Abstract
The application of three-dimensional (3D) cell cultures such as spheroids and organoids is growing in popularity both in academia and industry. However, morphology of the 3D architecture remains remarkably understudied. Here, we introduce an open-access Spheroid Light Microscopy Image Atlas (SLiMIA) that can serve as a training set for morphology studies of 3D cell cultures. We provide images with a variety of metadata: 9 microscopes, 47 cell lines, 8 culture media, 4 spheroid formation methods and multiple cell seeding densities; totalling approximately 8,000 images of spheroids. This comprehensive dataset can guide spheroid researchers and promote economizing of resources by advancing 3D cell culture optimization, standardization and implementation by the community at large. Considering the exponentially growing interest in spheroid morphometrical analyses and the emerging technological possibilities to do so, this atlas can be applied to train and develop image segmentation models to deepen our understanding of 3D spheroid morphometry in biomedical research.
Similar content being viewed by others
Background & Summary
When adherent cells are suspended into single cells and exposed to an environment to which they cannot attach, they tend to self-assemble into three-dimensional (3D) multicellular aggregates or spheroids. Compared to two-dimensional (2D) cell cultures, cells in spheroids experience in vivo-like intercellular interactions such as cell crowding, and gradients of oxygen, carbon dioxide, nutrients and waste products1. All these phenomena result in a phenotype that better resembles in vivo cells2,3. Similar to 2D cell cultures, 3D multicellular aggregates show a cell line specific morphometry. Single cell suspensions from different cell lines, seeded at equal numbers in ultra-low attachment (ULA) plates give rise to spheroids that vary in size, circularity, compactness and other morphometrical parameters. To demonstrate this, we performed a principal component analysis (PCA) of frequently used particle morphometrics measured on light microscopy images from spheroids (>1600) of 11 cell cultures used in the MISpheroID study. The analysis shows distinct clustering from large and compact HCT116 spheroids to small and loose SAR121 spheroids (Fig. 1).
Diversity of spheroid morphology based on cell type. (A) Scatter plot of the first two principal components shows the diversity in spheroid morphology between cell types. Each dot represents a spheroid. (B) Loading plot of PCA shows underlying relationship between AnaSP spheroid morphology metrics and how the metrics steer the direction of the principal components. (C) PCA scatter plot of the cell types morphology metric averages, supported by illustrative spheroid images. Visualized cell types (9 out of 11) are indicated on the spheroid image. Scale bar = 200 µm, representative for all images. Note: 1 A describes all data, 1 C averages; as a consequence, scales and PC values differ slightly.
Light microscopy is the most accessible way to visualize and assess spheroid morphometry. More than half (54.7%) of all reported (3,058) experiments in the MISpheroID knowledgebase use light microscopy images to analyze spheroid characteristics4. Note that light microscopy images are 2D projected images of the 3D object which may fail to capture the full complexity of spheroids. The widespread use of light microscopy imaging stimulated us to create SLiMIA: the Spheroid Light Microscopy Image Atlas. SLiMIA provides the largest open-access 3D cell culture image database with 7,990 images of spheroids from 47 cancer and non-cancer cell lines including methodological metadata such as cell source, cell seeding densities, culture media and spheroid formation methods (Fig. 2, Table 1), allowing researchers to search the dataset as needed. The dataset can be accessed, viewed and downloaded through Figshare5. Furthermore, multiple initiatives show increasing attention in morphological cell studies6,7,8. Accordingly, SLiMIA can be applied to compare spheroid diversity and to develop morphometric analysis software with potential applications on other 3D cultures such as organoids and patient-derived tissue fragments.
Cell lines represented in SLiMIA. A snapshot of spheroid images of 47 cancer and non-cancer cell lines available in SLiMIA. It is important to note that cell seeding densities, spheroid formation methods, culture media and timepoint of imaging are variable between presented spheroid images. White scalebar = 200 µm, red scalebar = 100 µm.
High-quality image datasets with a wide diversity in spheroid morphotypes are required to develop and optimize performant 3D cell aggregate segmentation platforms and convolutional networks. Reusing existing datasets could form an opportunity to economize both time and resources. Moreover, according to recent developments and guideline proposals, the reuse of light microscopy data will receive a strong boost from the bioimaging community9,10, consistent with the FAIR (Findable, Accessible, Interoperable, Reusable) principles11.
