Abstract
Pseudomonas lipase is a well-studied lipase. However, few studies have been conducted to examine the mechanisms underlying the regulation of the lipase expression. Hfq is a global regulatory protein that, among others, controls the expression of multiple genes, regulate bacterial peristalsis, and participates in the regulation of quorum-sensing (QS) system. In this study, the effects of Hfq on lipase expression were investigated by knocking out the hfq and rsmY genes or overexpressing of hfq and rsmY genes. We found that Hfq regulates the expression of lipA at both transcriptional and translational levels. The translational level was the main regulatory level of lipA. Hfq also regulates the expression and stability of rsmY. Additionally, using hfq/rsmY double gene knock-out, we showed that Hfq can directly bind to the rsmY to regulate lipA activity. In conclusion, our results indicate that Hfq regulates the expression of rsmY mainly at the translational level to influence the expression of lipA in Pseudomonas protegens Pf-5.
Similar content being viewed by others
Introduction
Lipase, which is present in a variety of animals, plants, and microorganisms, is an important industrial enzyme1, 2. However commercial lipase is derived mainly from microorganisms. Currently, lipase is used in the food, beverage, oil, detergent, cosmetics, paper, pollution control, and bioenergy industries. However, conventional culture and optimization of fermentation conditions have not been able to overcome problems related to inadequate production of bacterial lipase. Therefore, it is necessary to elucidate the molecular mechanisms underlying the regulation of expression of genes involved in lipase production in order to resolve these issues.
Previous studies have shown that the expression of bacterial lipase is regulated by a two-component regulatory system3, 4. For instance, in Pseudomonas alcaligenes, LipQ-LipR (lipQ/R) directly regulates lipase expression. In Pseudomonas aeruginosa, CbrA-CbrB (cbrA/B) is the two-component regulator of lipase expression. Moreover, las, rhl, and other two-component regulatory systems regulate the expression of the lipase quorum-sensing (QS) system. Our previous study showed that in Pseudomonas protegens Pf-5, two-component regulatory system GacS-GacA mediates lipA expression via rsmE rather than rsmA 5. However, it remains unclear whether other lipase-regulatory genes exist in P. protegens Pf-5.
Hfq was first found in Escherichia coli as an RNA-binding protein capable of affecting a series of phenotypes of bacteria, such as growth, virulence-factor expression, and resistance6. Further studies showed that the small regulatory RNA (sRNA) rsmY is directly bound to hfq in P. aeruginosa PAO1 to maintain the stability of rsmY, thereby enhancing its regulation of the expression of a series of genes7, 8. In other Pseudomonas sp., hfq also binds to the sRNA PhrS, to stimulate the expression of resistance genes. It has also been established that hfq binds to the 5′-noncoding region of pltR, pltT, and other genes to alter their expressions9. These findings identify hfq as a global regulatory factor capable of affecting various physiological processes essential to bacterial survival.
However, in P. protegens Pf-5, the function of hfq has not been studied, and it remains unclear whether it regulates lipase gene expression. In the present study, evidence is provided showing that hfq knockout significantly reduces lipase production, and that hfq directly interacts with rsmY to control the expression of lipase in P. protegens Pf-5.
Results
Hfq regulates the expression of lipA at the translational level
In P. protegens Pf-5, there is no classical QS regulatory system. In order to further understand the relationship between Hfq and lipA, we conducted hfq gene knockout (Fig. 1A). The effect of hfq on lipase expression was analyzed by measuring the activity of β-galactosidase and whole cell lipase. Figure 1B shows that growth of Pf-5 was decreased by the knockout of hfq (Fig. 1B). Furthermore, after the hfq knockdown, the relative activity of lipA and the mRNA expression of lipA were significantly decreased (Fig. 1C and D).
Effects of hfq mutation on lipA expression. (A) PCR confirmed hqf knockout. Lane 1: DNA markers. Lane 2: negative control (−). Lane 3, 4, 5, 6: mutant strains (PfΔhfq). Lane 7: Recombinant failed strains (B) Growth curve of Pseudomonas sp. Overnight bacterial cultures were performed in 50 mL LB. The initial OD value was adjusted to ~0.1. Bacterial cultures were shaken at 200 rpm at 28 °C, and OD value were determined once every 2 h. (C) qRT-PCR of relative lipA expression in P. protegens Pf-5 wild-type and the hfq mutant, and lipA mRNA levels were measured when bacterial growth reached the stationary phase. (D) Relative activity of whole-cell lipase in P. protegens Pf-5 wild-type and the hfq mutant. Whole-cell lipase activity was measured following bacterial culture in 50 mL LB to the stationary phase. (E) β-galactosidase activity in P. protegens Pf-5 wild-type and the hfq mutant. Bacteria were incubated in 50 mL LB to the stationary phase, and the enzyme activity of β-galactosidase was determined. Experiments were completed in triplicate. *P < 0.05, **P < 0.01 compared with the control group.
