Abstract
Preterm birth (gestational age < 37 weeks) with very low birth weight (VLBW, birth weight ≤ 1500 g) is associated with lifelong cognitive deficits, including in executive function, and persistent alterations in cortical and subcortical structures. However, it remains unclear whether “catch-up” growth is possible in the preterm/VLBW brain. Longitudinal structural MRI was conducted with children born preterm with VLBW (n = 41) and term-born peers participating in the Norwegian Mother and Child Cohort Study (MoBa) (n = 128) at two timepoints in early school age (mean ages 8.0 and 9.3 years). Images were analyzed with the FreeSurfer 5.3.0 longitudinal stream to assess differences in development of cortical thickness, surface area, and brain structure volumes, as well as associations with executive function development (NEPSY Statue and WMS-III Spatial Span scores) and perinatal health markers. No longitudinal group × time effects in cortical thickness, surface area, or subcortical volumes were seen, indicating similar brain growth trajectories in the groups over an approximately 16-month period in middle childhood. Higher IQ scores within the VLBW group were associated with greater surface area in left parieto-occipital and inferior temporal regions. Among VLBW preterm-born children, cortical surface area was smaller across the cortical mantle, and cortical thickness was thicker occipitally and frontally and thinner in lateral parietal and posterior temporal areas. Smaller volumes of corpus callosum, right globus pallidus, and right thalamus persisted in the VLBW group from timepoint 1 to 2. VLBW children had on average IQ 1 SD below term-born MoBa peers and significantly worse scores on WMS-III Spatial Span. Executive function scores did not show differential associations with morphometry between groups cross-sectionally or longitudinally. This study investigated divergent or “catch-up” growth in terms of cortical thickness, surface area, and volumes of subcortical gray matter structures and corpus callosum in children born preterm/VLBW and did not find group × time interactions. Greater surface area at mean age 9.3 in left parieto-occipital and inferior temporal cortex was associated with higher IQ in the VLBW group. These results suggest that preterm VLBW children may have altered cognitive networks, yet have structural growth trajectories that appear generally similar to their term-born peers in this early school age window.
Similar content being viewed by others
Introduction
Cognitive deficits among individuals born preterm (gestational age < 37 weeks) with very low birth weight (VLBW, birth weight ≤ 1500 g) can persist for decades1,2,3,4,5,6. Executive functions, which are foundational for academic performance and quality of life, are often impaired in the preterm-born VLBW population, even among those with otherwise typical cognitive ability7,8,9, starting in early childhood10 and lasting into adulthood11,12,13,14. Cognitive impairment following preterm birth may derive from altered connectivity that begins in utero15, as suggested by a recent fetal resting state functional magnetic resonance imaging (MRI) finding of reduced connectivity in a left hemisphere proto-language region16.
What is unclear is whether these structural and functional differences can potentially diminish over time (“catch up”), or whether they will persist. Finding a window for catch up development could be a therapeutic opportunity, yet there is limited evidence for catch-up growth in this population. A small number of studies have identified differential growth in corpus callosum volume17, cerebellar volume18, and cortical thickness in specific regions19. By contrast, much research has pointed to altered brain growth following preterm birth/VLBW20,21,22 and similar brain growth rates for preterm/VLBW and term-born children and adolescents, despite different starting points23,24,25,26. Smaller volumes, decreased general cognitive functioning, and altered frontal, thalamo-cortical, and subcortical connectivity are typical in this population27,28,29. Adolescents born extremely preterm/VLBW have shown a 1.6-year younger “brain age” based on T1-weighted whole brain structural data, compared to adolescents born after gestational week 2930.
As preterm-born children enter school age, they have been reported to have increased distractibility, worse inhibitory control, and poorer executive function skills that may contribute to poorer social competence31,32. A clearer understanding of the timing and extent of structural and functional plasticity in the preterm brain – and the potential for catch-up development – is thus needed33,34. Cross-sectional findings from an overlapping sample of this preterm/VLBW cohort and term-born participants in the Norwegian Mother and Child Cohort Study (MoBa)35,36 identified smaller cortical surface area bilaterally in frontal, temporal, and parietal lobes; thicker cortex in frontal and occipital regions; thinner cortex in posterior parietal areas; reduced volumes of subcortical structures including corpus callosum and hippocampus in the preterm/VLBW group; and only limited group differences in white matter tracts. This study is the first to present longitudinal findings comparing the preterm/VLBW and MoBa cohorts.
