Abstract
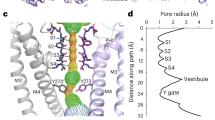
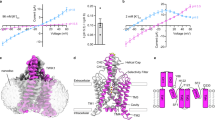
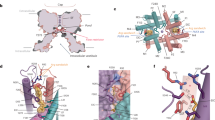
TWIK-related halothane-inhibited potassium channel (THIK1) maintains the resting membrane potential and regulates potassium efflux in microglia. It is a potential therapeutic target for neurodegenerative disorders, neuropathic pain and inflammation. However, the mechanism underlying its function remains unclear. Here we used cryo-electron microscopy to solve the structures of full-length human THIK1, revealing two inner gates and a C-type selectivity filter gate, distinct from other two-pore-domain potassium channels. One inner gate, formed by a short helix in the distal C terminus, introduces a unique gating mechanism involving the distal cytoplasmic domain. The other, beneath the selectivity filter, is constricted by Y273 in the M4 helix, dividing the cavity. In addition, the selectivity filter gate is modulated by polyunsaturated fatty acids. These structural insights into THIK1 gating, through the distal C-terminal helices, hydrophilic residues and selectivity filter, advance our understanding of THIK1’s role in microglial homeostasis and neuropathologies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
THIK1 protein sequence is available from UniProt under accession number Q9HB14. Structure coordinates and cryo-EM density maps were deposited to the PDB and EM Data Bank under accession numbers 9JGZ and EMD-61467 for THIK1WT and 9JH1 and EMD-61469 for THIK1S136P, respectively. Data and materials can be obtained from the corresponding author upon request. Source data are provided with this paper.
Change history
29 October 2025
In the version of this article, the first affiliation, Department of Anesthesiology, Zhongshan Hospital, Fudan University, Shanghai, China, was incomplete and is now amended in the HTML and PDF versions of the article.
References
Rajan, S. et al. THIK-1 and THIK-2, a novel subfamily of tandem pore domain K+ channels. J. Biol. Chem. 276, 7302–7311 (2001).
Kang, D., Hogan, J. O. & Kim, D. THIK-1 (K2P13.1) is a small-conductance background K+ channel in rat trigeminal ganglion neurons. Pflugers Arch. 466, 1289–1300 (2014).
Madry, C. et al. Microglial ramification, surveillance, and interleukin-1β release are regulated by the two-pore domain K+ channel THIK-1. Neuron 97, 299–312 (2018).
Izquierdo, P., Attwell, D. & Madry, C. Ion channels and receptors as determinants of microglial function. Trends Neurosci. 42, 278–292 (2019).
Izquierdo, P., Shiina, H., Hirunpattarasilp, C., Gillis, G. & Attwell, D. Synapse development is regulated by microglial THIK-1 K+ channels. Proc. Natl Acad. Sci. USA 118, e2106294118 (2021).
Ronzano, R. et al. Microglia-neuron interaction at nodes of Ranvier depends on neuronal activity through potassium release and contributes to remyelination. Nat. Commun. 12, 5219 (2021).
Drinkall, S. et al. The two pore potassium channel THIK-1 regulates NLRP3 inflammasome activation. Glia 70, 1301–1316 (2022).
Rifat, A. et al. Differential contribution of THIK-1 K+ channels and P2X7 receptors to ATP-mediated neuroinflammation by human microglia. J. Neuroinflammation 21, 58 (2024).
Hemonnot, A. L., Hua, J., Ulmann, L. & Hirbec, H. Microglia in Alzheimer disease: well-known targets and new opportunities. Front. Aging Neurosci. 11, 233 (2019).
Prinz, M., Jung, S. & Priller, J. Microglia biology: one century of evolving concepts. Cell 179, 292–311 (2019).
Blin, S. et al. Tandem pore domain halothane-inhibited K+ channel subunits THIK1 and THIK2 assemble and form active channels. J. Biol. Chem. 289, 28202–28212 (2014).
Renigunta, V., Zou, X., Kling, S., Schlichthorl, G. & Daut, J. Breaking the silence: functional expression of the two-pore-domain potassium channel THIK-2. Pflugers Arch. 466, 1735–1745 (2014).