In the wild-type P. protegens Pf-5, when hfq was reconstituted with hfq, relative lipase activity was restored in the hfq mutant, indicating that hfq knockout affected lipA expression at the promoter level and hence lipase activity. Moreover, measurement of lipase and β-galactosidase activities following hfq overexpression in hfq complementation-mutant strains revealed restoration of the activities of β-galactosidase and lipase to wild-type levels (Fig. 2A and B). These results suggest hfq involvement in regulating lipA expression and lipase activity through its influence on lipA at the transcription and translation levels.
Effects of hfq overexpression on lipA expression. (A) Relative activity of whole-cell lipase in P. protegens Pf-5 wild-type and the hfq mutant. Whole-cell lipase activity was measured after bacterial culture in 50 mL LB growth reached the stationary phase. (B) Influence of hfq overexpression on the expression of the chromosome-borne lipA′-′lacZ construct in different strains. Bacteria were cultured in 50 mL LB to the stationary phase, and β-galactosidase activity was determined. Pf-5F3/pBBRKm: P. protegens Pf-5 wild-type with pBBRKm; Pf-5F3/pBBRK-hfq: P. protegens Pf-5 wild-type overexpressing hfq; PfΔhfqF3/pBBRK: hfq mutant with pBBRKm; and PfΔhfqF3/pBBRK-hfq: PfΔhfq complementary hfq mutant. Experiments were completed in triplicate. *P < 0.05, **P < 0.01 compared with the control group.
Influence of Hfq on rsmY expression
According to the literature, hfq can be directly associated with phrs, rsmY, and previous studies from our laboratory showed that in Pf-5, rsmE rather than rsmA can directly binds to lipA and affect the expression of lipA. Therefore, we determined the activity of phrs, rsmA, rsmE, rsmY, rsmZ, respectively, to see which genes were affected by Hfq (Fig. 3). The results show that the activity of phrs, rsmA, rsmE did not change after hfq knockout. However, the activity of rsmZ had mild changes, and the activity of rsmY changed robustly after hfq knockout. These results indicate that hfq may affect the expression of lipA via rsmY or rsmZ.
Effects of hfq on the activity of regulatory RNAs. (A) phrS, (B) rsmA, (C) rsmE, (D) rsmY, and (E) rsmZ. (F) qRT-PCR of relative expression of rsmX, rsmY, and rsmZ in P. protegens Pf-5 and the hfq mutant. The level of expression of rsmX, rsmY, and rsmZ mRNAs were measured after the bacterial growth reached stationary phase. Experiments were completed in triplicate. *P < 0.05, **P < 0.01 compared with the control group.
Sequence interaction of hfq and rsmY
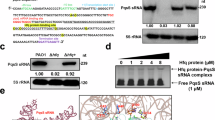
To further investigate the relationship between hfq and rsmY, the hfq protein was purified in order to determine whether Hfq binds to rsmY or not, using EMSA. The EMSA results (Fig. 4) show that hfq did not bind to the promoter sequences of rsmA, rsmE, rsmZ, but did bid to rsmY (Fig. 4A and B). Furthermore, competitive EMSA (Fig. 4C ) revealed that hfq concentrations and the amount of rsmY on the probe remained constant. Increasing the amount of free rsmY revealed direct association of rfq with rsmY. The different ratios of free rsmY to the rsmY bound to the probe were 1:1, 50:1, 100:1, and 150:1, in groups 1 through 4.
EMSA showing binding of hfq to the rsmY sequence. (A) Purified Hfq protein. (B) The 1-nM biotin-labeled DNA probe was incubated with purified Hfq protein in 20 μL binding buffer, and the Hfq-DNA complexes and free DNAs were cross-linked to the membrane by a 320-nm UV-light cross-linking instrument. Biotin-labeled bands were detected by chemiluminescent nucleic acid detection module. (C) Hfq protein directly bound to the rsmY sequence, but did not do so to rsmA, rsmE, and rsmZ sequences following increases in free rsmY. The different ratios of free rsmY to biotin-labeled rsmY used were 1:1, 50:1, 100:1, and 150:1 in groups 1 through 4.
