Abstract
Proteasomes degrade diverse proteins in different cellular contexts through incompletely defined regulatory mechanisms. Here we report the cryo-EM structure of human thioredoxin-like protein 1 (TXNL1) bound to the 19S regulatory particle of proteasomes via interactions with PSMD1 (Rpn2), PSMD4 (Rpn10) and PSMD14 (Rpn11). Proteasome binding is necessary for the ubiquitin-independent degradation of TXNL1 upon cellular exposure to metal- or metalloid-containing oxidative agents, thereby establishing a structural requirement for the stress-induced degradation of TXNL1.
Similar content being viewed by others
Main
Proteasomes are the primary protein degradation machinery in eukaryotes and comprise a 20S core particle harboring proteases capped by one or two 19S regulatory particles (RPs)1. Conventionally, proteasomes degrade poly-ubiquitylated proteins that are recruited by ubiquitin receptors on the 19S RP: PSMD2 (Rpn1), PSMD4 (Rpn10) and ADRM1 (Rpn13)2,3,4,5. These substrates are then unfolded by translocating through the AAA-ATPase motor at the base of the 19S RP before entering the core particle for proteolysis1,6,7. Successful proteasomal degradation also involves PSMD14 (Rpn11), an essential zinc metalloprotease on the 19S RP that deubiquitylates substrates to promote their efficient translocation8,9,10.
Some proteins can undergo proteasomal degradation without a requirement for ubiquitylation11,12,13,14,15. For example, the proteasomal adapter midnolin directly captures specific nuclear proteins to promote their degradation12,13. It is unclear whether and how other proteins engage proteasomes for ubiquitin-independent degradation. In this study, we report the cryo-EM structure of the human proteasome bound to thioredoxin-like protein 1 (TXNL1), a conserved thioreductase that interacts stably with proteasomes and, in Schizosaccharomyces pombe, shows genetic interactions with proteasome regulators16,17,18. Our structure reveals key binding interfaces between TXNL1 and proteasomal subunits required for the ubiquitin-independent degradation of TXNL1 upon cellular exposure to certain compounds that cause oxidative stress. Our findings identify the molecular interactions required for the stress-induced degradation of an abundant protein that may regulate proteasomal activity18.
Results and discussion
Cryo-EM structure of the TXNL1-bound proteasome
We determined the structure of the TXNL1-bound proteasome to an overall resolution of 3.0–3.3 Å from cryo-EM datasets of affinity-purified midnolin–proteasome complexes (Fig. 1a, Table 1 and Extended Data Figs. 1 and 2). In addition to revealing the structures of midnolin-bound proteasomes described elsewhere19, three-dimensional (3D) classifications revealed a subset of particles contributing to reconstructions of the proteasome or 19S RP with a well-resolved density that has not been previously reported (Fig. 1a and Extended Data Figs. 1 and 2). We identified this protein as TXNL1 based on amino acid sequences assigned to the density by ModelAngelo20.
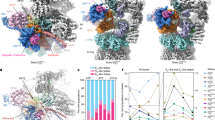
a, Cryo-EM map of TXNL1 on the proteasome. b, Model of the AAA-ATPase ring with density corresponding to a translocating substrate (light blue), viewed from above the substrate entry site. c,d, TXNL1 binding to PSMD1 (c) and PSMD14 (d) occurs through electrostatic interactions. e, The C-terminal tail of TXNL1 interacts with PSMD14 through hydrophobic interactions. f, H289 of TXNL1 coordinates the catalytic zinc ion associated with PSMD14. g, Proteasome-interacting domain of TXNL1 colored by conservation with residues at proteasomal interfaces labeled.
TXNL1 is an abundant (>1 µM)21, 32-kDa protein with an N-terminal thioredoxin domain and a C-terminal proteasome-interacting thioredoxin (PITH) domain16,17,18 (Extended Data Fig. 3a). In addition, native size fractionations revealed that a substantial proportion of endogenous TXNL1 co-migrated with proteasomes (Extended Data Fig. 3b). A high proteasomal occupancy may explain the presence of TXNL1-bound proteasomes in our cryo-EM datasets.
Although the thioredoxin domain of TXNL1 is not resolved in our map, we could model the entire PITH domain (residues 118–289) bound to PSMD1, a structural component of the 19S RP, the ubiquitin receptor PSMD4 and the deubiquitinase PSMD14. Below the TXNL1 binding site, the AAA-ATPase ring is in a state of active translocation, with a clear substrate polypeptide density that is likely an averaged mixture of endogenous proteasomal substrates and visible nucleotide density associated with each subunit (Fig. 1b and Extended Data Fig. 4a–d). The conformation of the AAA-ATPase is most similar to the human ED2 (root mean square (r.m.s.) deviation = 3.0 Å) or yeast 4D (r.m.s. deviation = 2.0 Å) proteasomal states6,7, with PSMC3 (Rpt5) at the top of the spiral AAA-ATPase configuration and PSMC6 (Rpt4) fully disengaged from the substrate (Extended Data Fig. 4b–d).
TXNL1 interactions with proteasomal components
Electrostatic interactions facilitate TXNL1 binding to PSMD1 and PSMD4 (Fig. 1c,d). Residues involved in these interactions include R234 of TXNL1, which interacts with an acidic patch on PSMD1 (Fig. 1c), and E136 and D162 of TXNL1, which interact with a basic interface on PSMD4 (Fig. 1d). The C-terminal unstructured tail of TXNL1 engages a hydrophobic groove on PSMD14 (Fig. 1e) and extends into the PSMD14 active site, where H289, the C-terminal residue of TXNL1, coordinates zinc along with H113 and H115 of PSMD14 (Fig. 1f,g). These interactions are consistent with another structural study of TXNL1-bound proteasomes22, and the amino acids on TXNL1 that interact with proteasomal subunits, particularly H289, are highly conserved (Fig. 1g).
To validate the interaction interfaces observed in our structure, we performed coimmunoprecipitations from TXNL1 knockout (KO) human embryonic kidney (HEK) 293T cells complemented with epitope-tagged TXNL1 variants (Fig. 2a). Wild-type (WT) TXNL1 associated with proteasomes, but the interaction was severely impaired by mutations to disrupt the interface with PSMD1 (R234D; lane 10), PSMD4 (E136R and D162R; lanes 3–6) or PSMD14 (lanes 11–14). With the exception of N139A TXNL1 (lane 4), we observed an inverse correlation between TXNL1 abundance and its ability to bind proteasomes, consistent with the previous observation that TXNL1 levels are increased upon proteasome inhibition16.
a, Immunoblotting of anti-FLAG IP (top) and input lysates (bottom) of TXNL1 KO HEK 293T cells transfected with 2xFLAG-tagged TXNL1 variants, representative of three independent experiments. b, Immunoblotting of purified HA-tagged TXNL1 variants immunoprecipitated in the presence of purified proteasomes, representative of three independent experiments. c, TXNL1 degradation upon arsenite treatment is proteasome-dependent but ubiquitin-independent. Immunoblotting of HEK 293T cells treated without or with 100 µM arsenite, 10 µM MG132, a proteasome inhibitor or 1 µM TAK-243, an inhibitor of the E1 ubiquitin-activating enzyme, representative of three independent experiments. d, TXNL1 degradation upon arsenite exposure requires proteasome binding and thioreductase activity. Immunoblotting from WT or TXNL1 KO HEK 293T cells stably complemented without or with WT and mutant versions of TXNL1 by lentiviral transduction, representative of three independent experiments. e, Volcano plots of showing the fold-change (log2(FC)) of protein levels upon treatment with arsenite for 3 h of TXNL1 KO HEK 293T cells complemented without or with WT or D162R R234D TXNL1. mTOR, mammalian target of rapamycin; Ub, ubiquitin.
