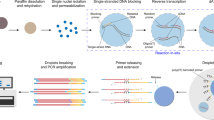
Abstract
Formalin-fixed paraffin-embedded (FFPE) samples represent a vast and valuable resource of patient material, often linked to extensive clinical history and follow-up data. However, achieving single-cell or single-nucleus RNA (sc/snRNA) profiling in these archived tissues remains challenging. To address this, we have developed snRandom-seq, a droplet- and random primer–based single-nucleus total RNA sequencing technology specifically designed for FFPE tissues. This method captures total RNAs by using random primers and demonstrates a low doublet rate (0.3%), increased RNA coverage and enhanced detection of non-coding and nascent RNAs compared to state-of-the-art high-throughput sc/snRNA-seq technologies. This protocol provides a comprehensive guide to isolating single nuclei from FFPE samples; performing in situ DNA blocking, reverse transcription and dA tailing reactions; barcoding single-nucleus droplets; and preparing sequencing libraries. The entire snRandom-seq process can be completed in 4 d. This platform serves as a powerful tool for snRNA-seq of clinical specimens, with broad applications in studying complex biological systems.
Key points
-
This protocol uses a mix of random and oligo(dT) primers to capture a broad range of RNAs from single nuclei prepared from formalin-fixed paraffin-embedded tissue samples for high-throughput sequencing by microfluidic droplet encapsulation and barcoding.
-
This technique expands the access of single-cell and single-nucleus RNA sequencing to a vast archive of patient samples with associated clinical data and, in combination with other high-throughput profiling methods, is particularly well suited for in-depth analysis of the tumor microenvironment.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The raw sequence data reported in this paper have been deposited in the Genome Sequence Archive (Genomics, Proteomics & Bioinformatics 2021) in the National Genomics Data Center (Nucleic Acids Res. 2024), China National Center for Bioinformation/Beijing Institute of Genomics, Chinese Academy of Sciences (GSA-Human: HRA005870) and are publicly accessible at https://ngdc.cncb.ac.cn/gsa-human. The raw sequence data reported in this paper are among the datasets published in our supporting primary research paper13. Source data are provided with this paper.
Code availability
The code for the preprocessing of snRandom-seq data is available at https://github.com/WangycLab/snRandom-seq21.
References
Gnanapragasam, V. J. Unlocking the molecular archive: the emerging use of formalin-fixed paraffin-embedded tissue for biomarker research in urological cancer. BJU Int. 105, 274–278 (2010).
Wang, K. et al. Archival single-cell genomics reveals persistent subclones during DCIS progression. Cell 186, 3968–3982.e15 (2023).
Evers, D. L., He, J., Kim, Y. H., Mason, J. T. & O’Leary, T. J. Paraffin embedding contributes to RNA aggregation, reduced RNA yield, and low RNA quality. J. Mol. Diagn. 13, 687–694 (2011).
Zheng, G. X. Y. et al. Massively parallel digital transcriptional profiling of single cells. Nat. Commun. 8, 14049 (2017).
Llora-Batlle, O. et al. 10x Genomics Gene Expression Flex is a powerful tool for single-cell transcriptomics of xenograft models. Preprint at https://www.biorxiv.org/content/10.1101/2024.01.25.577066v2 (2024).
Wang, T. et al. snPATHO-seq, a versatile FFPE single-nucleus RNA sequencing method to unlock pathology archives. Commun. Biol. 7, 1340 (2024).
Hattie, C. et al. SnFFPE-Seq: towards scalable single nucleus RNA-Seq of formalin-fixed paraffin-embedded (FFPE) tissue. Preprint at https://www.biorxiv.org/content/10.1101/2022.08.25.505257v1 (2022).
Isakova, A., Neff, N. & Quake, S. R. Single-cell quantification of a broad RNA spectrum reveals unique noncoding patterns associated with cell types and states. Proc. Natl Acad. Sci. USA 118, e2113568118 (2021).
Salmen, F. et al. High-throughput total RNA sequencing in single cells using VASA-seq. Nat. Biotechnol. 40, 1780–1793 (2022).
Rosenberg, A. B. et al. Single-cell profiling of the developing mouse brain and spinal cord with split-pool barcoding. Science 360, 176–182 (2018).