Bichet, D. et al. Silent but not dumb: how cellular trafficking and pore gating modulate expression of TWIK1 and THIK2. Pflugers Arch. 467, 1121–1131 (2015).
Natale, A. M., Deal, P. E. & Minor, D. L. Jr. Structural insights into the mechanisms and pharmacology of K2P potassium channels. J. Mol. Biol. 433, 166995 (2021).
Sakamaki, K. et al. Dysregulation of a potassium channel, THIK-1, targeted by caspase-8 accelerates cell shrinkage. Biochim. Biophys. Acta 1863, 2766–2783 (2016).
Tateyama, M. & Kubo, Y. Regulation of the two-pore domain potassium channel, THIK-1 and THIK-2, by G protein coupled receptors. PLoS ONE 18, e0284962 (2023).
Riel, E. B. et al. The versatile regulation of K2P channels by polyanionic lipids of the phosphoinositide and fatty acid metabolism. J. Gen. Physiol. 154, e202112989 (2022).
Chen, Z., Wei, H., Sagalajev, B., Koivisto, A. & Pertovaara, A. Amygdaloid administration of tetrapentylammonium attenuates development of pain and anxiety-like behavior following peripheral nerve injury. Pharm. Rep. 71, 54–60 (2019).
Abramson, J. et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 630, 493–500 (2024).
Rodstrom, K. E. J. et al. A lower X-gate in TASK channels traps inhibitors within the vestibule. Nature 582, 443–447 (2020).
Chatelain, F. C. et al. Silencing of the tandem pore domain halothane-inhibited K+ channel 2 (THIK2) relies on combined intracellular retention and low intrinsic activity at the plasma membrane. J. Biol. Chem. 288, 35081–35092 (2013).
Sterbuleac, D. Molecular determinants of chemical modulation of two-pore domain potassium channels. Chem. Biol. Drug Des. 94, 1596–1614 (2019).
Fan, C. et al. Ball-and-chain inactivation in a calcium-gated potassium channel. Nature 580, 288–293 (2020).
Demo, S. D. & Yellen, G. The inactivation gate of the Shaker K+ channel behaves like an open-channel blocker. Neuron 7, 743–753 (1991).
Hoshi, T., Zagotta, W. N. & Aldrich, R. W. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science 250, 533–538 (1990).
Venkataraman, G., Srikumar, D. & Holmgren, M. Quasi-specific access of the potassium channel inactivation gate. Nat. Commun. 5, 4050 (2014).
Zhou, M., Morais-Cabral, J. H., Mann, S. & MacKinnon, R. Potassium channel receptor site for the inactivation gate and quaternary amine inhibitors. Nature 411, 657–661 (2001).
Zhang, J. et al. N-type fast inactivation of a eukaryotic voltage-gated sodium channel. Nat. Commun. 13, 2713 (2022).
Lolicato, M. et al. K2P channel C-type gating involves asymmetric selectivity filter order-disorder transitions. Sci. Adv. 6, eabc9174 (2020).
Neelsen, L.C. et al. Ion occupancy of the selectivity filter controls opening of a cytoplasmic gate in the K2P channel TALK-2. Nat. Commun. 15, 7545 (2024).
Tu, T. H., Kim, H., Yang, S., Kim, J. K. & Kim, J. G. Linoleic acid rescues microglia inflammation triggered by saturated fatty acid. Biochem. Biophys. Res. Commun. 513, 201–206 (2019).
McClenaghan, C. et al. Polymodal activation of the TREK-2 K2P channel produces structurally distinct open states. J. Gen. Physiol. 147, 497–505 (2016).
Lolicato, M. et al. K2P2.1 (TREK-1)–activator complexes reveal a cryptic selectivity filter binding site. Nature 547, 364–368 (2017).
Deal, P. E. et al. Development of covalent chemogenetic K2P channel activators. Cell Chem. Biol. 31, 1305–1323 e9 (2024).
