Abstract
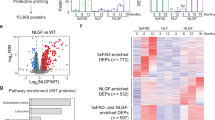
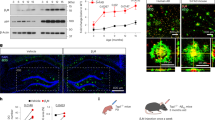
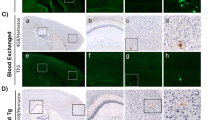
Proteomic profiling of Alzheimer disease (AD) brains has identified numerous understudied proteins, including midkine (MDK), that are highly upregulated and correlated with amyloid-β (Aβ) from the early disease stage but their roles in disease progression are not fully understood. Here, we present that MDK attenuates Aβ assembly and influences amyloid formation in the 5xFAD amyloidosis mouse model. MDK protein mitigates fibril formation of both Aβ40 and Aβ42 peptides according to thioflavin T fluorescence, circular dichroism, negative-stain electron microscopy and nuclear magnetic resonance analyses. Knockout of the Mdk gene in 5xFAD increased amyloid formation and microglial activation in the brain. Further comprehensive mass-spectrometry-based profiling of the whole proteome and detergent-insoluble proteome in these mouse models indicated significant accumulation of Aβ and Aβ-correlated proteins, along with microglial components. Thus, our structural and mouse model studies reveal a protective role of MDK in counteracting amyloid pathology in AD.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The NMR chemical shift assignments are available from the BMRB under accession number 17795. Information on all human cases is provided in Supplementary Data 2. The MS proteomics data were deposited to the ProteomeXchange Consortium through the PRIDE partner repository under dataset identifiers: PXD007985 (human whole-proteome dataset from previous publication16), PXD046539 and PXD061103 (whole proteome of AD mouse models) and PXD045746 and PXD061104 (detergent-insoluble proteome of the mice). Source data are provided with this paper.
References
Alzheimer’s Association 2023 Alzheimer’s disease facts and figures. Alzheimers Dement. 19, 1598–1695 (2023).
Bellenguez, C., Grenier-Boley, B. & Lambert, J. C. Genetics of Alzheimer’s disease: where we are, and where we are going. Curr. Opin. Neurobiol. 61, 40–48 (2020).
Bellenguez, C. et al. New insights into the genetic etiology of Alzheimer’s disease and related dementias. Nat. Genet. 54, 412–436 (2022).
Wightman, D. P. et al. A genome-wide association study with 1,126,563 individuals identifies new risk loci for Alzheimer’s disease. Nat. Genet. 53, 1276–1282 (2021).
Hardy, J. & Selkoe, D. J. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297, 353–356 (2002).
Ballatore, C., Lee, V. M. & Trojanowski, J. Q. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat. Rev. Neurosci. 8, 663–672 (2007).
Neumann, M. et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314, 130–133 (2006).
Nelson, P. T. et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain 142, 1503–1527 (2019).
Bai, B. et al. U1 small nuclear ribonucleoprotein complex and RNA splicing alterations in Alzheimer’s disease. Proc. Natl Acad. Sci. USA 110, 16562–16567 (2013).
Bai, B. et al. Integrated approaches for analyzing U1–70K cleavage in Alzheimer’s disease. J. Proteome Res 13, 4526–4534 (2014).
Hales, C. M. et al. U1 small nuclear ribonucleoproteins (snRNPs) aggregate in Alzheimer’s disease due to autosomal dominant genetic mutations and trisomy 21. Mol. Neurodegener. 9, 15 (2014).
Chen, P. C. et al. Alzheimer’s disease-associated U1 snRNP splicing dysfunction causes neuronal hyperexcitability and cognitive impairment. Nat. Aging 2, 923–940 (2022).
Zaman, M. et al. Dissecting detergent-insoluble proteome in Alzheimer’s disease by TMTc-corrected quantitative mass spectrometry. Mol. Cell Proteom. 22, 100608 (2023).
van Dyck, C. H. et al. Lecanemab in early Alzheimer’s disease. N. Engl. J. Med. 388, 9–21 (2023).