Xu, Z. et al. High-throughput single nucleus total RNA sequencing of formalin-fixed paraffin-embedded tissues by snRandom-seq. Nat. Commun. 14, 2734 (2023).
Sun, X. et al. A pathologically friendly strategy for determining the organ-specific spatial tumor microenvironment topology in lung adenocarcinoma through the integration of snRandom-seq and imaging mass cytometry. Adv. Sci. (Weinh.) 11, e2308892 (2024).
Xu, Z. et al. Single nucleus total RNA sequencing of formalin-fixed paraffin-embedded gliomas. Small Methods 8, e2301801 (2024).
Chen, H. et al. Pan-cancer single-nucleus total RNA sequencing using snHH-Seq. Adv. Sci. (Weinh.) 11, e2304755 (2024).
Niu, M. et al. Droplet-based transcriptome profiling of individual synapses. Nat. Biotechnol. 41, 1332–1344 (2023).
Amini, P. et al. An optimised protocol for isolation of RNA from small sections of laser-capture microdissected FFPE tissue amenable for next-generation sequencing. BMC Mol. Biol. 18, 22 (2017).
Bai, Z. et al. Spatially exploring RNA biology in archival formalin-fixed paraffin-embedded tissues. Cell 187, 6760–6779.e24 (2024).
Okubo, Y. et al. Effective preparation of FFPE tissue samples for preserving appropriate nucleic acid quality for genomic analysis in thyroid carcinoma. Endocr. Pathol. 35, 372–384 (2024).
Zong, C., Lu, S., Chapman, A. R. & Xie, X. S. Genome-wide detection of single-nucleotide and copy-number variations of a single human cell. Science 338, 1622–1626 (2012).
Zilionis, R. et al. Single-cell barcoding and sequencing using droplet microfluidics. Nat. Protoc. 12, 44–73 (2017).
Xu, Z., Lyu, Y., Chen, H., Chen, Y. & Wang, Y. Single-nucleus total RNA sequencing of formalin-fixed paraffin-embedded samples using snRandom-seq. Zenodo https://doi.org/10.5281/zenodo.15063307 (2024).
Acknowledgements
The project was supported by the National Natural Science Foundation of China (No. 32200073 to Y.W., No. 32250710678 to Y.W. and No. 82200977 to Z.X.); the Leading Innovative and Entrepreneur Team Introduction Program of Zhejiang (No. 2021R01012 to Y.W.); the ‘Pioneer’ R&D programs of Zhejiang Province (No. 2024C03005 to Y.W.); the Key R&D Program of Zhejiang (No. 2024SSYS0022 to Y.W.); the State Key Laboratory of Space Medicine, China Astronaut Research and Training Center (No. SKL2024K04 to Y.W.); and the Space Medical Experiment Project of CMSP (No. HYZHXMH03003 to Y.W.). We thank the core facilities of Zhejiang University and Liangzhu Laboratory for technical support. In particular, we thank Jingyao Chen and Chengcheng Zhang from the core facilities, Zhejiang University School of Medicine for their technical support.
Author information
Authors and Affiliations
Contributions
Y.W. conceived the study and managed the project’s progress. Z.X., Y.L. and H.C wrote the manuscript and revised the manuscript. Z.X. and H.C. developed the snRandom-seq procedure. Z.X., Y.L. and Y.C coordinated the experiments and analysis. All authors revised and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
Y.W. is a co-founder of M20 Genomics. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Chaoyong Yang, Paloma Cejas, and the other, anonymous, reviewer for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key references
Xu, Z. et al. Nat. Commun. 14, 2734 (2023): https://doi.org/10.1038/s41467-023-38409-5
Xu, Z. et al. Small Methods 8, e2301801 (2024): https://doi.org/10.1002/smtd.202301801
Extended data
Extended Data Fig. 1 Characterization of isolated FFPE RNA.