Rödström, K. E. J. et al. Cryo-EM structure of the human THIK-1 K2P K+ channel reveals a lower Y gate regulated by lipids and anesthetics. Nat. Struct. Mol. Biol. https://doi.org/10.1038/s41594-025-01497-6 (2025).
Roy-Chowdhury, S. et al. Structure of the human K2P13.1 channel reveals a hydrophilic pore restriction and lipid cofactor site. Nat. Struct. Mol. Biol. https://doi.org/10.1038/s41594-024-01476-3 (2025).
Li, B., Rietmeijer, R. A. & Brohawn, S. G. Structural basis for pH gating of the two-pore domain K+ channel TASK2. Nature 586, 457–462 (2020).
Turkaydin, B. et al. Atomistic mechanism of coupling between cytosolic sensor domain and selectivity filter in TREK K2P channels. Nat. Commun. 15, 4628 (2024).
Brohawn, S. G., del Marmol, J. & MacKinnon, R. Crystal structure of the human K2P TRAAK, a lipid- and mechano-sensitive K+ ion channel. Science 335, 436–441 (2012).
Brohawn, S. G., Campbell, E. B. & MacKinnon, R. Domain-swapped chain connectivity and gated membrane access in a Fab-mediated crystal of the human TRAAK K+ channel. Proc. Natl Acad. Sci. USA 110, 2129–2134 (2013).
Brohawn, S. G., Campbell, E. B. & MacKinnon, R. Physical mechanism for gating and mechanosensitivity of the human TRAAK K+ channel. Nature 516, 126–130 (2014).
Rietmeijer, R. A., Sorum, B., Li, B. & Brohawn, S. G. Physical basis for distinct basal and mechanically gated activity of the human K+ channel TRAAK. Neuron 109, 2902–2913 (2021).
Miller, A. N. & Long, S. B.Crystal structure of the human two-pore domain potassium channel K2P1. Science 335, 432–436 (2012).
Sorum, B., Docter, T., Panico, V., Rietmeijer, R. A. & Brohawn, S. G. Tension activation of mechanosensitive two-pore domain K+ channels TRAAK, TREK-1, and TREK-2. Nat. Commun. 15, 3142 (2024).
Plant, L. D., Rajan, S. & Goldstein, S. A. K2P channels and their protein partners. Curr. Opin. Neurobiol. 15, 326–333 (2005).
Gierten, J. et al. Regulation of two-pore-domain (K2P) potassium leak channels by the tyrosine kinase inhibitor genistein. Br. J. Pharmacol. 154, 1680–1690 (2008).
Lin, H., Li, J., Zhang, Q., Yang, H. & Chen, S. C-type inactivation and proton modulation mechanisms of the TASK3 channel. Proc. Natl Acad. Sci. USA 121, e2320345121 (2024).
Kimanius, D., Dong, L., Sharov, G., Nakane, T. & Scheres, S. H. W. New tools for automated cryo-EM single-particle analysis in RELION-4.0. Biochem. J. 478, 4169–4185 (2021).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 (2010).
Smart, O. S., Neduvelil, J. G., Wang, X., Wallace, B. A. & Sansom, M. S. HOLE: a program for the analysis of the pore dimensions of ion channel structural models. J. Mol. Graph. 14, 354–360, 376 (1996).
Wallace, A. C., Laskowski, R. A. & Thornton, J. M. LIGPLOT: a program to generate schematic diagrams of protein–ligand interactions. Protein Eng. 8, 127–134 (1995).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Acknowledgements
We thank Q. Chen for critical reading and discussing this paper. We thank the staff members of the Center of Cryo-EM of Fudan University for technical support and assistance and members of the B.L. laboratory for feedback on the paper. This work was supported in part by grants from The National Science and Technology Innovation 2030 Major Projects of China (STI2030-Major Projects-2022ZD0207800 to B.L. and J.W.), the National Natural Science Foundation of China (32371261 to B.L. and 32301011 to R.Z.) and the Fundamental Research Funds for the Central Universities (2632024ZD10 to J.W.).