Sims, J. R. et al. Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA 330, 512–527 (2023).
Bai, B. et al. Deep multilayer brain proteomics identifies molecular networks in Alzheimer’s disease progression. Neuron 105, 975–991 (2020).
Wang, H. et al. Integrated analysis of ultra-deep proteomes in cortex, cerebrospinal fluid and serum reveals a mitochondrial signature in Alzheimer’s disease. Mol. Neurodegener. 15, 43 (2020).
Higginbotham, L. et al. Integrated proteomics reveals brain-based cerebrospinal fluid biomarkers in asymptomatic and symptomatic Alzheimer’s disease. Sci. Adv. 6, eaaz9360 (2020).
Johnson, E. C. B. et al. Large-scale deep multi-layer analysis of Alzheimer’s disease brain reveals strong proteomic disease-related changes not observed at the RNA level. Nat. Neurosci. 25, 213–225 (2022).
Sathe, G. et al. Quantitative proteomic analysis of the frontal cortex in Alzheimer’s disease. J. Neurochem. 156, 988–1002 (2021).
Roberts, J. A. et al. A brain proteomic signature of incipient Alzheimer’s disease in young APOE ε4 carriers identifies novel drug targets. Sci. Adv. 7, eabi8178 (2021).
Bai, B. et al. Proteomic landscape of Alzheimer’s disease: novel insights into pathogenesis and biomarker discovery. Mol. Neurodegener. 16, 55 (2021).
Askenazi, M. et al. Compilation of reported protein changes in the brain in Alzheimer’s disease. Nat. Commun. 14, 4466 (2023).
Yarbro, J. M. et al. Human and mouse proteomics reveals the shared pathways in Alzheimer’s disease and delayed protein turnover in the amyloidome. Nat. Commun. 16, 1533 (2025).
Li, W. et al. Turnover atlas of proteome and phosphoproteome across mouse tissues and brain regions. Cell https://doi.org/10.1016/j.cell.2025.02.021 (2025).
Liao, L. et al. Proteomic characterization of postmortem amyloid plaques isolated by laser capture microdissection. J. Biol. Chem. 279, 37061–37068 (2004).
Xiong, F., Ge, W. & Ma, C. Quantitative proteomics reveals distinct composition of amyloid plaques in Alzheimer’s disease. Alzheimers Dement. 15, 429–440 (2019).
Drummond, E. et al. The amyloid plaque proteome in early onset Alzheimer’s disease and Down syndrome. Acta Neuropathol. Commun. 10, 53 (2022).
Rahman, M. M. & Lendel, C. Extracellular protein components of amyloid plaques and their roles in Alzheimer’s disease pathology. Mol. Neurodegener. 16, 59 (2021).
Ross-Munro, E. et al. Midkine: the who, what, where, and when of a promising neurotrophic therapy for perinatal brain injury. Front. Neurol. 11, 568814 (2020).
Yasuhara, O. et al. Midkine, a novel neurotrophic factor, is present in senile plaques of Alzheimer disease. Biochem. Biophys. Res. Commun. 192, 246–251 (1993).
Muramatsu, H. et al. Midkine as a factor to counteract the deposition of amyloid β-peptide plaques: in vitro analysis and examination in knockout mice. Int. Arch. Med. 4, 1 (2011).
Oakley, H. et al. Intraneuronal β-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. J. Neurosci. 26, 10129–10140 (2006).
LeVine, H. III Thioflavine T interaction with synthetic Alzheimer’s disease β-amyloid peptides: detection of amyloid aggregation in solution. Protein Sci. 2, 404–410 (1993).
Meisl, G. et al. Molecular mechanisms of protein aggregation from global fitting of kinetic models. Nat. Protoc. 11, 252–272 (2016).
Yang, Y. et al. Cryo-EM structures of amyloid-β 42 filaments from human brains. Science 375, 167–172 (2022).
Kollmer, M. et al. Cryo-EM structure and polymorphism of β amyloid fibrils purified from Alzheimer’s brain tissue. Nat. Commun. 10, 4760 (2019).