Example of an RNA eltropherogram of the RNA sample isolated from an FFPE glioma sample. The blue line represents the fluorescence intensity (relative fluorescence units, RFU) against fragment length (nt), with the x-axis showing fragment length and the y-axis representing fluorescence intensity. The gray bar beneath the electropherogram corresponds to fragment size distribution, with darker shading indicating higher concentrations of RNA fragments in that size range. The 18S and 28S ribosomal RNA associated areas are marked in red and green, respectively. The DV200 metric, calculated as the percentage of RNA fragments >200 nucleotides, is 77.0%, indicating that this sample is suitable for the next steps of this protocol, with expected cDNA library and sequencing data meeting quality requirements. k, thousand.
Extended Data Fig. 2 Assessment of single-nuclei suspension and droplet encapsulation.
a and b, Microscopic image of DAPI-stained single-nuclei suspension: a typical example of well-isolated single nuclei (a), and an example showing a higher proportion of fragmented nuclei (b). Scale bar: 100 μm. c, Microscopic image of DAPI-stained single-nuclei suspension after in situ reactions. Scale bar: 100 μm. The image shows monodispersed nuclei ready for droplet generation. d, Microscopic image of encapsulated droplets, each containing a single nucleus and a poly(T) barcoded bead. The black arrow indicates the bead. Scale bar: 100 μm.
Extended Data Fig. 3 Assessment of cDNA and library quality.
a, Example of a qPCR amplification curve showing the expected cycle threshold for high-quality extension products (11 cycles, indicated by the flat line crossing the fluorescence intensity curve). b, cDNA fragment size distribution analysis using a Qsep100 bio-fragment analyzer. The overall size distribution should range between 100 and 1,000 bp, with minimal content in the 100–200-bp range. c, Library fragment size distribution analysis using a Qsep100 bio-fragment analyzer. The analysis should show a single dominant peak with fragments predominantly between 300 and 800 bp. LM, lower marker (20 bp); UM, upper marker (1,000 bp).
Extended Data Fig. 4 Sequencing analysis results.
a, The table summarizes key mapping metrics, including raw reads (M), reads to align (M), uniquely mapped reads (%), multiply mapped reads (%), unmapped reads (%), mapped versus unmapped reads (%) and reads for clustering and UMAP visualization (%). The mapped reads is calculated as the sum of uniquely mapped reads and multiply mapped reads. The reads for clustering and UMAP visualization (%) represent the proportion of raw reads that were used for clustering and UMAP visualization. b and c, UMI-barcode plot (b) and gene-barcode plot (c) for the FFPE glioma sample. The x-axis represents the barcodes, ranked by count, and the y-axis represents the UMI/gene counts on a logarithmic scale. The dashed blue vertical line indicates the steep drop-off point and the threshold of cell barcode. d, The histogram illustrates the distribution of RNA molecules (UMI counts) detected per nucleus in the FFPE glioma sample. The red vertical line represents the threshold, calculated as the mean UMI counts per nucleus plus 2 s.d. e, The chart displays the percentage of reads mapped to various RNA types, including rRNA, coding RNA, lncRNA, introns, intergenic regions and antisense RNA. f, The table provides an overview of sequencing quality and transcriptomic diversity, including metrics such as Q30 Bases in RNA read, Sequencing saturation, estimated number of cells, UMIs in cells, median UMI per cell, median GeneFull per cell, total GeneFull detected and median filtered UMIs per cell for mRNA, lncRNA and rRNA. g, UMAP projection of snRandom-seq data from the FFPE glioma sample. The UMAP plot shows predicted cell types based on reference datasets or marker gene analysis, with each color corresponding to a specific cell type.
Supplementary information
Supplementary Information
Supplementary Fig. 1, Supplementary Tables 1–5
Supplementary Video 1
Video presenting the procedure for sample processing and nuclei isolation
Supplementary Video 2
Video presenting the procedure for microfluidic droplet barcoding
Supplementary Data 1
Computer-aided designss of bead generation and single-nucleus barcoding microfluidic chips
Source data
Source Data Extended Data Fig. 4
Statistical source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, Z., Lyu, Y., Chen, H. et al. Single-nucleus total RNA sequencing of formalin-fixed paraffin-embedded samples using snRandom-seq. Nat Protoc 20, 2771–2802 (2025). https://doi.org/10.1038/s41596-025-01170-8
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41596-025-01170-8
This article is cited by
-
Decoding osteosarcoma from heterogeneity to precision therapy
Discover Oncology (2025)