These interfaces also match AlphaFold3 predictions of 19S RP subunits with TXNL1 and PITHD1, which has a PITH domain but no thioreductase activity (Extended Data Fig. 5a,b). Although PITHD1 isolated proteasomes less efficiently than TXNL1, immunoprecipitations confirmed that mutations in the conserved interaction interfaces reduced proteasome binding to below detection in this assay (Extended Data Fig. 5c). In addition, when bound to TXNL1, the ubiquitin-interacting Insert-1 region of PSMD14 does not assume the β-hairpin conformation used to position ubiquitin for cleavage6,7,23 (Extended Data Fig. 5d). PITHD1 also has an extended C-terminal tail that is predicted to engage PSMD14 with a β-hairpin Insert-1 region and then enter the central pore of the AAA-ATPase ring24 (Extended Data Fig. 5b,d). Interestingly, extending the C-terminal tail of TXNL1 past H289 or replacing the C-terminal tail of TXNL1 with that of PITHD1 was sufficient to destabilize the protein in a proteasome-dependent, but ubiquitin-independent, manner (Extended Data Fig. 5e,f) (discussed below).
Next, we validated TXNL1–proteasome interactions with purified components. Immunoprecipitations of purified HA-TXNL1 variants incubated with proteasomes from TXNL1 KO HEK 293T cells confirmed that WT TXNL1 and a thioredoxin domain mutant (SxxS) isolated proteasomes, whereas D162R TXNL1, a proteasome binding-deficient mutant, did not (Fig. 2b and Extended Data Fig. 6a,b). TXNL1 binding to proteasomes was also impaired by preincubating proteasomes with 1,10-phenanthroline, a zinc chelator that inhibits PSMD14 deubiquitylation activity8, but not 1,7-phenanthroline, an inactive isomer (Extended Data Fig. 6c). This finding is consistent with our observation that H289 of TXNL1 coordinates the catalytic zinc of PSMD14 (Fig. 1f) and is important for proteasome engagement (Fig. 2a). However, unlike 1,10-phenanthroline, an excess of TXNL1 preincubated with purified proteasomes did not inhibit the in vitro deubiquitylation or degradation of a poly-ubiquitylated protein in an endpoint assay25 (Extended Data Fig. 6d).
Arsenite-induced TXNL1 degradation requires proteasomal contacts
It has been reported that exposing cells to arsenite, a chemotherapeutic used to treat acute promyelocytic leukemia that causes oxidative stress, reduces TXNL1 protein levels17,26. We confirmed that TXNL1 is rapidly destabilized in cells treated with arsenite, but not hydrogen peroxide (Extended Data Fig. 7a,b). The proteasome inhibitor MG132 abrogated the arsenite-triggered degradation of TXNL1, but TAK-243, an inhibitor of the E1 ubiquitin-activating enzyme27, did not (Fig. 2c). Complementing TXNL1 KO cells with TXNL1 variants showed efficient arsenite-induced degradation of WT TXNL1, but not of D162R R234D TXNL1 deficient in proteasome binding (Fig. 2d and Extended Data Fig. 7c). The SxxS thioredoxin mutant of TXNL1 also was not destabilized, even though it could still bind proteasomes, in the presence of arsenite (Fig. 2d and Extended Data Fig. 7c,d). We observed similar results using auranofin, a gold-containing oxidizing agent28,29,30, in place of arsenite (Extended Data Fig. 7e,f). These observations indicate that metal- or metalloid-containing oxidative agents trigger a cellular response leading to ubiquitin-independent degradation of TXNL1 that requires both the proteasome binding activity and catalytic cysteines of TXNL1.
To further investigate this response and the general role of TXNL1, we used multiplexed proteomics to analyze TXNL1 KO cells complemented without or with WT or D162R R234D TXNL1 (Fig. 2e, Extended Data Fig. 8 and Supplementary Table 1). We also performed RNA sequencing of the same cell lines as well as TXNL1 KO cells complemented with SxxS TXNL1 (Extended Data Fig. 9 and Supplementary Table 2). These datasets revealed that re-expressing any TXNL1 variant in KO cells minimally impacted the proteome and transcriptome (Extended Data Figs. 8 and 9a). Supporting our immunoblotting results, arsenite treatment selectively destabilized WT, but not D162R R234D TXNL1 protein levels and induced a dramatic transcriptional response and post-transcriptional upregulation of the stress-induced transcription factor ATF4 in all cell lines (Fig. 2e and Extended Data Fig. 9b). However, we did not observe specific differences in the arsenite-dependent upregulation or downregulation of any protein or transcript other than TXNL1 across the cell lines. Thus, elucidation of the purpose and impact of TXNL1 degradation in cellular physiology remains to be determined.
Implications
Altogether, our study reveals specific interactions required for proteasome binding and the stress-induced degradation of TXNL1. We hypothesize that arsenite may react with the thioredoxin domain of TXNL1 and induce conformational changes, such as unfolding or a tighter interaction with a proteasomal substrate, that initiates the translocation and degradation of TXNL1 in proximity to the AAA-ATPase. This idea may be supported by our observation that simply extending the C-terminal of tail of TXNL1 toward the AAA-ATPase ring is sufficient to induce constitutive ubiquitin-independent degradation of TXNL1 (Extended Data Fig. 5d,e).
In addition, based on the high abundance of TXNL1, its thioreductase activity and its position above the entry to the AAA-ATPase motor of actively translocating proteasomes, it is tempting to speculate that TXNL1 normally functions to reduce oxidized substrate proteins to facilitate their degradation. In this model, TXNL1 may cooperate with deubiquitylation by PSMD14 to promote efficient substrate translocation, which may involve TXNL1 coming on and off the proteasome when substrate-conjugated ubiquitins engage PSMD14 for removal. Because putative substrates that require TXNL1 for degradation are probably heterogeneous and constitute only a small fraction of proteins in a typical cell, like many proteins regulated by quality control pathways, identifying them may be challenging. Future insights, facilitated by the molecular interactions reported here, will require discovering specific substrates, cell types or cellular conditions that are particularly reliant on TXNL1.
Methods
Cryo-electron microscopy sample preparation, data collection, image processing and model building
Plasmid DNA encoding epitope-tagged midnolin variants was transiently transfected into HEK 293T cells for immunoprecipitation. The affinity purifications were partitioned by size-exclusion chromatography (SEC) and the fractions corresponding to proteasomes were collected. Then 3.5 µl of 4 mg ml−1 proteasomal complexes was applied to glow-discharged R0.6/1 UltrAuFoil 300 mesh grids (Quantifoil) and frozen in liquid ethane using a Vitrobot Mark IV (Thermo Fisher Scientific) set at 4 °C and 100% humidity with a 10 s wait time, 3 s blot time and +8 blot force.
