 and A127) in pre-1S, in which two base triples C100-A127-C538 and G101-C125-G220 were formed. b. Secondary structure, cryo-EM density map and model of D6 with bulged A127 interacting with G101-C125 in 1S. c-d. Analogous bulged A structure in c. canonical group II intron E.r. intron pre-1F (8T2S) and d. yeast S.c. spliceosome (7B9V). Blue dashed lines indicate hydrogen bonds.
Extended Data Fig. 7 Other tertiary interactions in Cte 1 intron compared to canonical group II introns.
a. Projected secondary structures of Cte 1 intron. Mutations that disrupt novel tertiary interactions are denoted. Cte 1 intron (gray) tertiary interactions of b. θ-θ‘ compared to 8T2T, c. ε-ε‘ compared to 6ME0, d. α-α‘ compared to 4E8K (magenta), e. ω-ω‘ compared to 4E8K, f. σ-σ‘ compared to 6ME0, g. ρ-ρ‘ compared to 4R0D (gold). Mutations that disrupt novel tertiary interactions are denoted in the secondary structure on top.
Extended Data Fig. 8 Metal ion distribution in Cte 1 intron and comparison with O.i. group IIC intron (4E8Q).
a. Metal ion distribution shows that most metal ions reside in D1 domain. b. The metal core in D1d domain. c. Comparison with O.i. group IIC intron reveals analogous metal ion-binding sites in the catalytic core. Black dashed lines indicate metal ion coordination.
Extended Data Fig. 9 Covariation analysis facilitates discovery of novel CP group II introns in other bacteria.
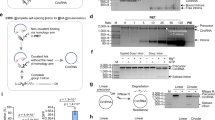
a. Consensus sequence and secondary structure model of CP group II intron, highlighting over one hundred covarying mutation pairs (green boxes). b. In vitro splicing assay demonstrates catalytic activity of five newly identified CP group II introns from Ape, Glu, Kpn, Pla, and Pni with incubation at 37 °C for 30 minutes. This is a representative gel electrophoresis from three independent experiments with consistent results. c. 2.9 Å cryo-EM map of the Pla CP-GII intron.
Extended Data Fig. 10 The Pla CP intron structure reveals conserved features as those in Cte 1 CP intron.
a. The overall structure of Pla CP intron. b. 5′- and 3′-exon base-pairing interaction. c. The 2-bp EBS3-IBS3 interaction motif. d. M35 stabilizing the 5′-exon. Black and blue dashed lines indicate metal ion coordination and hydrogen bonds, respectively.
Supplementary information
Supplementary Information
Supplementary Tables 1–3 and Data 1.
Supplementary Video 1
Dynamics of Cte 1 CP intron back-splicing.
Supplementary Data 1
The multiple sequence alignment of CP group II intron in Stockholm format.
Source data
Source Data Fig. 3
Unprocessed gels.
Source Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 1
Unprocessed gels.
Source Data Extended Data Fig. 9
Unprocessed gels.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, L., Xie, J., Zhang, C. et al. Structural basis of circularly permuted group II intron self-splicing. Nat Struct Mol Biol 32, 1091–1100 (2025). https://doi.org/10.1038/s41594-025-01484-x
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41594-025-01484-x