Lu, J. X. et al. Molecular structure of β-amyloid fibrils in Alzheimer’s disease brain tissue. Cell 154, 1257–1268 (2013).
Sawaya, M. R., Hughes, M. P., Rodriguez, J. A., Riek, R. & Eisenberg, D. S. The expanding amyloid family: structure, stability, function, and pathogenesis. Cell 184, 4857–4873 (2021).
Yang, Y. et al. Cryo-EM structures of amyloid-β filaments with the Arctic mutation (E22G) from human and mouse brains. Acta Neuropathol. 145, 325–333 (2023).
Evans, R. et al. Protein complex prediction with AlphaFold-Multimer. Preprint at bioRxiv https://doi.org/10.1101/2021.1110.1104.463034 (2022).
Roche, J., Shen, Y., Lee, J. H., Ying, J. & Bax, A. Monomeric Aβ(1–40) and Aβ(1–42) peptides in solution adopt very similar Ramachandran map distributions that closely resemble random coil. Biochemistry 55, 762–775 (2016).
Wallin, C. et al. The neuronal tau protein blocks in vitro fibrillation of the amyloid-β (Aβ) peptide at the oligomeric stage. J. Am. Chem. Soc. 140, 8138–8146 (2018).
Yao, Z. et al. A transcriptomic and epigenomic cell atlas of the mouse primary motor cortex. Nature 598, 103–110 (2021).
Styren, S. D., Hamilton, R. L., Styren, G. C. & Klunk, W. E. X-34, a fluorescent derivative of Congo red: a novel histochemical stain for Alzheimer’s disease pathology. J. Histochem. Cytochem. 48, 1223–1232 (2000).
Ito, D. et al. Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res Mol. Brain Res. 57, 1–9 (1998).
Schwabenland, M. et al. Analyzing microglial phenotypes across neuropathologies: a practical guide. Acta Neuropathol. 142, 923–936 (2021).
Bai, B. et al. Deep profiling of proteome and phosphoproteome by isobaric labeling, extensive liquid chromatography, and mass spectrometry. Methods Enzymol. 585, 377–395 (2017).
Wang, Z. et al. 27-plex tandem mass tag mass spectrometry for profiling brain proteome in Alzheimer’s disease. Anal. Chem. 92, 7162–7170 (2020).
Niu, M. et al. Extensive peptide fractionation and y1 ion-based interference detection method for enabling accurate quantification by isobaric labeling and mass spectrometry. Anal. Chem. 89, 2956–2963 (2017).
Langfelder, P. & Horvath, S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 9, 559 (2008).
Sousa, C. et al. Single-cell transcriptomics reveals distinct inflammation-induced microglia signatures. EMBO Rep. 19, e46171 (2018).
Zhang, Y. et al. Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse. Neuron 89, 37–53 (2016).
Saha, O. et al. The Alzheimer’s disease risk gene BIN1 regulates activity-dependent gene expression in human-induced glutamatergic neurons. Mol. Psychiatry 29, 2634–2646 (2024).
Voskobiynyk, Y. et al. Alzheimer’s disease risk gene BIN1 induces tau-dependent network hyperexcitability. Elife 9, e57354 (2020).
Arvidsson, U., Riedl, M., Elde, R. & Meister, B. Vesicular acetylcholine transporter (VAChT) protein: a novel and unique marker for cholinergic neurons in the central and peripheral nervous systems. J. Comp. Neurol. 378, 454–467 (1997).
Rasmussen, N. L., Kournoutis, A., Lamark, T. & Johansen, T. NBR1: the archetypal selective autophagy receptor. J. Cell Biol. 221, e202208092 (2022).
Cohen, S. I. et al. Proliferation of amyloid-β42 aggregates occurs through a secondary nucleation mechanism. Proc. Natl Acad. Sci. USA 110, 9758–9763 (2013).
Lee, J., Culyba, E. K., Powers, E. T. & Kelly, J. W. Amyloid-β forms fibrils by nucleated conformational conversion of oligomers. Nat. Chem. Biol. 7, 602–609 (2011).