Datasets were collected using a Titan Krios (Thermo Fisher Scientific) operating at 300 kV and equipped with a BioQuantum K3 imaging filter with a 20 eV slit width and a K3 summit direct electron detector (Gatan) in counting mode at a nominal magnification of ×105,000 corresponding to a calibrated pixel size of 0.825 Å. Semi-automated data collection was performed using SerialEM v.4.0.5 for four datasets with the following parameters. Dataset 1: 2.597-s exposures were fractionated into 50 frames, resulting in a total exposure of 52.98 e−/Å2. The defocus targets were −1.0 to −2.2 µm. Dataset 2: 2.652-s exposures were fractionated into 48 frames, resulting in a total exposure of 50.54 e−/Å2. The defocus targets were −1.0 to −2.2 µm. Dataset 3: 2.65-s exposures were fractionated into 50 frames, resulting in a total exposure of 53 e−/Å2. The defocus targets were −1.0 to −2.2 µm. Dataset 4: 3.02-s exposures were fractionated into 54 frames, resulting in a total exposure of 53.79 e−/Å2. The defocus targets were −0.8 to −1.8 µm.
Data processing was performed in cryoSPARC v.4.3.1. After patch-based motion correction and contrast transfer function (CTF) estimation, micrographs with severe contamination or poor CTF fits were removed. Then 3,914 (Dataset 1), 3,396 (Dataset 2), 4,095 (Dataset 3) or 8,035 (Dataset 4) micrographs were subjected to automated particle picking using templates generated from blob-based picking. The particles were extracted with a box size of 800 and downsampled to a box size of 200. After two-dimensional classification, 380,637 (Dataset 1), 380,842 (Dataset 2), 285,529 (Dataset 3) and 658,210 (Dataset 4) particles were selected for heterogeneous refinement using multiple reference volumes generated by ab initio reconstruction. Particles in the best classes were subjected to homogeneous refinement with C2 symmetry, followed by symmetry expansion. Afterwards, all particles were re-centered on the 19S particle, extracted with a box size of 440 and downsampled to a box size of 220. Good 19S particles after two-dimensional classification were then subjected to heterogeneous refinements and 3D classification without alignment using a mask over the TXNL1 binding site based on initial observation of density in this region from heterogeneous refinements of Dataset 1 (Extended Data Fig. 1).
In total, 418,061 particles from the four datasets in classes with density corresponding to the TXNL1 were unbinned, local motion corrected, combined and subjected to nonuniform and an additional round of heterogeneous refinement. Classes corresponding to intact proteasomes were selected and subjected to nonuniform refinement and 3D classification with a mask focused on TXNL1. The 103,163 particles with clear TXNL1 density were used for final nonuniform and local refinements using the same mask. TXNL1 was identified by BLASTing the sequence assigned to this density by ModelAngelo20. An initial model for TXNL1–proteasome complex was generated primarily by rigid body fitting individual AlphaFold models31 of each proteasomal subunit and of TXNL1, in addition to cross-referencing the output from ModelAngelo. The model was manually adjusted in Coot32 v.0.9.8 in between iterative rounds of real space refinement in Phenix33 v.1.19.2. Figures were made using ChimeraX34 v.1.5–v.1.7.
Expression plasmids
Human TXNL1 cDNA (complementary DNA) oligonucleotide, flanked with attB1 or attB2 sites, was synthesized by Integrated DNA Technologies (IDT) for Gateway cloning into pDONR221 via the BP reaction (Thermo Fisher Scientific, cat. no. 11789020). Mutations to the TXNL1 entry clone were introduced using the Q5 Site-Directed Mutagenesis kit (NEB, cat. no. E0554S). The PCR primers were designed using the NEBaseChanger program and synthesized by IDT. WT and mutant versions of TXNL1 were subcloned into a pHAGE CMV 2xFLAG-N destination vector for N-terminal tagging or a pHAGE CMV destination vector conferring puromycin resistance13 via LR reactions (Thermo Fisher Scientific, cat. no. 11791100).
The tail-extended TXNL1 variant appended the following C-terminal residues of PITHD1—ASANPADHRVHQVTPQTHFIS. The tail-replaced TXNL1 variant exchanged the TXNL1 tail sequence (VQATNMNDFKRVVGKKGESH) with the entire C-terminal tail of PITHD1—LRRHEVTICNYEASANPADHRVHQVTPQTHFIS.
HA-TXNL1 (WT, D162R, SxxS) were codon-optimized for expression in bacteria and synthesized by IDT to contain overhangs for Gibson assembly (NEB, cat. no. E2611S). A pET 6xHis-3C bacterial expression vector was obtained from the Dana-Farber Cancer Institute Crystallography core facility and was digested with BamHI and NotI before Gibson assembly of the synthetic oligonucleotides.
Cell culture and cell line generation
HEK 293T cells (ATCC, CRL-3216, RRID:CVCL_0063) were incubated at 37 °C and 5% CO2 in DMEM (Thermo Fisher Scientific, cat. no. 11965118) supplemented with 100 units ml−1 of penicillin, 0.1 mg ml−1 of streptomycin (Thermo Fisher Scientific, cat. no. 15070063) and 10% fetal bovine serum (Cytiva, cat. no. SH30088.03). Cells were treated with 10 µM MG132 (Selleckchem, cat. no. S2619), 1 µM TAK-243 (MedChemExpress, cat. no. HY-100487), 100 µM arsenite (Sigma, cat. no. S7400), 5 µM auranofin (MedChemExpress, cat. no. HY-B1123) or 250 µM hydrogen peroxide (Sigma, cat. no. 216763) from stock solution in DMSO or water.
To knockout TXNL1, WT HEK 293T cells were transfected using PolyJet (SignaGen, cat. no. SL100688) with a lentiCRISPRv2 plasmid expressing a blue-fluorescent protein (BFP) and the guide RNA targeting TXNL1 (GAATGACCCTGGAAGCAATG). Five days post-transfection, BFP-positive cells were single-cell sorted into 96-well plates and grown out for 2 weeks. Isogenic clones were expanded and screened for TXNL1 KO.
To generate lentivirus, HEK 293T cells were cultured in six-well plates to 90% confluency. Using PolyJet, the cells were transfected with 1 µg of total plasmid DNA encoding Tat, Rev, Gag-Pol and VSV-G (mixed equimolar) and a lentiviral transfer vector. The media was replaced 1-day after transfection. The lentiviral supernatant was collected 2 days post-transfection, passed through a 0.45 µm filter, and applied directly onto cells.
Antibodies
The following primary antibodies were used for immunoblotting, all at a 1:1,000 dilution: rabbit anti-PSMD14 (Cell Signaling Technology (CST), cat. no. 4197S, RRID:AB_11178935), rabbit anti-PSMD4 (CST, cat. no. 12441S, RRID:AB_2797916), mouse anti-PSMD1 (Santa Cruz, cat. no. sc-166038, RRID:AB_2172797), rabbit anti-PSMD2 (CST, cat. no. 25430, RRID:AB_2798903), rabbit anti-PSMA2 (CST, cat. no. 2455, RRID:AB_2171400), rabbit anti-PSMB5 (CST, cat. no. 12919, RRID:AB_2798061), rabbit anti-FLAG (CST, cat. no. 14793, RRID:AB_2572291), rabbit anti-HA (CST, cat. no. 3724S, RRID:AB_1549585), rabbit anti-mTOR (CST, cat. no. 2983, RRID:AB_2105622), rabbit anti-TXNL1 (Abcam, cat. no. ab188328, RRID:AB_2687563) and rabbit anti-ubiquitin (CST, cat. no. 43124S, RRID:AB_2799235). Secondary antibodies used at a 1:2,000 dilution were: anti-rabbit immunoglobulin G, horseradish peroxidase-linked (CST, cat. no. 7074, RRID:AB_2099233) or anti-mouse immunoglobulin G, horseradish peroxidase-linked (CST, cat. no. 7076S, RRID:AB_330924).