Influence of Hfq on RsmY stability
It is reported that the binding of Hfq and rsmY can maintain the stability of rsmY. However, in Pf-5, Hfq has not been studied. So we examined whether the stability of rsmY is different after rifampicin treatment. The results show that the stability of rsmY in hfq mutant was significantly lower than that of wild type after rifampicin treatment (Fig. 5). These results showed that Hfq affects the expression of rsmY by regulating the stability of rsmY, which in turn affects the expression of lipA.
Influence of rsmY on lipA expression and lipase activity
After knocking out the rsmY gene (Fig. 6A), lipA expression was measured to verify the role of rsmY in regulating lipA expression. It was observed that lipA expression was reduced in cells where hfq was knocked out but the expression was considerably reduced in cells in which both hfq and rsmY knockout were effected (Fig. 6B and C). In addition, in the hfq mutant, re-incorporation of rsmY resulted in moderate recovery of lipA expression to near wild-type levels, and overexpression of rsmY resulted in lipA expression exceeding levels observed in wild-type strain and in the hfq mutant.
Effect of rsmY on hfq regulation of lipA expression. (A) Verification of rsmY knockout by PCR. Lane 1: DNA markers. Lane 2: wild type strain. Lane 3: mutant strains (PfΔrsmY). (B) Relative activity of whole-cell lipase activity following hfq and rsmY single or double knockout. Whole-cell lipase activity was measured following bacterial culture to the stationary phase. The relative lipase activity decreased to a greater extent following double knockout, than that observed in the single-gene knockout strain, indicating that hfq and rsmY both regulate lipase expression and activity, and that RsmY is capable of partially compensating for the effects of hfq knockout. (C) β-galactosidase activity following hfq and rsmY single or double knockout. β-galactosidase activity was determined following bacterial culture to the stationary phase. β-galactosidase activity was much lower in the double-knockout strain, and overexpression of rsmY in the hfq mutant enhanced β-galactosidase activity, indicating that the effects of hfq knockout on lipA expression was compensated for by rsmY expression. Experiments were completed in triplicate. *P < 0.05, **P < 0.01 compared with the control group.
Discussion
Many studies have shown that hfq is involved regulating the expression of multiple genes. It influences the activity of housekeeping genes containing Fe-S clusters, those controlling pyochelin and pyocin production. It also influences the activities of enzymes involved in degradation of aromatic compounds, alcohol dehydrogenase metabolism, and the ABC transport systems. Furthermore, hfq regulates QS-controlled genes, thereby regulating several virulence factors10. The expressions of global transcriptional regulators, like Fur 11, RpoS 12 and H-NS 13, are modulated by hfq in E. coli. However, the details regarding how these genes are regulated by hfq have remained unclear, and there are few reports regarding the regulation of lipA expression by hfq. The present study investigated the regulation lipA expression by hfq in P. protegens Pf-5 by knocking out the hfq gene. It was found that both lipA expression and lipase activity decreased significantly following hfq knockout. The hfq gene was also over-expressed in mutant PfΔhfq and wild-type P. protegens Pf-5. It was found that complementation of hfq restored lipA expression and lipase activity, and that hfq overexpression enhanced lipA expression and lipase activity in wild-type P. protegens Pf-5. Examination of the growth curve revealed that hfq knockout altered bacterial growth. These results evidently indicate that hfq regulates lipA expression and lipase activity in P. protegens Pf-5.
Electron microscope studies of E. coli hfq proteins14,15,16 and X-ray crystallography examination of the hfq protein from P. aureus 14 and P. aeruginosa 17, showed that 72 N-terminal amino acids are identical18. This finding led to the conclusion that the proteins belong to the Sm-like protein family which exhibit hexameric ring-shaped structures and recognize short U-rich stretches in primary RNA transcripts19. In eukaryotic cells, they are involved in RNA processing. In primary transcripts, a common hfq-binding motif exists, which is a stem-loop structure that precedes or follows an A/U-rich region20,21,22. Similar to P. Protegens CHAO, is GacS/GacA which regulates the expression of genes involved in secondary metabolism via rsmX, rsmZ, and rsmY in P. protegens Pf-55. Transcriptions of rsmZ and rsmY are directly regulated by PAO1 and GacA to upregulate the expression of hundreds of genes in P. aeruginosa 23. In P. aeruginosa, PAO1, hfq and rsmA competitively associate with the GGA-repeat site of rsmY to regulate its expression24, and in P. protegens CHAO, the GGA-repeat site of rsmY is also a key rsmA-binding site25. Interestingly, the nucleotide sequence of P. protegens Pf-5 rsmY is exactly the same as that of rsmY from P. protegens CHAO. Therefore, it is reasonable to speculate that hfq regulates rsmY expression by associating with its GGA site. However, further studies will be necessary in order to accept or reject this hypothesis.