Belsare, K. D. et al. Soluble TREM2 inhibits secondary nucleation of Aβ fibrillization and enhances cellular uptake of fibrillar Aβ. Proc. Natl Acad. Sci. USA 119, e2114486119 (2022).
Gao, Y., Schedin-Weiss, S. & Tjernberg, L. O. A closer look at pathogenic amyloid-β in Alzheimer’s disease using cryo-electron microscopy: a narrative review. Adv. Technol. Neurosci. 1, 177–187 (2024).
Maeda, N. et al. A receptor-like protein-tyrosine phosphatase PTPζ/RPTPβ binds a heparin-binding growth factor midkine. Involvement of arginine 78 of midkine in the high affinity binding to PTPζ. J. Biol. Chem. 274, 12474–12479 (1999).
Muramatsu, H. et al. LDL receptor-related protein as a component of the midkine receptor. Biochem. Biophys. Res. Commun. 270, 936–941 (2000).
Muramatsu, H. et al. α4β1- and α6β1-integrins are functional receptors for midkine, a heparin-binding growth factor. J. Cell Sci. 117, 5405–5415 (2004).
Ichihara-Tanaka, K., Oohira, A., Rumsby, M. & Muramatsu, T. Neuroglycan C is a novel midkine receptor involved in process elongation of oligodendroglial precursor-like cells. J. Biol. Chem. 281, 30857–30864 (2006).
Stoica, G. E. et al. Midkine binds to anaplastic lymphoma kinase (ALK) and acts as a growth factor for different cell types. J. Biol. Chem. 277, 35990–35998 (2002).
Huang, Y. et al. Midkine induces epithelial–mesenchymal transition through Notch2/JAK2–STAT3 signaling in human keratinocytes. Cell Cycle 7, 1613–1622 (2008).
Yamazaki, Y., Zhao, N., Caulfield, T. R., Liu, C. C. & Bu, G. Apolipoprotein E and Alzheimer disease: pathobiology and targeting strategies. Nat. Rev. Neurol. 15, 501–518 (2019).
Levites, Y. et al. Integrative proteomics identifies a conserved Aβ amyloid responsome, novel plaque proteins, and pathology modifiers in Alzheimer’s disease. Cell Rep. Med 5, 101669 (2024).
Chishti, M. A. et al. Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. J. Biol. Chem. 276, 21562–21570 (2001).
Beach, T. G. et al. Arizona study of aging and neurodegenerative disorders and brain and body donation program. Neuropathology 35, 354–389 (2015).
Connelly, J. P. & Pruett-Miller, S. M. CRIS.py: a versatile and high-throughput analysis program for CRISPR-based genome editing. Sci. Rep. 9, 4194 (2019).
Narina, S., Connelly, J. P. & Pruett-Miller, S. M. High-throughput analysis of CRISPR–Cas9 editing outcomes in cell and animal models using CRIS.py. Methods Mol. Biol. 2631, 155–182 (2023).
Chen, Z. L. et al. A high-speed search engine pLink 2 with systematic evaluation for proteome-scale identification of cross-linked peptides. Nat. Commun. 10, 3404 (2019).
Delaglio, F. et al. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 6, 277–293 (1995).
Lee, W., Tonelli, M. & Markley, J. L. NMRFAM-SPARKY: enhanced software for biomolecular NMR spectroscopy. Bioinformatics 31, 1325–1327 (2015).
Schanda, P., Kupce, E. & Brutscher, B. SOFAST-HMQC experiments for recording two-dimensional heteronuclear correlation spectra of proteins within a few seconds. J. Biomol. NMR 33, 199–211 (2005).
Bankhead, P. et al. QuPath: open source software for digital pathology image analysis. Sci. Rep. 7, 16878 (2017).
Xu, P., Duong, D. M. & Peng, J. Systematical optimization of reverse-phase chromatography for shotgun proteomics. J. Proteome Res. 8, 3944–3950 (2009).