Lastly, further research is needed to translate spheroid morphotypes (e.g. circularity/sphericity, compactness, and size) into methodological metadata such as cell source and cell seeding density. In previous work we have shown that spheroid size impacts biological metadata (e.g. cell death, ATP production, cytokine release)4 and studies are needed to investigate which spheroid morphotype parameters influence spheroid biology. Future atlases may focus on images from drug-treated spheroids that are disintegrated or are obscured by a rim of cell debris12. Z-projected images of spheroids allow investigation of the 3D geometry of spheroids and may even further differentiate in distinguishing cell source and spheroid biology7,13. In conclusion, SLiMIA is a dataset comprising approximately 8,000 images and has an enormous potential to assist in the development, training and validation of spheroid segmentation models and to connect spheroid morphometry with methodological metadata.
Methods
Cell origin and culture
For uniformity and clarity, cell line names are written in their simplest and most economic notation, with respect to the synonyms registered in the Cellosaurus database14.
The following cell lines: 4T1 (CRL-2539), A549 (CCL-185), BEAS2B (CRL-3588), BT20 (HTB-19), DLD1 (CCL-221), FaDu (HTB-43), H1299 (CRL-5803), HCT116 (CCL-247), HCT15 (CCL-225), HEK293 (CRL-1573), HEPG2 (HB-8065), Hs578T (HTB-126), MCF7 (HTB-22), MCF10A (CRL-10317), MDAMB231 (HTB-26), MDAMB361 (HTB-27), MRC5 (CCL-171), NCIH226 (CRL-5826), NCIH460 (HTB-177), PANC1 (CRL-1469), SKOV3 (HTB-77), SW837 (CCL-235), SW1353 (HTB-94), T47D (HTB-133), U87MG (HTB-14) and VcaP (CRL-2876) were purchased from the American Type Culture Collection (ATCC, catalog number between brackets). ASC (adipose-derived stem cells) (SCC038) was purchased from Sigma Aldrich (Germany), CAL33 (ACC447) was purchased from DSMZ (Germany), CT5.3 hTERT are hTERT-immortalized cancer-associated fibroblasts of which primary culture was originally isolated from colorectal carcinoma15,16,17. D2A1LUCmCherry was a gift from C. Isacke (London, England)18, HCA2hTERT are hTERT-immortalized fibroblast of which primary culture was originally isolated from human foreskin (a gift from C. Jones; Cardiff, UK)19, HCC2998, HOP62 and OVCAR8 were obtained from National Cancer Institute (NCI, Frederick, USA). HeLa (940003-P-T) was purchased from Tebu-Bio (Paris, France), HSC4 (JCRB0624) and SAS (JCRB0260) was purchased from Japanese Collection of Research Bioresources (JCRB, Japan). HuH7 (300156), MDCK (602280), U138MG (300363) and U251MG (300385) were purchased from Cell Line Service (CLS) GmbH (Germany). SAR030 and SAR121 are early passage soft tissue sarcoma cell cultures as previously described4,20.
For clarity, 2D cell culture conditions prior to spheroid formation are summarized in Table 2.
All human cell lines were authenticated via STR profiling (ATCC, United States; DSMZ, Germany; Eurofins, Luxembourg; Genomic Scientific Platforms, i3S, Portugal; Institute of Legal Medicine, TU Dresden, Germany) and routinely tested to exclude Mycoplasma contamination.
Spheroid formation
-
a.
Liquid overlay: Ultra-Low Attachment (ULA) plate
4T1, A549, BEAS2B, CT5.3 hTERT, D2A1LUCmCherry, HCA2hTERT, HCT116, HEPG2, MCF7, PANC1, SAR030, SAR121, SKOV3, SW1353 and U87MG
The U-shaped, 96-well (CLS7007-24EA, Sigma-Aldrich, Saint Louis MO, USA) and 384-well (MS-9384UZ, S-bio, Hudson NH, USA) ultra-low attachment (ULA) plates were seeded in a suspension of respectively 200 µl or 80 µl cell culture media with different seeding densities (Table 1). Culture media were DMEM high glucose (HG) (41965039, ThermoFisher), DMEM/F12 (1:1) (41965039 and 21765029, ThermoFisher), RPMI1640 (21875091, ThermoFisher), DMEM low glucose (LG) (31885023, ThermoFisher), EMEM (10-009-CV, Corning) and MEM (10370-047, ThermoFisher), all supplemented with 10% FBS, 100IU/ml penicillin and 100 mg/ml streptomycin (P/S). The ULA plates were sealed with Breathe-Easy semipermeable tape (BEM-1, Dedham MA, USA) to prevent evaporation. The spheroids were cultured at 37 °C in an atmosphere of 5% CO2 under normoxia.