For trans-acting hfq-binding sRNAs in enteric bacteria, their regulatory function is associated with the RNA chaperone hfq at the post-transcriptional level. It is fairly well understood that the activity sRNA cannot be accomplished without the functional roles of hfq. The primary role of hfq is mostly to act as negative effector of translation by facilitating base-pairing between target mRNAs and sRNAs. However, it also acts as a positive effector in some cases6, 26, 27. The role it adopts, and the mechanism by which it accomplished that role depend on the specific bacterial system. For example, the majority of genes identified in microarray studies conducted following hfq knockout were not regulated by hfq in P. aeruginosa or in E. coli 11. Another role of hfq is to destabilize sRNA–mRNA hybrids on the one hand, through recruitment of RNase E near target mRNAs28. On the other hand, hfq stabilizes sRNAs by protecting them from ribonucleases29, 30. For example, in P. aeruginosa, sRNAs PrrF1 and PrrF2 play destabilizing roles on target mRNAs. Given that hfq can modulate Fur expression11 and that Fur controls prrF1 and prrF2 transcription, it follows that hfq affects the target genes of sRNAs indirectly31. The present study has shown that in P. protegens Pf-5, hfq not only regulates rsmY expression, but also destabilizes rsmY. However, the specific mechanism underlying this phenomenon remains unclear and warrants further investigations.
In a previous study, it was shown that rsmE rather than rsmA directly binds to the lipA promoter region to activate lipA transcription5. Therefore, further research is needed to investigate the interactions between the promoter region of lipA and rsmX, rsmY, rsmZ, rsmA, and rsmE, so as to elucidate the mechanisms underlying lipA expression via rsmY. The results obtained in the present study indicate that lipA expression in P. protegens Pf-5 is regulated at the transcriptional level by hfq through a mechanism involving direct binding of hfq to rsmY.
Materials and Methods
Bacteria, plasmids, and culture conditions
The bacteria and plasmids used in this study are listed in Table 1. E. coli was cultured by incubation at 37 °C. At the same time, P. protegens was cultured at 28 °C in lysogeny broth (LB; solid medium plus 1.5% agar). The antibiotics and concentrations used for P. protegens and E. coli culture were as follows: 40 μg/mL of kanamycin, 50 μg/mL of gentamicin, and 100 μg/mL of ampicillin. The concentration of sucrose used was 10% (w/v) when gene knockout was performed using the suicide plasmid pJQ200SK. Other components were isopropyl-β-D-thiogalactopyranoside (IPTG, 0.5 mM), ortho-nitrophenyl-β-D-galactopyranoside (4 mg/mL) and Taqaq (TaKaRa, Shiga, Japan). DNA ligase, plasmid preparation, restriction endonucleases, RNA reverse transcriptase, DNA gel extraction, and KOD Plus DNA polymerase (TaKaRa) were performed based on manufacturer’s protocol described in the commercial kits (Omega Bio-Tek, Doraville, GA, USA). Primers (synthetic oligonucleotides) were purchased from Anygene Biological Technology Co., Ltd. (Wuhan, China). Shanghai Sunny Biotechnology Co., Ltd. (Shanghai, China) provided DNA sequencing services. All molecular biology procedures were used based on standard methods.
Gene knockout and complementation of hfq and rsmY in P. protegens Pf-5
The rsmY genes (900-bp and-800-bp, respectively) and the upstream and downstream fragments of the hfq (1000-bp and 900-bp, respectively) were fused by polymerase chain reaction (PCR) and digested with XbaI/HindIII along with the suicide plasmid pJQ200SK prior to their ligation to and construction of the vectors pJQΔhfq and pJQΔrsmY. The knockout vectors pJQΔhfq and pJQΔrsmY were then transferred into P. protegens Pf-5, and mutants were selected on 10% sucrose LB plates. The P. protegens Pf-5 harboring plasmid pJQ200SK was unable to grow on 10% sucrose plates, indicating that the double-recombination of the strains resulted in loss of plasmid pJQ200SK. Polymerase chain reaction (PCR) and sequencing confirmed the knockout of hfq and rsmY genes, and the strains were named PfΔhfq and PfΔrsmY, respectively. The hfq and rsmY double-gene knockout was achieved using the same methods. The knockout vector pJQΔrsmY was transferred into PfΔhfq, followed by selection for the rsmY-knockout mutant, and the double-gene knockout strain was named pfΔhfqΔrsmY. Here, pRK2073 was used as a helper plasmid, which was transferred into P. protegens Pf-5 using tri-parental hybridization.