Wang, H. et al. Systematic optimization of long gradient chromatography mass spectrometry for deep analysis of brain proteome. J. Proteome Res. 14, 829–838 (2015).
Wang, X. et al. JUMP: a tag-based database search tool for peptide identification with high sensitivity and accuracy. Mol. Cell Proteom. 13, 3663–3673 (2014).
Peng, J., Elias, J. E., Thoreen, C. C., Licklider, L. J. & Gygi, S. P. Evaluation of multidimensional chromatography coupled with tandem mass spectrometry (LC/LC–MS/MS) for large-scale protein analysis: the yeast proteome. J. Proteome Res. 2, 43–50 (2003).
Elias, J. E. & Gygi, S. P. Target–decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat. Methods 4, 207–214 (2007).
Ritchie, M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 (2015).
Mertens, B. J. A. Transformation, normalization, and batch effect in the analysis of mass spectrometry data for omics studies. In Statistical Analysis of Proteomics, Metabolomics, and Lipidomics Data Using Mass Spectrometry (eds Datta, S. & Mertens, B. J. A.) (Springer, 2016).
Zhang, B. & Horvath, S. A general framework for weighted gene co-expression network analysis. Stat. Appl. Genet. Mol. Biol. 4, Article17 (2005).
Yu, G., Wang, L. G., Han, Y. & He, Q. Y.clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16, 284–287 (2012).
Acknowledgements
We thank I. Chen for critical readings and comments. We also thank the St. Jude Shared Resources and Core Facilities, including the Protein Technology Center, Biomolecular NMR Center, Cryo-EM and Tomography Center, Cell and Tissue Imaging Center, Animal Research Center, Center for Advanced Genome Engineering and Center for Proteomics and Metabolomics. This work was partially supported by National Institutes of Health grants R01AG053987, RF1AG064909, RF1AG068581, U19AG069701 and P30CA021765 and the American Lebanese Syrian Associated Charities foundation. The Banner Sun Health Research Institute Brain and Body Donation Program was supported by National Institutes of Health grants U24NS072026, P30AG019610 and P30AG072980, the Arizona Department of Health Services, the Arizona Biomedical Research Commission and the Michael J. Fox Foundation for Parkinson’s Research.
Author information
Authors and Affiliations
Contributions
J.P., P.-C.C., G.Y. and M.Z. conceptualized the project. M.Z., Y.Y, J.M.Y., Y.H., A.S.T., R.K., C.R.G., M.T. and S.C. engaged in the biophysical experiments. S.Y., Z. Wang, K.E.H., H.S., A.H., S.H., Y.J. and P.-C.C. performed the biological experiments. P.-C.C., S.Y., Y.J., S.M.P.-M. and V.S. generated and bred the mouse models. S.Y., Z. Wang, D.L., Y, H., Z. Wu, A.A.H. and X.W. contributed to the MS-based proteomics analysis. G.E.S. and T.G.B. characterized and provided the human brain samples. M.Z., Y.H., P.-C.C. and J.P. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks Stephen Ginsberg, Todd Golde and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available. Primary Handling Editor: Katarzyna Ciazynska, in collaboration with the Nature Structural & Molecular Biology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Bottom-up MS analysis of recombinant MDK proteins and their effect on Aβ fibrillation.
a, Protein sequences of MDK proteins expressed in mammalian 293 or E. coli cells. The N-terminal His-tag was cleaved after expression by TEV protease. b, Workflow of bottom-up LC-MS/MS analysis to characterize the positions of disulfide (S-S) bonds. Proteins were subjected to IAA alkylation, trypsin digestion and LC-MS/MS analysis. Crosslinked peptides containing single or double S-S bonds were identified. c, Relative peak intensities of top five crosslinked peptides (n = 3 replicates). Data are shown as mean ± SEM. d-e, Effect of 293- or E. coli-expressed MDK (10 µM) on Aβ40/42 (5 µM) fibrillation kinetics in ThT fluorescence assays. Results represent the average of 3 replicates. Full statistical information is in Source Data Statistics.