MCF10A: BME, Matrigel
To culture MCF10A mammary 3D spheroids, MCF10A cells were seeded in 384-well U-shaped ULA (#MS-9384UZ, S-bio, Hudson, NH, USA) plate. We have previously reported the use of 384-well culture platforms to form MCF10A spheroids and refer to the culture protocol21,22,23. Briefly, MCF10A cells were cultured in growth media containing DMEM/F12 (11330-032, Gibco) and supplemented with 5% horse serum (16050-122, Gibco), 20 ng/ml HB-EGF (100-47, Peprotech), 0.5 µg/ml hydrocortisone (H0888, Sigma), 100 ng/ml Cholera toxin (C8052, Sigma), and 10 µg/ml insulin (I1882, Sigma). 2D cultures were maintained at 37 °C and 5% CO2 in T75 culture flasks, supplemented with 1% P/S (15140-163, Gibco), and routinely passaged at 70–80% confluence. To culture 3D spheroid formation in 384-well ULA plate 3 × 103 MCF10A cells were seeded in each well in a final volume of 25 μl22. The cells were supplemented with 0.24% methocel A4M (94378, Sigma), 10% FBS (900-108, GemiBio), 120 µg/ml MatrigelTM (256231, Corning) or 100 µg/ml Cultrex UltiMatrix Basement Membrane Extract (BME001-01, R&D system). On day 3 of spheroid culture, the media was exchanged 3 times to wash out the seeding supplements using a CyBio FeliX liquid handling machine (Analytik Jena). For routine culture, media was exchanged 2 times every 2-3 days. While the spheroids were maintained in ULA culture, they were imaged using an Incucyte S3 (Sartorious) in-incubator microscope system.
A549-MRC5, H1299, MRC5, VcaP
U-bottom 96-well (CLS7007-24EA, Sigma-Aldrich, Saint Louis MO, USA) and 384-well (MS-9384UZ, S-bio, Hudson NH, USA) ULA plates were seeded with a suspension of 80 µl cell culture media with 2 × 10³ (H1299, VCAP cells) and 10 × 103 (MRC5 cells). About the heterotypic multicellular spheroids composed by A549 and MRC5 cells, a mixed suspension of 80 µl cell culture media with both cell lines in a 1:1 ratio was used (i.e. 2 × 103 A549:2 × 103 MRC5). Culture media were: RPMI (ECB9006, Euroclone) supplemented with 1% L-glutamine, 0.1% Insulin for H1299 cells; EMEM (30–2003, ATCC) for MRC5 and A549-MRC5 spheroids and DMEM high glucose (41965039, ThermoFisher) for VCAP. All culture media were supplemented with 10% FBS and without antibiotic/antimycotic. The ULA plates were sealed with Breathe-Easy semipermeable tape (BEM-1, Dedham MA, USA) to prevent evaporation. The spheroids were cultured at 37 °C in an atmosphere of 5% CO2 under normoxia.
-
b.
Liquid overlay: agarose-coated plate
BT20, BT474, CAL33, DLD1, FaDu, HCC2998, HCT15, HEK293, HOP62, Hs578T, HSC4, MDAMB361, NCIH226, NCIH460, OVCAR8, PaTu8902, SAS, U138MG, U251MG
Spheroids from these cells lines derive from spheroid experiments using the liquid-overlay approach in flat-bottom 96-well plates coated with 50 µl/well of 1.5% agarose in serum-free medium (A8539-100G, Sigma Aldrich) as described in Friedrich et al.24, with all materials and distributors as detailed. In brief, all epithelial cancer cell types were routinely grown in DMEM with physiological (low) glucose concentration (LG), with or without 25 mM HEPES (P04-05551 and P04-01550 respectively, Pan Biotech), supplemented with 10% heat-inactivated FBS, 100 IU/ml penicillin and 100 mg/ml streptomycin (1% P/S) (Pan Biotech). U138-MG and U251 glioblastoma cell lines and spheroids were cultured in MEM Eagle with EBSS (P04-08250, Pan Biotech), supplemented with 10% FBS and antibiotics (1% P/S). Spheroids were formed by seeding single cell suspensions on the agarose-coated wells and were kept at 37 °C, normoxia, 5% or 8% CO2, as highlighted in Tables 1, 2.