Sucrose gradient ultracentrifugation
About 2 million HEK 293T cells were lysed in 50 µl of lysis buffer containing 0.5% CHAPS, 100 mM NaCl, 40 mM HEPES pH 7.4 and 1× protease-phosphatase inhibitor cocktail. Twenty microliters of clarified lysate supernatant was loaded onto a gradient containing five 40-µl steps of 30%, 25%, 20%, 15% and 10% sucrose in 100 mM NaCl and 40 mM HEPES pH 7.4 and subjected to ultracentrifugation at 55,000 rpm (258,719g) for 1 h using a TLS55 rotor. After centrifugation, 20-µl aliquots were diluted in Tris-Glycine sample buffer containing 10% 2-mercaptoethanol.
Immunoprecipitations from cells
HEK 293T cells were seeded at 1 million cells in 10-cm culture dishes and transfected with 3 µg of DNA encoding 2xFLAG-TXNL1 variants using PolyJet 2 days post-seeding. The media was replaced with fresh media 1 day post-transfection and incubated overnight. The cells were rinsed once with ice-cold PBS and 700 µl of lysis buffer containing 0.5% CHAPS, 100 mM NaCl, 40 mM HEPES pH 7.4, 1× protease-phosphatase inhibitor cocktail (Thermo Fisher Scientific, cat. no. 78441) was applied directly to the dish. The cells were collected in Eppendorf tubes by scraping, and were incubated for 15 min at 4 °C with end-to-end rotation. The lysates were then centrifuged at 21,000g for 15 min at 4 °C. While centrifuging, 15 µl per plate of anti-FLAG magnetic beads (Sigma, cat. no. M8823, RRID:AB_2637089) were rinsed three times with the same lysis buffer. After centrifuging, 30 µl of lysate was diluted into 200 µl of Tris-Glycine SDS sample buffer (Thermo Fisher Scientific, cat. no. LC2676) supplemented with 10% 2-mercaptoethanol. The remaining supernatant was applied directly to the anti-FLAG beads and incubated end-to-end for 1 h at 4 °C. After incubation, the beads were rinsed three times with 700 µl of lysis buffer and resuspended in 50 µl of sample buffer. Proteins were eluted from the beads and denatured for 3 min at 95 °C. An aliquot of immunoprecipitate (IP) sample was diluted 1:20 in sample buffer to blot for the abundant bait protein. To perform immunoblotting, 15 µl of lysate, diluted IP or total IP was loaded into 15-well gels.
Purification of recombinant HA-TXNL1
Rosetta (DE3) cells (Millipore, cat. no. 71397-3) were transformed with pET plasmids encoding 6xHis-3C-HA-TXNL1 (WT, D162R, SxxS) and grown overnight at 37 °C on plates containing kanamycin + chloramphenicol. Single colonies were then grown out in 50 ml of LB medium containing kanamycin + chloramphenicol overnight at 37 °C with 200 rpm shaking. The following day, the LB containing bacteria was transferred to 1 liter of fresh LB solution containing kanamycin + chloramphenicol and grown at 37 °C with 200 rpm shaking to an optical density of 0.6. The bacteria were kept at 4 °C for 10 min before supplementing the LB medium with 0.5 mM IPTG (Sigma, cat. no. I5502-10G) to induce TXNL1 expression. Cultures were incubated at 16 °C for 16 h with 200 rpm shaking.
The following day, the liquid cultures were transferred to 1-liter centrifuge bottles, and bacteria were pelleted by centrifuging at 4,000g for 15 min at 4 °C. The pellets were resuspended in 35 ml of buffer containing 50 mM HEPES pH 7.4, 150 mM NaCl and 10 mM imidazole (Sigma, cat. no. 68268-500ML-F). The bacteria were again pelleted by centrifugation and the supernatant was discarded. The bacteria were then resuspended in 35 ml of lysis buffer containing 50 mM HEPES pH 7.4, 150 mM NaCl, 10 mM imidazole, 1 mM DTT (Thermo Fisher Scientific, cat. no. R0862), benzonase (Millipore, cat. no. 71206-3) and protease inhibitor tablets (Sigma, cat. no. 11873580001). A microfluidizer (700 bar, 4 cycles, 20 ml min−1 flow rate) was then used to lyse cells. The lysate was centrifuged at 21,000g for 20 min at 4 °C. During the centrifugation, 4.8 ml of Ni-NTA agarose (Qiagen, cat. no. 30210) was rinsed three times using lysis buffer. Post-centrifugation, the supernatant was incubated with the washed Ni-NTA resin in 50-ml conical tubes for 30 min at 4 °C with end-to-end rotation. The solution was then passed through a column (Bio-Rad, cat. no. 7321010) to remove the liquid by gravity. The conical tubes and column were rinsed with 50 ml total volume of lysis buffer by gravity flow at 4 °C. Once rinsed, the proteins were eluted from the resin by sequentially incubating the resin with 500 µl of elution buffer containing 50 mM HEPES, 150 mM NaCl and 200 mM imidazole four times for 5 min at room temperature. Using a NanoDrop, the elutions were measured for absorbance at 280 nm to determine whether the protein eluted from the resin.
Elutions containing proteins were pooled and concentrated using an Amicon Ultra-15, 3 kDa MWCO (Millipore, cat. no. UFC9003). The concentrated sample was centrifuged for 1 min at 21,000g to remove large particles. A Superdex 200 was equilibrated using a buffer containing 50 mM HEPES, 150 mM NaCl and 1 mM DTT, and the concentrated sample was injected into a 0.5-ml injection loop. Fractions corresponding to TXNL1 were collected, pooled and concentrated using an Amicon Ultra-15, 3 kDa MWCO. Protein samples were flash-frozen using liquid nitrogen for storage at −80 °C.
Purification of TXNL1-deficient proteasomes
TXNL1 KO HEK 293T cells were seeded in fifty 15-cm plates at 3.5 million cells per plate and grown for 2 days. Cells were then transfected with 10 µg of plasmid DNA encoding 2xFLAG-midnolin per plate using PolyJet. The medium was replaced 1 day post-transfection and cells were incubated overnight. The cells were then rinsed once with ice-cold PBS before applying 1 ml of buffer containing 40 mM HEPES, 100 mM NaCl, 2 mM MgCl2, 5 mM ATP, 1× HALT protease-phosphatase inhibitor and 0.5% CHAPS to each 15-cm plate. The cells were collected by scraping and the lysate was incubated at 4 °C with end-to-end rotation for 20 min. The lysate was then centrifuged at 21,000g for 20 min at 4 °C. During centrifugation, 3.6 ml of anti-FLAG M2 agarose resin (Millipore, cat. no. A2220) was rinsed four times using lysis buffer. After centrifugation, the supernatant was incubated in a 50-ml conical tube containing the washed anti-FLAG resin with end-to-end rotation for 1.5 h at 4 °C. The solution was then passed through a column (Bio-Rad, cat. no. 7321010) by gravity at 4 °C. The conical tube and the column were rinsed with 50 ml of lysis buffer total by gravity flow at 4 °C. The column was also rinsed once with 20 ml of SEC buffer containing 40 mM HEPES, 100 mM NaCl, 2 mM MgCl, 5 mM ATP, 0.05% CHAPS at 4 °C.