The recombinant-expression plasmid pBBR-hfq was created to construct an hfq complementation strain following knockout. pBBR-hfq was constructed by ligating the promoter sequence and the 547-bp sequence containing the hfq gene into the shuttle plasmid pBBR1MCS-5 of Pseudomonas–E. coli following BamHI/HindIII digestion. Then pBBR-hfq was transferred into the PfΔhfq strain to generate the complementation strain pfΔhfq/pBBR-hfq. The plasmid pBBRK-hfq was constructed by ligating the 547-bp sequence containing the hfq gene and the promoter sequence into plasmid pBBRKm following BamHI/HindIII digestion. The same methods were then used to construct pBBR-rsmX, pBBR-rsmY, pBBR-rsmZ, pBBRK-rsmX, pBBRK-rsmY, and pBBRK-rsmZ.
Construction of the promoter-lacZ reporter gene
The promoter-lacZ reporter gene was constructed for studying the regulation of lipase gene expression by hfq, by fusing the lipA promoter sequence with the lacZ sequence. PCR was used to amplify lacZ from the genomic DNA of E. coli BL21 (DE3), with the ‘lacZ amplicon (bp 22–3110 from the start site of translation) lacking the first seven codons and the sequence of Shine–Dalgarno (SD), whereas the amplicon of wild-type lacZ (bp 18–3110 from the start site of translation) contained the SD sequence. Into the plasmid pBBR1MCS-5, lacZ and ‘lacZ were inserted HindIII and BamHI cleavages, and cloned to generate the transcriptional-fusion plasmid pBBR02 and the translational-fusion plasmid PBBR01, respectively. The lipA gene was amplified using PCR, and following KpnI and HindIII cleavages, the lipA amplicons (bp 613–18 from the start site of translation) were cloned into plasmid pBBR01 and cloned to generate plasmid pBBR03. Similarly, the inserted lipA′ amplicons (bp 613–12 from the start site of translation) in plasmid pBBR02 generated plasmid pBBR04 (Table 1). Into plasmid pJQ200SK, lipA′-‘lacZ and lipA-lacZ were inserted following pBBR03 and pBBR04 cleavages with BamHI and SphI, and cloned to generate plasmids pJQ003 and pJQ004, respectively. The same methods were utilized to construct rsmZ’-‘lacZ, rsmY’-‘lacZ, rsmE’-‘lacZ, rsmA’-‘lacZ, and phrs’-‘lacZ.
Reverse transcription (RT)-PCR analysis
P. protegens Pf-5 was cultured until the level of growth attained optical density value of about 5.5 at 600 nm (OD600) and thereafter, RNA was extracted using RNA extraction kit (CWBIO, Beijing, China). Following purification, 2 μg of the RNA was reverse-transcribed using random hexamer primers as described in Revert Aid kit instruction leaflet for first-strand cDNA synthesis (Thermo Fisher Scientific, Waltham, MA, USA). Real-time PCR machine (ABI 7500; Applied Biosystems, Foster City, CA, USA) was employed for quantitative RT-PCR (qRT-PCR) in 96-well plate with its default program (2 min at 50 °C and 10 min at 95 °C, followed by 40 cycles at 94 °C for 15 s and at 60 °C for 60 s). A total of 20 μL reaction mixture volume was used. The reaction mixture contained 6.4 μL of RNase-free water, 10 ng of final cDNA, 10 pM of each primer, and SYBR Green master mix (10 μL; Roche, Basel, Switzerland). There was a control with an aliquot of RNase-free water of 2.0 μL in each plate. Each plate contained three technical replicates. Prior to qRT-PCR evaluation of the P. protegens Pf-5 genes, PCR-efficiency curves as well as specific verification of the dissociated PCR-amplified candidate reference gene were determined. Using rpoD as an internal reference, differences in mRNA expression were determined.