Extended Data Fig. 2 Characterization of the effect of MDK on Aβ fibrillation.
a, Diagram of the secondary nucleation model illustrating Aβ assembly. The rate constants (k+ and k2) are defined with units, reflecting their dependency on protein concentration and time. b-c, Analysis of Aβ40 and Aβ42 elongation constants (k+) by fitting the ThT data. d, Effect of MDK (0-3 µM) on Aβ40 fibrillation kinetics (n = 3 replicates, averaged data shown). e, Effect of MDK (0-3 µM) on Aβ42 fibrillation kinetics (n = 3 replicates, averaged data shown). f, Time course CD spectra of Aβ40 alone (0-24 h). g, Time course CD spectra of Aβ40 with MDK (0-24 h). h, Time course CD spectra of Aβ42 alone (0-24 h). i, Time course CD spectra of Aβ42 with MDK (0-24 h). Full statistical information is in Source Data Statistics.
Extended Data Fig. 3 Characterization of ubiquitin protein by MS and its lack of effect on Aβ40/42 fibrillation.
a, Purified Ub protein on a stained SDS gel. b-c, Top-down mass spectrum and deconvoluted results of Ub protein showing different charge states. Similar results were obtained in two independent experiments. d, Bottom-up LC-MS/MS analysis of Ub, covering the full Ub sequence except two short tryptic peptides (in grey). e, ThT fluorescence assay measuring Aβ40 fibril formation at different Ub concentrations (n = 3 replicates, averaged data shown). f, CD spectroscopy of Aβ40 with or without Ub, with ellipticity reported in millidegrees (mdeg). g, Negative stain EM of Aβ40/Ub samples with a scale bar (100 nm). h, ThT fluorescence assay measuring Aβ42 fibril formation at different Ub concentrations (n = 3 replicates, averaged data shown). i. CD spectroscopy of Aβ42 with or without Ub. j, Negative stain EM of Aβ42/Ub samples with a scale bar (100 nm). Similar results were obtained in two independent experiments. Full statistical information is in Source Data Statistics.
Extended Data Fig. 4 MDK or ubiquitin cannot independently assemble into fibrils.
a, ThT assay of MDK alone at different concentrations (n = 3 replicates, averaged data shown). b, Time course CD spectra of MDK (0-24 h). c, Negative stain EM of the MDK samples, with a scale bar (100 nm). Similar results were obtained in two independent experiments. d, ThT assay of Ub alone at different concentrations (n = 3 replicates, averaged data shown). e, Time course CD spectra of Ub (0-24 h). f, Negative stain EM of the Ub samples, with a scale bar (100 nm). Similar results were obtained in two independent experiments. Full statistical information is in Source Data Statistics.
Extended Data Fig. 5 Structural prediction of Aβ-MDK interaction.
a, The alignment of human and mouse MDK protein sequences. MDK is a secreted protein containing a signal peptide for secretion (aa 1–22) and the main protein chain (aa 23–143). b, AlphaFold2-multimer predicted structures of the Aβ-MDK potential interacting interfaces. The confidence of the predicted 3D structures is evaluated by the predicted Local Distance Difference Test (pLDDT). Full statistical information is in Source Data Statistics.
Extended Data Fig. 6 Ubiquitin, used as a control, does not prevent the loss of NMR signals in Aβ peptides.
a, 1H-15N HSQC spectra of Aβ40 (10 μM) with or without Ub (10 μM) in 50 mM Tris buffer (pH 7.5). Spectra were collected before incubation (left panel) and after 48 h incubation (right two panels). b, Relative cross-peak intensities for each residue, excluding D1 and H14. Intensities were normalized to a maximum value of 1. c-d, NMR analysis of Aβ42 under similar conditions as Aβ40, but with 24 h incubation.