The resin was then incubated with 1.8 ml of SEC buffer containing 0.5 mg ml−1 of 3xFLAG peptide (APExBIO, cat. no. A6001) for 20 min at room temperature. A total of four elutions were performed sequentially and the protein content in each elution was determined by obtaining the absorbance at 280 nm using a NanoDrop. The eluted proteins were pooled and concentrated to 500 µl using an Amicon Ultra-15, 10 kDa MWCO concentrator. The concentrated sample was centrifuged for 1 min at 21,000g to remove large particles. A Superose6 column was equilibrated using SEC buffer for SEC and the sample was manually injected into a 0.5-ml injection loop. Fractions corresponding to the proteasome were collected, pooled and concentrated using Amicon Ultra-15, 10 kDa MWCO. Protein samples were flash-frozen using liquid nitrogen for storage at −80 °C.
Binding and degradation assays with purified components
To test TXNL1 binding to proteasomes in vitro, 1 µl of each TXNL1 protein (1.7 mg ml−1) was diluted in 199 µl of sample buffer, and 1 µl of proteasomes (3 mg ml−1) was diluted in 99 µl of sample buffer as input. Ten microliters of anti-HA beads (Thermo Fisher Scientific, cat. no. 88836, RRID:AB_2749815) per reaction were washed three times using 700 µl of CHAPS buffer (100 mM NaCl, 40 mM HEPES pH 7.4, 0.5 % CHAPS, 1× HALT protease-phosphatase inhibitor). After washing, the beads were resuspended in 700 µl of CHAPS buffer containing 4% BSA (40 mg ml−1). The beads were blocked for 1 h at 4 °C with end-to-end rotation. After blocking, the beads were washed three times using 700 µl of CHAPS buffer. The beads were resuspended in CHAPS buffer and 10 µl of beads were aliquoted into pre-lubricated Eppendorf tubes (Millipore, cat. no. CLS3207). HA-TXNL1 protein (15 µg per reaction) was added directly to the beads to a final volume of 500 µl in the CHAPS buffer. The beads were incubated for 1 h at 4 °C with end-to-end rotation to immobilize the TXNL1. The beads were then washed three times using 700 µl of CHAPS buffer and resuspended to 500 µl in CHAPS buffer. Four microliters of proteasomes (3 mg ml−1) were added to each reaction. For the chelator experiment, proteasomes were preincubated with 6 mM 1,10-phenanthroline (Sigma, cat. no. 516705) or 1,7-phenanthroline (Sigma, cat. no. 301841) for 30 min at room temperature before adding the mixture to the TXNL1-bound beads. The beads were incubated with end-to-end rotation for 1 h at 4 °C, and then washed three times using 700 µl of CHAPS buffer before being resuspended in 25 µl of Tris-Glycine sample buffer containing 10% 2-mercaptoethanol. Proteins were eluted from the beads by denaturing at 95 °C for 3 min.
To assay for protein degradation, 50 nM purified proteasomes were preincubated at room temperature for 30 min with a negative control buffer, 6 mM 1,10-phenanthroline, or 50 µM TXNL1 variants in 100 mM NaCl and 40 mM HEPES pH 7. Then, 50 nM poly-ubiquitinated substrate was added directly to the proteasome mixture along with 5 mM ATP (Sigma, cat. no. A2383) in a 10-µl reaction volume, incubated for 10 min at 37 °C, and quenched with 10 µl of Tris-Glycine sample buffer containing 10% 2-mercaptoethanol. Proteins were denatured at 95 °C for 3 min and 8 µl of sample was loaded into 4%–20% Tris-Glycine 15-well pre-cast gels (Thermo Fisher Scientific, cat. no. XP04205BOX). In-gel fluorescent images were acquired using an Odyssey LI-COR.
Multiplexed mass spectrometry
Cells were washed twice with 1× PBS, harvested on ice using a cell scraper in 1× PBS, pelleted via centrifugation for 5 min at 1,000g at 4 °C, and washed with 1× PBS before resuspension in in 8 M urea (Sigma, cat. no. U5378), 50 mM NaCl, 50 mM EPPS (Sigma, cat. no. E9502) and 1× Protease Inhibitor Cocktail (Roche, cat. no. 4906845001). After 10 s of sonication, lysed cells were pelleted, and the protein concentration of the clarified sample was determined using a BCA kit (Thermo Fisher Scientific, cat. no. 23225). Then, 100 µg of each sample was incubated for 30 min at 37 °C with 5 mM TCEP (Gold Biotechnology) for disulfide bond reduction with subsequent alkylation with 20 mM iodoacetamide (Sigma, cat. no. I6125) for 20 min at room temperature followed by quenching with 15 mM DTT for 15 min under gentle shaking. MeOH-chloroform precipitation of samples was performed as follows: to each sample, four parts MeOH was added followed by vortexing, one part chloroform was added followed by vortexing, and finally three parts H2O was added. After vortexing, the suspension was centrifugated for 5 min at 14,000g and the aqueous phase around the protein precipitate was removed using a loading tip. The precipitate was washed twice with MeOH and resuspended in 200 mM EPPS (pH 8.0) (Sigma, cat. no. E9502) and digested with Trypsin/Lys-C (Promega, cat. no. V5073) digestion (1:100) at 37 °C overnight with gentle shaking.
One hundred and fifty microliters of digested samples were labeled by adding 10 µl of TMT reagent (Thermo Scientific, cat. no. A52045; stock: 20 mg ml−1 in acetonitrile (ACN), Millipore Sigma, cat. no. 34851) together with 50 µl of ACN to yield a final ACN concentration of approximately 25% (v/v) for 1 h at room temperature before quenching the reaction with hydroxylamine (Thermo Fisher Scientific, cat. no. 90115) to a final concentration of 0.2% (v/v). The TMTpro-labeled samples were pooled at a 1:1 ratio, resulting in a consistent peptide amount across all channels. Pooled samples were vacuum centrifuged for 1 h at room temperature to remove ACN, followed by reconstitution in 1% formic acid (FA), desalting using C18 solid-phase extraction (200 mg, Sep-Pak, Waters, cat. no. WAT054960) and vacuum centrifugation until near dryness. We fractionated the pooled, labeled peptide sample using basic pH reversed-phase HPLC35 and an Agilent 1260 pump equipped with a degasser and a UV detector (wavelength set at 220 and 280 nm). Peptides were subjected to a 50-min linear gradient from 5% to 35% ACN in 10 mM ammonium bicarbonate pH 8 at a flow rate of 0.6 ml min−1 over an Agilent 300Extend C18 column (3.5 μm particles, 4.6 mm internal diameter and 220 mm in length). The peptide mixture was fractionated into 96 fractions, which were consolidated into 24 super-fractions36, of which 12 nonadjacent fractions were analyzed. Samples were subsequently acidified with 1% FA (Sigma, cat. no. 94318) and vacuum centrifuged to near dryness. Each super-fraction was desalted via StageTip, dried again via vacuum centrifugation and reconstituted in 10 µl 5% ACN, 5% FA for LC–MS/MS processing.