Hela, HuH7, LNCap, MDCK
Spheroid formation was carried out using a modified version of the liquid overlay technique25. 10 µl of a heated 1.5% w/v agarose (in DMEM (11880036, ThermoFischer), without FBS) solution was dispended by liquid dispensers (Multidrop Combi, Thermo Scientific) into sterile 384-well clear bottom imaging plates (6057308, Perkin Elmer) and cooled down at room temperature. Next, single cell suspensions were seeded in 40 µl RPMI1640 (21875-034, Gibco) containing 10% FBS supplemented with 1% P/S using a liquid dispenser. The plates were then incubated under standard cell culture conditions at 37 °C and 5% CO2 in humidified incubators.
-
c.
Liquid overlay: Microchip
4T1LUC, ASC, MDAMB231
Agarose microchips with 400 µm diameter pores were prepared as follows: 3 g UltraPure Agarose (16500-500, Invitrogen) was dissolved in 100 ml sterile PBS- by heating, resulting in a 3% w/v agarose solution. This solution was poured over a negative polydimethylsiloxane (PDMS) mold (height 3 mm, diameter 18 mm) which contains 1585 (400 µm) pores. Once solidified, the agarose microchips were separated from their molds and placed in a 12-well plate. Next, 500 µl of cell suspension was seeded onto the 400 µm pore microchip.
SW837
Agarose microchips with 800 µm diameter pores were prepared as follows: 2 g SeaKem® LE Agarose (50004, Lonza) was dissolved in 100 ml sterile 0.9% NaCl by heating, resulting in a 2% w/v agarose solution. This solution was poured over a MicroTissues® 3D Petri Dish micro-mold (Z764000-6EA, Merck) which contains 81 pores. Once solidified, the agarose microchips were separated from their molds and placed in a 12-well plate. Next, 190 µl of cell suspension SW837 was seeded onto the microchip.
-
d.
Hanging drop
In the hanging drop method, 2 × 10³ cells were plated under the lids of petri dishes (A19618, Novolab, Geraardsbergen, Belgium) in 20 µl drops of DMEM HG (41965039, ThermoFisher) or DMEM LG (31885023, ThermoFisher). The bottom of the petri dishes was filled with 10 ml PBSD− to limit evaporation.
Light microscope
Each applied microscopic set-up (microscope, objectives, camera system and acquisition software) is summarized in Table 3.
Image processing and statistics
AnaSP (v1.4) has been applied to segment the spheroid images and extract morphological parameters26. Principal component analysis (PCA) was performed in Graphpad Prism (v9.3.1) with 11 cell lines which are representative for the various morphological types of spheroids and of which sufficient biological (≥3) and technical (≥6) were obtained. These spheroids were all cultured in ULA plates (cfr. supra).
Manual segmentation
Each light microscopy image underwent manual segmentation. In AnaSP (v1.4)26 the authors and contributors manually segmented the spheroid images, distinguishing foreground (spheroid) from background. For the manual segmentation the spheroid images are enlarged to optimally distinguish foreground from background. This segmentation results in a binary (B/W) ome.tiff image with the identical name as the original raw image. These manual segmentation images can be thus considered as ground truth images.
Image conversion
To retain a maximum amount of metadata, to facilitate handling of multidimensional pixel information (e.g. for annotation and deep learning workflows) and to create uniformity the microscopic images were converted from a .jpg- or .tiff- to an ome.tiff-format by use of bftools (Bio-Formats, v5.6.0).