Extended Data Fig. 7 MDK expression in KO mice, cell-type specificity, and effects on Aβ pathology.
a, Predicted mouse MDK protein sequences in WT and CRISPR-mediated KO mice. A 23 bp deletion in Mdk gene disrupts the open reading frame in KO mice, potentially generating a truncated protein with distinct C-terminus (blue). Peptides detected by MS in brain proteomic analysis are marked with lines. The two shared peptides (aa 30-47 and aa 31-47, red line) allow quantification of MDK full length proteins and the tentative N-terminal truncation. b, Quantification of shared peptides in brain lysates (FAD n = 5, FAD/KO n = 7), normalized to the highest value (100). The absence of signals in FAD/KO mice suggests that the tentative N-terminal truncation was not expressed at a detectable level. c, Uniform Manifold Approximation and Projection plots showing expression of Mdk, Gfap (astrocyte marker) and Pdgfra (oligodendrocyte progenitor cell marker) in mouse brain, from single-cell RNA sequencing of more than 1.2 million cells in the whole cortex and hippocampus cells using the 10x Genomics Chromium platform44. Expression levels are indicated by color intensity (red). Mdk is highly expressed in astrocyte (377_Astro; trimmed mean (25-75%) Log2(CPM + 1) = 4.13), and oligodendrocyte (366_Oligo; trimmed mean value = 0.72). d, ELISA analysis of Aβ40/Aβ42 ratio in the Sarkosyl-soluble and -insoluble cortical fractions (n = 10 per group). e, Quantification of X34-positive amyloid plaque density in the cortices of male (FAD n = 5; FAD/KO n = 5) and female (FAD n = 5, FAD/KO n = 6) mice. f, Quantification of X34-positive amyloid plaque area in the same mice as in e; value normalized to the FAD mean (set to 1). g, Example images of X34 staining showing amyloid plaque quantification in the cortices of FAD and FAD/KO mice. Cortices were outlined by the white lines. Experiments were independently repeated three times. Scale bar, 500 µm. b, d-f. Results were analyzed using a two-tailed unpaired Student’s t-test when equal variances were confirmed, or Welch’s t-test when unequal variances were identified based on preliminary variance testing. Data are shown as mean ± SEM. Full statistical information is available in Source Data.
Extended Data Fig. 8 Proteomic comparison of four genotypes derived from FAD and Mdk KO mice.
a, Boxplot of whole proteome data showing equal loading across samples, with WT, KO, FAD and FAD/KO represented in purple, blue, orange, and green, respectively. b, PCA of whole proteome data using DEPs, showing genotype-specific separation. c, Representative volcano plot showing DEPs between FAD and FAD/KO mice, with FDR values from limma analysis and log2 ratios converted to z values. d, Selected DEPs in four whole proteome clusters (WPC), with each protein represented by a colored box after log2 conversion and Z score analysis. e, Boxplot of insoluble proteome data. f, PCA of insoluble proteome data using DEPs. g, Volcano plot comparing the insoluble proteome of FAD and FAD/KO mice. h, Heatmap of selected DEPs shared between the insoluble and whole proteomes. i, Full-length APP protein diagram showing the position of a representative non-Aβ tryptic peptide. j, Quantification of the non-Aβ peptide levels in whole proteome across four genotypes (WT n = 8, KO n = 8, FAD n = 15, FAD/KO n = 17), with statistical significance determined by a two-tailed Student’s t-test. Data are shown as mean ± SEM.
Supplementary information
Source data
Source Data Figures and Extended Data Figures
Uncropped western blots for Figs. 1b and 5c.
Source Data Figures and Extended Data Figures
Statistical source data for Figs. 1a,b, 2e,f, 3a–c,f, 4c–f and 5b,d–f and Extended Data Figs. 1c–e, 2d–h,i, 3e,i, 4a,b,d,e, 5b,d and 7b,d–f.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zaman, M., Yang, S., Huang, Y. et al. Midkine attenuates amyloid-β fibril assembly and plaque formation. Nat Struct Mol Biol (2025). https://doi.org/10.1038/s41594-025-01657-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41594-025-01657-8