Mass spectrometric data were collected on Orbitrap Fusion Lumos instruments coupled to a Proxeon NanoLC-1200 UHPLC. The 100-µm capillary column was packed with 35 cm of Accucore 150 resin (2.6 μm, 150 Å; Thermo Fisher Scientific) at a flow rate of 340 nl min−1. The scan sequence began with an MS1 spectrum (Orbitrap analysis, resolution 60,000, mass range 350–1,350 Th, automatic gain control target 100%, maximum injection time 118 ms). Data were acquired at ~90 min per fraction. The hrMS2 stage consisted of fragmentation by higher energy collisional dissociation (normalized collision energy 36%) and analysis using the Orbitrap (automatic gain control 200%, maximum injection time 120 ms, isolation window 0.6 Th, resolution 50,000). Data were acquired using the FAIMSpro interface with the dispersion voltage set to 5,000 V, the compensation voltages set at −40, −60 and −80 V, and the TopSpeed parameter set at 1 s per compensation voltage.
Spectra were converted to mzXML via MSconvert37. Database searching included all entries from the mouse UniProt reference database (downloaded August 2021). The database was concatenated with one composed of all protein sequences for that database in the reversed order. Searches were performed using a 50-ppm precursor ion tolerance for total protein-level profiling. The product ion tolerance was set to 0.03 Da. These wide mass tolerance windows were chosen to maximize sensitivity in conjunction with Comet searches and linear discriminant analysis38,39. TMTpro labels on lysine residues and peptide N termini (+304.207 Da), as well as carbamidomethylation of cysteine residues (+57.021 Da) were set as static modifications, while oxidation of methionine residues (+15.995 Da) was set as a variable modification. Peptide–spectrum matches (PSMs) were adjusted to a 1% false discovery rate40,41. PSM filtering was performed using a linear discriminant analysis, as described previously39 and then assembled further to a final protein-level false discovery rate of 1% (ref. 40). Proteins were quantified by summing reporter ion counts across all matching PSMs, also as described previously42. Reporter ion intensities were adjusted to correct for the isotopic impurities of the different TMTpro reagents according to manufacturer specifications. The signal-to-noise measurements of peptides assigned to each protein were summed and these values were normalized so that the sum of the signal for all proteins in each channel was equivalent to account for equal protein loading. Finally, each protein abundance measurement was scaled, such that the summed signal-to-noise for that protein across all channels equals 100, thereby generating a relative abundance measurement.
MSstatsTMT43 was performed on peptides with >200 summed signal-to-noise ratios across TMT channels. For each protein, the filtered PSM TMTpro raw intensities were summed and log2 normalized to calculate protein quantification values (weighted average) and normalized to total TMT channel intensity across all quantified PSMs (adjusted to median total TMT intensity for the TMT channels)44. The log2 normalized summed protein reporter intensities were compared using a Student’s t-test and P values were corrected for multiple hypotheses using the Benjamini–Hochberg adjustment45.
RNA sequencing
HEK 293T cells were grown to 80% confluency in six-well plates, treated with oxidizing agents (100 µM arsenite or 600 µM hydrogen peroxide) in triplicate for 6 h, and total RNA was isolated using an RNEasy Plus mini kit (Qiagen, cat. no. 74134). Messenger RNA sequencing libraries were prepared by Innomics and sequenced on a DNBseq platform to obtain 100-bp paired-end reads. Reads were aligned to the GRCh38 primary assembly obtained from gencodegenes.org using qAlign from the QuasR package. Raw counts were filtered to discard transcripts with fewer than ten total reads in the three untreated TXNL1 KO replicates, and then the DESeq2 package in R was used to obtain differential expression data and statistics.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
EM maps and models are available under accession numbers EMD-44949, EMD-44952 and PDB 9BW4. The mass spectrometry proteomics data are available via ProteomeXchange with identifier PXD052933. RNA sequencing data are available through GEO under the accession number GSE271951. Genome assembly GRCh38 (GCF_000001405.26) and the UniProt reference database (downloaded August 2021) were used for data analysis. All other data are included in the paper and its Supplementary Information. Source data are provided with this paper.
Code availability
No new code was generated in this study.
References
Bard, J. A. M. et al. Structure and function of the 26S proteasome. Annu. Rev. Biochem. 87, 697–724 (2018).
Husnjak, K. et al. Proteasome subunit Rpn13 is a novel ubiquitin receptor. Nature 453, 481–488 (2008).
Elsasser, S. et al. Proteasome subunit Rpn1 binds ubiquitin-like protein domains. Nat. Cell Biol. 4, 725–730 (2002).
Elsasser, S., Chandler-Militello, D., Müller, B., Hanna, J. & Finley, D. Rad23 and Rpn10 serve as alternative ubiquitin receptors for the proteasome. J. Biol. Chem. 279, 26817–26822 (2004).
Shi, Y. et al. Rpn1 provides adjacent receptor sites for substrate binding and deubiquitination by the proteasome. Science 351, aad9421 (2016).
de la Peña, A. H., Goodall, E. A., Gates, S. N., Lander, G. C. & Martin, A. Substrate-engaged 26S proteasome structures reveal mechanisms for ATP-hydrolysis-driven translocation. Science 362, eaav0725 (2018).
Dong, Y. et al. Cryo-EM structures and dynamics of substrate-engaged human 26S proteasome. Nature 565, 49–55 (2019).
Verma, R. et al. Role of Rpn11 metalloprotease in deubiquitination and degradation by the 26S proteasome. Science 298, 611–615 (2002).
Yao, T. & Cohen, R. E. A cryptic protease couples deubiquitination and degradation by the proteasome. Nature 419, 403–407 (2002).
Matyskiela, M. E., Lander, G. C. & Martin, A. Conformational switching of the 26S proteasome enables substrate degradation. Nat. Struct. Mol. Biol. 20, 781–788 (2013).
Erales, J. & Coffino, P. Ubiquitin-independent proteasomal degradation. Biochim. Biophys. Acta 1843, 216–221 (2014).
Du, J. et al. Stuxnet facilitates the degradation of polycomb protein during development. Dev. Cell 37, 507–519 (2016).
Gu, X. et al. The midnolin-proteasome pathway catches proteins for ubiquitination-independent degradation. Science 381, eadh5021 (2023).
Makaros, Y. et al. Ubiquitin-independent proteasomal degradation driven by C-degron pathways. Mol. Cell 83, 1921–1935 (2023).
Murakami, Y., Matsufuji, S., Hayashi, S., Tanahashi, N. & Tanaka, K. Degradation of ornithine decarboxylase by the 26S proteasome. Biochem. Biophys. Res. Commun. 267, 1–6 (2000).
Andersen, K. M. et al. Thioredoxin Txnl1/TRP32 is a redox-active cofactor of the 26S proteasome. J. Biol. Chem. 284, 15246–15254 (2009).
Wiseman, R. L. et al. Thioredoxin-related protein 32 is an arsenite-regulated thiol reductase of the proteasome 19S particle. J. Biol. Chem. 284, 15233–15245 (2009).
Andersen, K. M. et al. Txl1 and Txc1 are co-factors of the 26S proteasome in fission yeast. Antioxid. Redox Signal. 14, 1601–1608 (2011).
Nardone, C. et al. Structural basis for the midnolin-proteasome pathway and its role in suppressing myeloma. Mol. Cell https://doi.org/10.1016/j.molcel.2025.05.030 (2025).
Jamali, K. et al. Automated model building and protein identification in cryo-EM maps. Nature 628, 450–457 (2024).
Cho, N. H. et al. OpenCell: endogenous tagging for the cartography of human cellular organization. Science 375, eabi6983 (2022).