The aim of this study was therefore to determine whether the cortical and subcortical deviations found at the first timepoint35,36 persisted longitudinally, and whether VLBW children showed different growth trajectories of brain structures compared to term-born peers. To our knowledge, this study is the first to investigate longitudinal morphometric changes in the preterm brain at early school age. Moreover, we assessed executive function at early school age and examined possible interactions with brain development over time, in this sensitive window where demands on executive function escalate37. We expected that preterm-born/VLBW children would continue to show altered brain structure, as well as associations between MRI findings and both cognitive scores and perinatal morbidity markers.
Methods
Participants
Preterm-born VLBW participants (n = 41) born between 2003 and 2007 were recruited based on admittance to the Neonatal Intensive Care Unit at St. Olav’s University Hospital in Trondheim, Norway. Term-born control participants from central Norway (n = 128) born between 2001 and 2007 were recruited from the national Norwegian Mother and Child Cohort Study (MoBa) study, coordinated by the Norwegian Institute of Public Health38,39. Cerebral MRI and cognitive data were collected at two timepoints in childhood: first at mean age 8.0 years (range: 4.9–10.6) then at mean age 9.3 years (range: 6.1–12.0).
Exclusion criteria were severe cerebral palsy (unable to complete neuropsychological testing and MRI), severe sensory impairments, and/or MRI contraindications. Birth weight and gestational age for MoBa participants were retrieved from registry data (not available for 2 participants, for whom parent-reported birth weight was used); birth weight > 2500 g and gestational age ≥ 37 completed weeks were inclusion criteria for term-born participants in this study. Among the VLBW participants, five children with retinopathy of prematurity, one with epilepsy and mild cerebral palsy, one with intraventricular hemorrhage (grade 1) and mild cerebral palsy, and 2 others without cerebral palsy with intraventricular hemorrhage (grades 1 and 3) who successfully completed the neuropsychological assessments and MRI were included in the analyses; IQ range of these participants was 87 to 117. Four participants (2 VLBW and 2 term-born) had ADHD, and one term-born child had a history of concussion. Overall 120 participants (VLBW n = 30) had two successful MRI scans, and 49 (VLBW n = 11) with only one successful scan were also included in this study. Six preterm participants and no controls had twins. Morphometry findings at the first timepoint in an overlapping sample of this cohort were previously reported using the FreeSurfer cross-sectional processing stream35,36. Birth weight, gestational age, Apgar scores at 1 and 5 minutes, number of days in the NICU, and number of neonatal days on ventilator were the clinical variables in the VLBW group assessed for partial correlations with MRI data.
MRI
MRI data were collected using a 12-channel head coil on a 1.5 T Siemens Avanto scanner (Siemens, Erlangen, Germany). The total scan time was on average 30 minutes. The pulse sequence used for morphometric analyses was a 3D T1-weighted magnetization prepared rapid acquisition gradient echo (MPRAGE) scan with the following parameters: TR = 2400 ms, TE = 3.61 ms, TI = 1000 ms; flip angle 8°, FOV 240 × 240 mm2, and TA = 4 minutes and 18 minutes. Each volume consisted of 160 sagittal slices with voxel sizes of 1.25 × 1.25 × 1.20 mm3. All subjects had between one and four MPRAGE T1 scans. Each MPRAGE series was visually inspected using FreeSurfer’s tkregister2 tool and Aeskulap Viewer (http://aeskulap.nongnu.org) to identify artifacts and evaluate Talairach alignment, and only scans with no or minimal movement artifacts were included. The FreeSurfer package QA Tools was run on all subjects for visual inspection of segmentation. Ten subjects did not have any satisfactory MPRAGE T1–weighted scans due mostly to motion artifacts or other objects such as braces.
Image analysis
All image analysis was performed with the freely available FreeSurfer image analysis suite version 5.3.0 (http://surfer.nmr.mgh.harvard.edu). The technical details of the FreeSurfer image processing procedures are described in prior publications40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55. Images in this study were processed automatically using FreeSurfer’s longitudinal stream to extract reliable volume and thickness estimates across the timepoints55. Specifically, an unbiased within-subject template image was created using robust, inverse consistent registration54. Several processing steps, such as skull stripping, Talairach transforms, atlas registration as well as spherical surface maps and parcellations were then initialized with common information from the within-subject template, significantly increasing reliability and statistical power55. The subcortical brain structures included in the analyses are based on the automated segmentation and labeling procedure in FreeSurfer46,48. The cortical parcellation scheme in FreeSurfer by Desikan et al.50 was used for the table in Section 3.4, and the naming based on the Destrieux et al.53 cortical parcellation scheme was used to provide additional detail in the text. Ventricular system volume is the aggregate of lateral, inferior, third, and fourth ventricle volumes.