Data Records
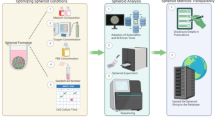
SLiMIA is accessible at Figshare5. Figure 3 demonstrates that SLiMIA consists of 3 levels. In the first level, the atlas is subdivided in 9 folders named by microscope (e.g. Axiovert 200, Incucyte S3) where each microscope folder contains a folder with images and a mirroring folder with the corresponding manual segmentations. In the second level, folders are named by the cell line of the spheroids (e.g. BT20, BT474, DLD1). In the third level, folders are described by the cell seeding density applied to form spheroids (e.g. 800, 1000, 2000). Except for Incucyte S3 where T1-T7 represent technical replicates of spheroids followed over time. The last level holds the spheroid images. To provide as much metadata as possible per image, each image file is named as follows:
Structure of SLiMIA. SLiMIA is subdivided into 9 microscopes where each microscope folder contains a folder with images and a mirroring folder with the corresponding manual segmentations. In the second level each microscope folder is subdivided in cell line folders. One level lower, folders are named by the cell seeding density per well/drop. Except for Incucyte S3 where T1-T7 represent technical replicates of spheroids followed over time (0–374 h).
“CELLLINENAME_CULTUREMEDIUM_FormationMethod_CellSeedingDensity_TimepointOfImaging_BiologicalReplicateNumber_TechnicalReplicateNumber_Magnification.ome.tiff”
For example:
D2A1LUCmCherry_DMEMLG_HangingDrop_2000cells_72h_B1_T5_10x.ome.tiff
The images contain raw data only. No processing has occurred apart from image conversion to grayscale and to ome.tiff.
Technical Validation
Two independent observers (AP, SK) visually verified the quality of the images with special attention for focus, inclusion of all spheroid borders, absence of non-cellular (e.g. dust) particles which could interfere with spheroid structure. Images that did not qualify were removed from the dataset. Brightness and contrast were evaluated on a second level since these parameters are easily adjustable through most image software packages.
Code availability
No custom code was utilized in this work. Some versions of ImageJ need the Bio-Formats plugin to open ome.tiff files (https://www.openmicroscopy.org/bio-formats/downloads/).
References
Hirschhaeuser, F. et al. Multicellular tumor spheroids: an underestimated tool is catching up again. J. Biotechnol. 148, 3–15 (2010).
Han, K. et al. CRISPR screens in cancer spheroids identify 3D growth-specific vulnerabilities. Nature 580, 136–141 (2020).
Folkesson, E. et al. High-throughput screening reveals higher synergistic effect of MEK inhibitor combinations in colon cancer spheroids. Sci. Rep. 10, 11574 (2020).
Peirsman, A. et al. MISpheroID: a knowledgebase and transparency tool for minimum information in spheroid identity. Nat. Methods 18, 1294–1303 (2021).
Blondeel, E. et al. SLiMIA: the Spheroid Light Microscopy Image Atlas. Figshare https://doi.org/10.6084/m9.figshare.c.7486311 (2024).
You, L. et al. Linking the genotypes and phenotypes of cancer cells in heterogenous populations via real-time optical tagging and image analysis. Nat. Biomed. Eng. 6, 667–675 (2022).
Mousavikhamene, Z., Sykora, D. J., Mrksich, M. & Bagheri, N. Morphological features of single cells enable accurate automated classification of cancer from non-cancer cell lines. Sci. Rep. 11, 24375 (2021).
Falk, T. et al. U-net: deep learning for cell counting, detection, and morphometry. Nat. Methods 15, 67–70 (2019).
Hammer, M. et al. Towards community-driven metadata standards for light microscopy: tiered specifications extending the OME model. Nat. Methods 18, 1427–1440 (2021).
Boehm, U. et al. QUAREP-LiMi: a community endeavor to advance quality assessment and reproducibility in light microscopy. Nat. Methods 18, 1423–1426 (2021).
Wilkinson, M. D. et al. The FAIR guiding principles for scientific data management and stewardship. Sci. Data 3, 160018 (2016).
Streller, M. et al. Image segmentation of treated and untreated tumor spheroids by fully convolutional networks. Preprint at https://arxiv.org/pdf/2405.01105 (2024).
Li, S. et al. Simultaneous 2D and 3D cell culture array for multicellular geometry, drug discovery and tumor microenvironment reconstruction. Biofabrication 13 (2021).