Arkinson, C., Gee, C. L., Zhang, Z., Dong, K. C. & Martin, A. Structural landscape of AAA+ ATPase motor states in the substrate-degrading human 26S proteasome reveals conformation-specific binding of TXNL1. Preprint at bioRxiv https://doi.org/10.1101/2024.11.08.622731 (2024).
Worden, E. J., Dong, K. C. & Martin, A. An AAA motor-driven mechanical switch in Rpn11 controls deubiquitination at the 26S proteasome. Mol. Cell 67, 799–811 (2017).
Amann, S. J. et al. PITHD1: an endogenous inhibitor of the 26S proteasome during cellular dormancy. Preprint at bioRxiv https://doi.org/10.1101/2024.12.04.626795 (2024).
Li, H., Ji, Z., Paulo, J. A., Gygi, S. P. & Rapoport, T. A. Bidirectional substrate shuttling between the 26S proteasome and the Cdc48 ATPase promotes protein degradation. Mol. Cell 84, 1290–1303 (2024).
Alimoghaddam, K. A review of arsenic trioxide and acute promyelocytic leukemia. Int. J. Hematol. Oncol. Stem Cell Res. 8, 44–54 (2014).
Hyer, M. L. et al. A small-molecule inhibitor of the ubiquitin activating enzyme for cancer treatment. Nat. Med. 24, 186–193 (2018).
Sabatier, P. et al. Comprehensive chemical proteomics analyses reveal that the new TRi-1 and TRi-2 compounds are more specific thioredoxin reductase 1 inhibitors than auranofin. Redox Biol. 48, 102184 (2021).
Chiappetta, G. et al. Redox proteome analysis of auranofin exposed ovarian cancer cells (A2780). Redox Biol. 52, 102294 (2022).
Saei, A. A. et al. ProTargetMiner as a proteome signature library of anticancer molecules for functional discovery. Nat. Commun. 10, 5715 (2019).
Varadi, M. et al. AlphaFold Protein Structure Database: massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 50, D439–D444 (2021).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D 66, 486–501 (2010).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Wang, Y. et al. Reversed‐phase chromatography with multiple fraction concatenation strategy for proteome profiling of human MCF10A cells. Proteomics 11, 2019–2026 (2011).
Paulo, J. A. et al. Quantitative mass spectrometry-based multiplexing compares the abundance of 5000 S. cerevisiae proteins across 10 carbon sources. J. Proteomics 148, 85–93 (2016).
Chambers, M. C. et al. A cross-platform toolkit for mass spectrometry and proteomics. Nat. Biotechnol. 30, 918–920 (2012).
Beausoleil, S. A., Villén, J., Gerber, S. A., Rush, J. & Gygi, S. P. A probability-based approach for high-throughput protein phosphorylation analysis and site localization. Nat. Biotechnol. 24, 1285–1292 (2006).
Huttlin, E. L. et al. A tissue-specific atlas of mouse protein phosphorylation and expression. Cell 143, 1174–1189 (2010).
Elias, J. E. & Gygi, S. P. Target–decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat. Methods 4, 207–214 (2007).
Elias, J. E. & Gygi, S. P. Proteome bioinformatics. Methods Mol. Biol. 604, 55–71 (2009).
McAlister, G. C. et al. Increasing the multiplexing capacity of TMTs using reporter ion isotopologues with isobaric masses. Anal. Chem. 84, 7469–7478 (2012).
Huang, T. et al. MSstatsTMT: statistical detection of differentially abundant proteins in experiments with isobaric labeling and multiple mixtures. Mol. Cell. Proteomics 19, 1706–1723 (2020).
Plubell, D. L. et al. Extended multiplexing of tandem mass tags (TMT) labeling reveals age and high fat diet specific proteome changes in mouse epididymal adipose tissue. Mol. Cell. Proteomics 16, 873–890 (2017).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 57, 289–300 (2018).
Acknowledgements
Cryo-EM screening and data collection were performed at the Harvard Center for Cryo-Electron Microscopy (HC2EM). Data processing was supported by SBGrid. We thank H Li and T Rapoport (Harvard Medical School) for providing the poly-ubiquitylated protein for degradation assays, and members of the Elledge and Shao labs for useful discussions. This work was supported by a Robin Reed Memorial Fellowship (J.G.), a National Science Foundation Graduate Research Fellowship (C.N.), an American Heart Association predoctoral fellowship (M.C.J.Y.), a Takeda Foundation Fellowship (H.C.), the National Mah Jongg League Fellow of the Damon Runyon Cancer Research Foundation (grant no. DRG-2469-22; X.G.), NIH grant no. R01GM132129 (J.A.P.), a Packard fellowship (S.S.), NIH grant no. R01AG073277 (S.S.) and NIH grant no. R01AG011085 (S.J.E.). S.J.E. is a member of the Ludwig Center at Harvard. S.S. and S.J.E. are Investigators with the Howard Hughes Medical Institute. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
J.G. and M.C.J.Y. prepared cryo-EM samples and collected and processed cryo-EM data. C.N. generated cell lines and performed most cellular and biochemical experiments with help from H.C., X.G. and M.J.R. J.G., M.C.J.Y. and S.S. modeled and interpreted the structure. H.C. prepared samples for proteomics analysis. C.N. and X.G. purified proteasomal complexes used for cryo-EM. C.N. and Z.M. performed RNA-seq analysis. J.A.P. oversaw proteomics analysis. C.N., J.G., S.J.E. and S.S. wrote the paper with input from all authors. S.J.E. and S.S. supervised the project.
Corresponding authors
Ethics declarations
Competing interests
S.J.E. is a founder of and holds equity in TScan Therapeutics, MAZE Therapeutics and Mirimus, and serves on the scientific advisory board of TScan Therapeutics, Infinity Bio and MAZE Therapeutics. The other authors declare no competing interests.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Dimitris Typas, in collaboration with the Nature Structural & Molecular Biology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Cryo-EM data processing.
Summary of cryo-EM data processing and classification strategy showing a representative micrograph, 2D class images, and 3D reconstructions.
Extended Data Fig. 2 Cryo-EM map and model quality.
a, Fourier shell correlation (FSC) vs. resolution (1/Å) curves for the indicated cryo-EM maps. b, The whole (top) and focused (bottom) cryo-EM map colored by local resolution. Arrow denotes TXNL1. c, Model vs. map FSC curves.
Extended Data Fig. 3 Characteristics of TXNL1.
a, AlphaFold prediction of TXNL1 showing the N-terminal thioredoxin domain and C-terminal proteasome-interacting thioredoxin (PITH) domain. b, A substantial portion of TXNL1 co-migrates with proteasomal components in size fractionations of HEK-293T cell lysates by sucrose gradient ultracentrifugation, assayed by SDS-PAGE and immunoblotting, representative of 2 independent experiments.
Extended Data Fig. 4 Characteristics of the TXNL1-bound proteasome.
a, Structure of the TXNL1-bound proteasome with TXNL1, PSMD14, and AAA-ATPase subunits colored as in Fig. 1bb, Clipped side view of the AAA-ATPase ring (top), colored by subunit, with the position of aromatic pore loop residues shown in spheres and substrate density in blue. Scheme (bottom) depicting the configuration and nucleotide (T – ATP, D – ADP)-bound states of the ATPase subunits relative to the substrate (blue). c, Superposition of the AAA-ATPase ring with that in the ED2 state of the human proteasome (PDB 6MSK; transparent dark blue; middle) or the 4D state of the yeast proteasome (PDB 6EF3; transparent dark purple, right). d, Map density (mesh) and model of the nucleotide binding site in the indicated AAA-ATPase subunit (from top to bottom: PSMC2, PSMC1, PSMC5, PSMC4, PSMC6, and PSMC3).