Expression and purification of Hfq protein
The 305-bp DNA fragment containing the entire hfq open reading frame sequence (261-bp) was amplified by PCR using P. protegens Pf-5 as a template. After cleavage with the restriction enzymes NdeI/HindIII, the generated fragment was inserted in the expression vector pET28a to produce the hfq-expression vector pET28a-hfq (Table 1). Into E. coli BL21 (DE3) cells, pET28a-hfq was transferred and the host cultured at 37 °C in LB containing 0.5 mM IPTG. Each E. coli BL21 (DE3) was allowed to grow and attain an OD600 of ~0.8. Then it was incubated for 20 h at 16 °C. The cells were thereafter pelleted by centrifugation and re-suspended in nickel A buffer [25 mM Tris–HCl (pH 8.0), 300 mM NaCl, and 20 mM imidazole] supplemented with 50 μM phenylmethyl sulfonyl, 1 μg/mL aprotinin, and 1 μg/mL leupeptin. After shaking the suspension for 30 min at 4 °C, an ultrasonic cell disruptor was used to lyse the cells. The lysate was allowed to percolate completely into a column of nickel-nitrilotriacetic acid agarose (GE Healthcare, Pittsburgh, PA, USA). The column was washed twice with 5 mL portions of nickel eluting buffer containing 500 mM imidazole, to elute the hfq protein. The purified hfq protein was then stored in a buffer containing 1 mM ethylenediaminetetraacetic acid (EDTA), 1 mM dithiothreitol (DTT), 200 mM NaCl and 20 mM Tris–HCl.
RNA electrophoretic mobility shift assay (REMSA)
Light-shift chemiluminescent RNA EMSA kit (Thermo Fisher Scientific), REMSA was used for REMSA. RNA fragments of rsmA, rsmE, rsmY, rsmZ were synthesized in vitro and labeled with biotin. A 2-μL probe solution containing the respective biotin-labeled RNA fragment was mixed with 3 μL of purified hfq protein (1 mg/mL) in 10 μL of binding buffer [10 mM DTT, 10 mM MgCl2, 200 mM KCl, and 100 mM HEPES, pH 7.3], and placed for 10 min at room temperature to prevent non-specific binding of the protein and probe. In studies on competitive binding, unlabeled probe concentrations were 50-fold, 100-fold, and 150-fold higher than that of the labeled-probe. Binding buffer (1 μL; colorless; 10 × ) was added and mixed immediately. Pre-electrophoresis was conducted for 30 min with 0.5 × TBE (Tris/borate/EDTA) as the electrophoretic solution at 80 V. The electrophoretically-separated protein-RNA conjugates were bound to a positively-charged nylon membrane (Ambion; Thermo Fisher Scientific). The membrane was cross-linked by the free RNAs and transferred hfq-RNA complexes when exposed to UV light at 320 nm. The biotin-labeled nucleic acid bands on the membrane were detected by chemiluminescence (Thermo Fisher Scientific). While on nylon membrane, the transferred biotin-labeled RNAs were visualized by using the activated conjugate of stabilized streptavidin and horseradish peroxidase (HRP). In order to produce light of high sensitivity, the HRP was allowed to act on luminol-based substrate. The luminescent membrane was exposed to X-ray film after remaining in a film cassette for 20–30 seconds.
Determination of rsmY abundance and stability
The strains P. protegens Pf-5 and PfΔhfq were used to determine the stability and steady-state level of rsmY. It was added at an OD600 value of 4.0 to 500 µg/mL of rifampicin (final concentration). Rifampicin was also added to the total RNA isolated from 4 ml aliquot at 0, 10, 20, 30, 40, and 60 min. Aliquots (4-mL portions) were withdrawn. With 2 µg of total RNA, primer extension technique was used to determine rsmY concentrations with AMV reverse transcriptase (Promega, Durham, NC, USA).
Northern blot
Denaturing gel composed of urea and polyacrylamide (8.3 M urea, 8% acrylamide, and 0.2% bisacrylamide) was used for electrophoretic separation of RNA and subjected to northern blot in 1 × TBE buffer [50 mM Tris-borate (pH 8.3) and 1 mM EDTA]. The molecular-weight markers (low-range RNA ladder; Fermentas, Waltham, MA, USA) corresponding to the band in a lane was excised and stained with 5 mg/mL of ethidium bromide. It was then photographed under UV light beside a reference ruler. The remaining gel was electroblotted for 20 min in 1 × TBE buffer onto a Hybond-N membrane at 150 mA (15–25 V). Nucleic acids in the membranes were cross-linked by exposure to UV light for 5 min. Then 2 × SSC (1 × SSC contains 0.15 M NaCl and 15 mM sodium citrate) was used to wash all membranes (Sambrook and Russell, 2001). Northern hybridizations were performed according to recommended protocols (DIG filter hybridization; Roche) for using digoxigenin (DIG)-labeled DNA probes.
β-Galactosidase assay
The β-galactosidase activity assay was performed as previously described36. The enzyme activity was normalized in Miller units of bacterial culture to the OD600 value. In order to induce the expression of strains containing pBBR1MCS-5 or pET-28a derivatives, 0.1 mM IPTG was added to cultures.