Author information
Authors and Affiliations
Contributions
B.L., R.Z. and J.W. conceptualized and supervised the project. X.F. prepared the samples. X.F., R.Z. and B.L. performed data acquisition, image processing and structure determination. J.W. and H.J. performed electrophysiology and analyzed the data. B.L. wrote the paper with the input from R.Z., J.W., X.F. and H.J.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks Youxing Jiang, Marcos Matamoros and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available. Primary Handling Editor: Katarzyna Ciazynska, in collaboration with the Nature Structural & Molecular Biology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Inclusion and ethics
We support inclusive, diverse and equitable conduct in the research community.
Extended data
Extended Data Fig. 1 Cryo-EM analysis of THIK1WT.
a, Cryo-EM data processing steps in Relion 4.0 and cryoSPARC4.4.1. See Methods for details. b, A representative cryo-EM micrograph of THIK1WT. Scale bar, 200 Å. c, 2D class average images of THIK1WT. d, Local resolution distribution for the density map of THIK1WT. e, Euler angle distribution of the set of particles that contributed to the final density map of THIK1WT. f, The GSFSC curve for the reconstruction of THIK1WT. g, Cryo-EM densities for various TMs in the THIK1WT.
Extended Data Fig. 2 Sequence alignment of human K2P channels.
Alignment of human K2P channels colored by conservation. THIK1 secondary structure is drawn above the sequences, and selectivity filters as orange lines.
Extended Data Fig. 3 The full-length THIK1 structure predicted by AlphaFold 3.
viewed from the membrane plane (a) and the intracellular side (b).
Extended Data Fig. 4 Molecular dynamics simulation of C-terminus helix gate.
a, MD simulation started from the cryo-EM structure. The plots of the root mean square deviations (RMSD) for all Cα atoms (green) and selected Cα atoms of residues 393-404 (black) are shown. b, MD simulation of the cryo-EM structure with missing loop added. The conformation of the missing loop is derived from AlphaFold3. The RMSD for Cα atoms of residues in the cryo-EM structure (green) and residues 393-404 (black) are shown.
Extended Data Fig. 5 Cryo-EM analysis of THIK1 S136P.
a, cryo-EM data processing steps in Relion 4.0 and cryoSPARC4.4.1. See Methods for details. b, A representative cryo-EM micrograph of THIK1S136P. Scale bar, 200 Å. c, 2D class average images of THIK1S136P. d, Local resolution distribution for the density map of THIK1S136P. e, Euler angle distribution of the set of particles that contributed to the final density map of THIK1S136P. f, The GSFSC curve for the reconstruction of THIK1S136P. g, Cryo-EM densities for various TMs in the THIK1S136P.
Extended Data Fig. 6 Conformational changes of TM2 and TM4 induced by the S136P mutation.
a-c, View from the membrane plane showing one TM2 from THIK1WT, THIK1S136P, and both structures overlaid. The dashed line in (c) indicates the central axis of the helix, and the rotation degree is labeled. d, Overlaid structures of THIK1WT and THIK1S136P. The dashed arrows indicate the motion directions of TM2 and TM4.
Extended Data Fig. 7 Lipid binding in the modulator pocket.
a, Hydrophobic surface representation of the modulator pocket in THIK1WT (blue represents the most hydrophilic regions, transitioning to white, and then to orange-red for the most hydrophobic regions). b, Ligplot of the lipid in the modulator pocket. Key residues are labeled.
Extended Data Fig. 8
Densities for the lipid and surrounding residues in THIK1WT (a) and THIK1S136P (b).
Extended Data Fig. 9 Conformational changes of the selectivity filter.
a, d, Ion occupancy in the selectivity filter for THIK1WT and THIK1S136P. b-c, e-f, Inter-carbonyl distances at K+-binding sites S3 and S4 for THIK1WT and THIK1S136P.
Supplementary information
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fang, X., Jin, H., Wang, J. et al. Gating mechanism of the two-pore-domain potassium channel THIK1. Nat Struct Mol Biol 32, 1175–1182 (2025). https://doi.org/10.1038/s41594-025-01542-4
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41594-025-01542-4