Extended Data Fig. 5 Common PITH domain interaction interfaces.
a, Cryo-EM structure showing TXNL1 PITH domain interactions with the proteasome. b, Alphafold3 models of TXNL1 (left) or PITHD1 (right) with PSMD1, PSMD4, PSMD14, and the AAA-ATPase subunits show shared interaction interfaces. Note: PITHD1 has an extended C-terminal tail that may enter the central pore of the AAA-ATPase ring. c, Immunoblotting of anti-FLAG immunoprecipitates (IP, top) and input lysates (bottom) of TXNL1 knockout (KO) HEK-293T cells transfected with 2xFLAG-tagged PITHD1 and TXNL1 variants, representative of 2 independent experiments. WT, wild type. D73 and K142 of PITHD1 are equivalent to D162 and R234 of TXNL1, respectively. d, Comparison of ubiquitin (red, left, PDB 6EF3), TXNL1 (teal, middle), and PITHD1 (purple, right, Alphafold3 model) engagement with PSMD14/Rpn11. The Insert-1 region of PSMD14/Rpn11 that assumes a β-hairpin when bound to ubiquitin is colored green. The C-terminal sequences of each factor that engage PSDM14/Rpn11 are highlighted in yellow and shown below. Note: ubiquitin and PITHD1, but not TXNL1, induce the β-hairpin Insert-1 conformation, and PITHD1 contains a C-terminal extension (purple) past the segment predicted to bind PSMD14. e, Same assay as in c, but from cells expressing TXNL1 variants in which the C-terminal tail was either extended (Tail-ext.) or replaced (Tail-repl.) with the PITHD1 C-terminal tail, as aligned in d, representative of 2 independent experiments. Cells were pre-treated with 10 µM MG-132 for 4 hrs. f, Immunoblotting of lysates of TXNL1 KO cells that were transduced with lentivirus to stably express wild-type or mutant variants of TXNL1 and pre-treated with the indicated drugs for 4 hrs, representative of 2 independent experiments. *, nonspecific band.
Extended Data Fig. 6 In vitro assays of TXNL1.
a, SDS-PAGE and Coomassie staining of purified HA-tagged TXNL1 variants (left) and TXNL1-deficient human proteasomes (right), representative of 2 independent purifications. b, Coomassie stain of samples from Fig. 2b, representative of 3 independent experiments. IP, immunoprecipitation. c, Purified HA-TXNL1 variants were immunoprecipitated in the presence of purified proteasomes pre-incubated with 6 mM of the indicated compound for 30 min at room temperature before immunoblotting, representative of 2 independent experiments. d, Proteasomes were pre-incubated with TXNL1 or the PSMD14/Rpn11 inhibitor 1,10-phenanthroline for 30 min at room temperature before the addition of a polyubiquitylated (polyUb) sfGFP protein substrate for 10 min at 37 °C, representative of 2 independent experiments. Note: 1,10-phenanthroline inhibits deubiquitylation and degradation of the polyUb-substrate (lane 3), but an excess of TXNL1 does not (lane 4).
Extended Data Fig. 7 Requirements for stress-induced TXNL1 degradation.
a, TXNL1 is rapidly degraded upon cellular exposure to arsenite but not hydrogen peroxide. Immunoblotting of HEK-293T cells treated with the indicated concentration of oxidative agents for 24 hrs, representative of 3 independent experiments. b, Immunoblotting of HEK-293T cells treated with 100 µM arsenite or 250 µM hydrogen peroxide over a time course, representative of 3 independent experiments. c, Immunoblotting from TXNL1 knockout (KO) HEK-293T cells that were stably complemented with wild type (WT) and mutant versions of TXNL1 by lentiviral transduction, representative of 3 independent experiments. D162, R234, and M275 reside at the interaction interfaces with PSMD1, PSMD4, and PSMD14, respectively. SxxS mutates the catalytic cysteines in the thioredoxin domain of TXNL1. Note: D162R TXNL1 does not co-purify proteasomes (Fig. 2b) but only weakly impairs arsenite-induced degradation, indicating that it still has some affinity for proteasomes, likely through the interface with PSMD1 that is disrupted by the R234D mutation. d, Immunoblotting of anti-FLAG immunoprecipitates (IP, top) and input lysates (bottom) of TXNL1 KO HEK-293T cells transfected with 2xFLAG-tagged TXNL1 variants and treated with 100 µM arsenite and 10 µM MG132 for 3 hrs, representative of 3 independent experiments. Note: WT and SxxS TXNL1 still interact with proteasomes in the presence of arsenite. e, TXNL1 degradation upon auranofin treatment requires proteasome binding and thioredoxin activity. Assay as in Fig. 2c but with auranofin treatment in place of arsenite treatment, representative of 3 independent experiments. f, Assay as in Fig. 2d but with auranofin treatment in place of arsenite treatment, representative of 3 independent experiments.
Extended Data Fig. 8 Proteome changes upon arsenite exposure with TXNL1 variants.
Selective degradation of TXNL1 upon arsenite exposure requires proteasome binding. Volcano plots of multiplexed proteomics data showing the fold-change (Log2FC) of protein levels in TXNL1 KO HEK-293T cells complemented with the indicated TXNL1 variant compared to KO cells without (left) and with (right) arsenite treatment. Two-sided Student’s t-test p-values were adjusted for multiple comparisons using the Benjamini and Hochberg method. Note: arsenite treatment selectively lowers the level of WT but not D162R/R234D TXNL1.
Extended Data Fig. 9 Transcriptional changes upon cellular arsenite exposure.
a, Volcano plots of RNA sequencing data showing the fold-change (Log2FC) of transcript levels in TXNL1 knockout (KO) HEK-293T cells complemented with wild type (WT) TXNL1, D162R/R234D TXNL1 deficient in proteasome binding, or SxxS TXNL1 deficient in thioreductase activity compared to KO cells without (top) or with (bottom) arsenite treatment. Two-sided Wald test p-values were adjusted for multiple comparisons using the Benjamini and Hochberg method. Note: TXNL1 is the main transcript that is significantly changed in both conditions. b, Volcano plots of RNA sequencing data as in a but showing the fold-change (Log2FC) of transcript levels in TXNL1 KO cells without (left) or with complementation with WT TXNL1 or the indicated TXNL1 mutants upon arsenite treatment. Note: changes in stress-responsive transcript (purple) levels are not impacted by TXNL1 expression.
Supplementary information
Supplementary Table 1
TMT-MS of untreated and arsenite-treated TXNL1 KO HEK 293T cells complemented without or with wild-type or R162D R234D TXNL1.
Supplementary Table 2
RNA sequencing of untreated, arsenite-treated, or hydrogen peroxide-treated TXNL1 KO HEK 293T cells complemented without or with wild-type, D162R R234D (Prot) or SxxS (Thio) TXNL1.
Source data
Source data
Unprocessed western blots and gels for Fig. 2 and Extended Data Figs. 3, 5, 6 and 7.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gao, J., Nardone, C., Yip, M.C.J. et al. Structure of the TXNL1-bound proteasome. Nat Struct Mol Biol (2025). https://doi.org/10.1038/s41594-025-01639-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41594-025-01639-w