Lipase-activity assay
In view of the fact that LipA is an intracellular lipase, LipA activity was measured as the activity of whole-cell lipase. According to previously described methods [5], bacterial samples were prepared and 30 μL of p-nitrophenyl caprylate [pNPC; 2.9 mL 50 mM Tris–HCl (pH 9.0) and pNPC (10 mM pNPC in acetonitrile)] was used to determine lipase activity. The reaction mixture containing 70 μL of the cell sample was pre-heated for 5 min at 55 °C and centrifuged at 12,000 rpm for 2 min at 4 °C. The amount of pNP released in the supernatant was determined spectrophotometrically by measuring absorbance at 600 nm. One unit of enzyme activity (U) was defined as the amount required to release 1 μmol of p-nitrophenol/min. Lipase activity was expressed as U/mL*OD600.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
References
Houde, A., Kademi, A. & Leblanc, D. Lipases and their industrial applications: an overview. Appl. Biochem. Biotechnol. 118, 155–70 (2004).
Soberon-Chavez, G. & Palmeros, B. Pseudomonas lipases: molecular genetics and potential industrial applications. Crit. Rev. Microbiol. 20, 95–105 (1994).
Krzeslak, J., Gerritse, G., van Merkerk, R., Cool, R. H. & Quax, W. J. Lipase expression in Pseudomonas alcaligenes is under the control of a two-component regulatory system. Appl. Environ. Microbiol. 74, 1402–11 (2008).
Rosenau, F. & Jaeger, K. Bacterial lipases from Pseudomonas: regulation of gene expression and mechanisms of secretion. Biochimie 82, 1023–32 (2000).
Zha, D., Xu, L., Zhang, H. & Yan, Y. The two-component GacS-GacA system activates lipA translation by RsmE but not RsmA in Pseudomonas protegens Pf-5. Appl. Environ. Microbiol. 80, 6627–37 (2014).
Vogel, J. & Luisi, B. F. Hfq and its constellation of RNA. Nat. Rev. Microbiol. 9, 578–89 (2011).
Storz, G., Vogel, J. & Wassarman, K. M. Regulation by small RNAs in bacteria: expanding frontiers. Mol. Cell. 43, 880–91 (2011).
Balasubramanian, D., Schneper, L., Kumari, H. & Mathee, K. A dynamic and intricate regulatory network determines Pseudomonas aeruginosa virulence. Nucleic Acids Res. 41, 1–20 (2013).
Wang, G. et al. The RNA chaperone Hfq regulates antibiotic biosynthesis in the rhizobacterium Pseudomonas aeruginosa M18. J. Bacteriol. 194, 2443–57 (2012).
nSonnleitner, E. et al. Reduced virulence of a hfq mutant of Pseudomonas aeruginosa O1. Microb. Pathog. 35, 217–28 (2003).
Vecerek, B., Moll, I., Afonyushkin, T., Kaberdin, V. & Blasi, U. Interaction of the RNA chaperone Hfq with mRNAs: direct and indirect roles of Hfq in iron metabolism of Escherichia coli. Mol. Microbiol. 50, 897–909 (2003).
Muffler, A., Fischer, D. & Hengge-Aronis, R. The RNA-binding protein HF-I, known as a host factor for phage Qbeta RNA replication, is essential for rpoS translation in Escherichia coli. Genes Dev. 10, 1143–51 (1996).
Lease, R. A. & Belfort, M. Riboregulation by DsrA RNA: trans-actions for global economy. Mol. Microbiol. 38, 667–72 (2000).
Schumacher, M. A., Pearson, R. F., Moller, T., Valentin-Hansen, P. & Brennan, R. G. Structures of the pleiotropic translational regulator Hfq and an Hfq-RNA complex: a bacterial Sm-like protein. EMBO J. 21, 3546–56 (2002).
Moller, T. et al. Hfq: a bacterial Sm-like protein that mediates RNA-RNA interaction. Mol. Cell. 9, 23–30 (2002).
Zhang, A., Wassarman, K. M., Ortega, J., Steven, A. C. & Storz, G. The Sm-like Hfq protein increases OxyS RNA interaction with target mRNAs. Mol. Cell. 9, 11–22 (2002).
Nikulin, A. et al. Structure of Pseudomonas aeruginosa Hfq protein. Acta Crystallogr D Biol. Crystallogr. 61, 141–6 (2005).
Sauter, C., Basquin, J. & Suck, D. Sm-like proteins in Eubacteria: the crystal structure of the Hfq protein from Escherichia coli. Nucleic Acids Res. 31, 4091–8 (2003).