Bairoch, A. The cellosaurus, a cell-line knowledge resource. J. Biomol. Tech. 29, 25–38 (2018).
De Boeck, A. et al. Differential secretome analysis of cancer-associated fibroblasts and bone marrow-derived precursors to identify microenvironmental regulators of colon cancer progression. Proteomics 13, 379–388 (2013).
De Wever, O. et al. Tenascin-C and SF/HGF produced by myofibroblasts in vitro provide convergent proinvasive signals to human colon cancer cells through RhoA and Rac. FASEB J. 9, 1016–1018 (2004).
De Vlieghere, E. et al. Tumor-environment biomimetics delay peritoneal metastasis formation by deceiving and redirecting disseminated cancer cells. Biomaterials 54, 148–157 (2015).
Jungwirth, U. et al. Generation and characterisation of two D2A1 mammary cancer sublines to model spontaneous and experimental metastasis in a syngeneic BALB/c host. Dis. Model Mech. 11 (2018).
Wyllie, F. et al. Telomerase prevents the accelerated cell ageing of Werner syndrome fibroblasts. Nat. Genet. 24, 16–17 (2000).
Fischer, S. et al. Post‐operative minimal residual disease models to study metastatic relapse in soft‐tissue sarcoma patient‐derived xenografts. Clin. Transl. Med. 13, e1290 (2023).
Parigoris, E. et al. Cancer cell invasion of mammary organoids with basal-in phenotype. Adv. Healthc. Mater. 10, 2000810 (2021).
Lee, S. et al. High-throughput formation and image-based analysis of basal-in mammary organoids in 384-well plates. Sci. Rep. 12, 317 (2022).
Djomehri, S. I., Burman, B., Gonzalez, M. E., Takayama, S. & Kleer, C. G. A reproducible scaffold-free 3D organoid model to study neoplastic progression in breast cancer. J. Cell Commun. Signal. 13, 129–143 (2019).
Friedrich, J., Seidel, C., Ebner, R. & Kunz-Schughart, L. A. Spheroid-based drug screen: considerations and practical approach. Nat. Protoc. 4, 309–324 (2009).
Wenzel, C. et al. 3D high-content screening for the identification of compounds that target cells in dormant tumor spheroid regions. Exp. Cell Res. 323, 131–143 (2014).
Piccinini, F. AnaSP: A software suite for automatic image analysis of multicellular spheroids. Comput. Methods Programs Biomed. 119, 43–52 (2015).
Acknowledgements
Thanks to Marit Wondrak for the technical assistance in spheroid culturing. Anouk Vanderstricht, Taylor N. Brinsfield, Mariachiara Stellato, Noah Borges, Carolien Sels, Oscar Lemmens and Sabina Shamieva helped in the manual annotation of the atlas. ODW acknowledges support by the Concerted Research Actions from Ghent University, Stichting Tegen Kanker (Foundation against Cancer), Kom Op Tegen Kanker (Stand up to Cancer), the Flemish cancer society, and Fund for Scientific Research Flanders. FP and GC acknowledge support from the MAECI Science and Technology Cooperation Italy-South Korea Grant Years 2023–2025 by the Italian Ministry of Foreign Affairs and International Cooperation (CUP project: J53C23000300003).
Author information
Authors and Affiliations
Contributions
A.P. and O.D.W. designed the study. O.D.W., A.T., S.T., P.S., L.K.S. and M.J.O. supervised experiments. E.B., A.P., F.D.V., S.A.J., M.B., T.C., D.E., S.L., N.M. and N.Z. collected data. F.P. and G.C. designed software. S.K., S.T., S.L., N.M., M.B., E.B., F.P., G.C., F.D.V., A.P., D.E., T.C., M.J.O. and S.V. manually annotated images. A.P., S.V. and S.D. processed the data. A.P., E.B., S.V., F.P., P.B., G.C., T.C., L.D.M., M.J.O., L.K.S., P.S., S.T., A.T. and O.D.W. edited the manuscript. E.B., A.P. and S.V. contributed equally.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Blondeel, E., Peirsman, A., Vermeulen, S. et al. The Spheroid Light Microscopy Image Atlas for morphometrical analysis of three-dimensional cell cultures. Sci Data 12, 283 (2025). https://doi.org/10.1038/s41597-025-04441-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41597-025-04441-x