Achsel, T., Stark, H. & Luhrmann, R. The Sm domain is an ancient RNA-binding motif with oligo(U) specificity. Proc. Natl. Acad. Sci. USA 98, 3685–9 (2001).
Brescia, C. C., Mikulecky, P. J., Feig, A. L. & Sledjeski, D. D. Identification of the Hfq-binding site on DsrA RNA: Hfq binds without altering DsrA secondary structure. RNA. 9, 33–43 (2003).
Moll, I., Afonyushkin, T., Vytvytska, O., Kaberdin, V. R. & Blasi, U. Coincident Hfq binding and RNase E cleavage sites on mRNA and small regulatory RNAs. RNA. 9, 1308–14 (2003).
Geissmann, T. A. & Touati, D. Hfq, a new chaperoning role: binding to messenger RNA determines access for small RNA regulator. EMBO J. 23, 396–405 (2004).
Brencic, A. et al. The GacS/GacA signal transduction system of Pseudomonas aeruginosa acts exclusively through its control over the transcription of the RsmY and RsmZ regulatory small RNAs. Mol. Microbiol. 73, 434–45 (2009).
Sorger-Domenigg, T., Sonnleitner, E., Kaberdin, V. R. & Blasi, U. Distinct and overlapping binding sites of Pseudomonas aeruginosa Hfq and RsmA proteins on the non-coding RNA RsmY. Biochem. Biophys. Res. Commun. 352, 769–73 (2007).
Van Assche, E., Van Puyvelde, S., Vanderleyden, J. & Steenackers, H. P. RNA-binding proteins involved in post-transcriptional regulation in bacteria. Front. Microbiol. 6, 141 (2015).
Gottesman, S. & Storz, G. Bacterial small RNA regulators: versatile roles and rapidly evolving variations. Cold Spring Harb. Perspect. Biol. 3, a003798 (2011).
Lenz, D. H. et al. The small RNA chaperone Hfq and multiple small RNAs control quorum sensing in Vibrio harveyi and Vibrio cholerae. Cell. 118, 69–82 (2004).
Morita, T., Maki, K. & Aiba, H. RNase E-based ribonucleoprotein complexes: mechanical basis of mRNA destabilization mediated by bacterial noncoding RNAs. Genes Dev. 19, 2176–86 (2005).
Gottesman, S. The small RNA regulators of Escherichia coli: roles and mechanisms*. Annu. Rev. Microbiol. 58, 303–28 (2004).
Gesteland, R.F., Cech, T. & Atkins, J.F. The RNA world: the nature of modern RNA suggests a prebiotic RNA world. 3rd ed. Cold Spring Harbor monograph series. Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory Press. xxiii, 768 p (Spring, 2006).
Wilderman, P. J. et al. Identification of tandem duplicate regulatory small RNAs in Pseudomonas aeruginosa involved in iron homeostasis. Proc. Natl. Acad. Sci. USA 101, 9792–7 (2004).
Howell, C. R. Control of Rhizoctonia solani on Cotton Seedlings with Pseudomonas fluorescens and With an Antibiotic Produced by the Bacterium. Phytopathology. 69, 480–482 (1979).
Leong, S. A., Ditta, G. S. & Helinski, D. R. Heme Biosynthesis inRhizobium: identification of a cloned gene coding for δ-aminolevulinic acid synthetase from rhizobium meliloti. J. Biol. Chem. 257, 8724–8730 (1982).
Quandt, J. & Hynes, M. F. Versatile suicide vectors which allow direct selection for gene replacement in gram- negative bacteria. Gene. 127, 15–21 (1993).
Kovach, M. E. et al. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene. 166, 175–176 (1995).
Miller, J. H. Experiments in molecular genetics. Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory. xvi, 466 (Spring,1972).
Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (No. J1103514), the National High Technology Research and Development Program of China (2011AA02A204), the Innovation Foundation of Shenzhen Government (JCYJ2012083111657864), and the National Natural Science Foundation of Hubei Province (No. 2015CFA085).
Author information
Authors and Affiliations
Contributions
Wu Liu and Menggang Li designed the experimental scheme and did the most of the preparation and experiments. Jinyong Yan contributed to the analysis of the experimental data. Yunjun Yan revised the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, W., Li, M., Yan, J. et al. The role of Hfq in regulation of lipA expression in Pseudomonas protegens Pf-5. Sci Rep 7, 10356 (2017). https://doi.org/10.1038/s41598-017-10808-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-10808-x