We used a method described by Hansen and Brezova et al.56 to measure intracranial volume (ICV). Briefly, ICV was estimated with an automated reverse brain mask method using the “new segment” approach of the SPM8 toolbox (release 5236) (www.fil.ion.ucl.ac.uk/spm) inside the cranium, including the brain, meninges, and cerebrospinal fluid. The pituitary gland is excluded by a straight line through the upper pituitary stalk in the axial plane. The lowest point of the cerebellum defines the caudal border. All ICV segmentations were visually inspected, and none were rejected or manually adjusted.
Cognitive measures
Comprehensive neuropsychological assessment and IQ testing were performed in the two groups. At neuropsychological assessment, parents reported whether children had received or planned to receive special education, such as aid of an assistant or help with specific subjects, at school/preschool. Executive function scores deemed invalid or incomplete by test administrators were not included in the analyses, leading to different participant numbers for the different tests.
IQ in VLBW group
In the VLBW group, children ≥ 6 years of age were assessed with Wechsler Intelligence Scale for Children, fourth edition (WISC-IV)57, which comprises four indices: Verbal Comprehension Index, Perceptual Reasoning Index, Working Memory Index, and Processing Speed Index. Children < 6 years of age were assessed with the complete version of the Wechsler Preschool and Primary Scale of Intelligence, third edition (WPPSI-III)58. Since most participants were tested twice, scores from their first cognitive assessment were used here to avoid practice effects; however, for 11 VLBW participants, IQ scores from the first timepoint were not available (e.g., due to incomplete testing), so scores from the second timepoint were used instead. Due to incomplete testing, Verbal Comprehension Index was used as a substitute for Full-scale IQ Index for four participants, and Perceptual Reasoning Index was used as a substitute for one participant. Full-scale IQ Index scores were used for this study.
IQ in control group
Cognitive abilities in the controls who were ≥ 6.5 years of age were assessed with the Wechsler Abbreviated Scale of Intelligence (WASI)59. WASI is a validated screening test used to assess verbal knowledge, visual information processing, spatial and nonverbal reasoning, and general intelligence. Three IQ scores can be measured using the WASI: Verbal IQ and Performance IQ, which when combined provide an estimated Full-scale IQ score. The controls < 6.5 years of age completed a short form of the WPPSI-III58, including the vocabulary, similarities, block design, and matrices subtests. Full-scale IQ Index scores from timepoint 1 were used for this study.
NEPSY Statue
The NEPSY Statue subtest of the Developmental NEuroPSYchological Assessment, Norwegian version (NEPSY)60, is designed to assess motor control and inhibition by asking the child to maintain a body position for 75 seconds and ignore distracting sounds that they are not informed about before the test starts. Points are awarded per five-second interval: two points for full response inhibition, one point for one inappropriate response, and zero points for more than one inappropriate response61. This study used raw scores, where a higher score reflects better response inhibition.
Spatial Span
The Spatial Span subtest of the Wechsler Memory Scale, third edition (WMS-III)62, is designed to evaluate visual working memory. The examiner points to blue blocks on a white board and asks the participant to point to the blocks in the same order, with increasing difficulty. Later, the participant is instructed to point in reverse order, also with increasing difficulty. For this study, we used the raw total outcome score of correctly replicated items.
Socio economic status
Hollingshead’s63 two factor index of social position based on education and occupation of one parent or the mean index of both parents was used to calculate socioeconomic status.
Statistical analysis
IBM SPSS 24 (Chicago, USA) was used to evaluate group differences and correlations between demographic, clinical, morphometric, and cognitive measures, with significance threshold at p < 0.05. One-way ANOVA was used to compare demographic variables with normal distribution, with p < 0.05 indicating significant group differences. Mann-Whitney U Test was used for age at scan, which had a nonparametric distribution as assessed by Shapiro-Wilk’s test (p < 0.05). Chi-square (χ2) testing for association was used for socioeconomic status. Drop-out analysis used independent samples t-tests within the preterm and term-born groups in terms of gestational age, birth weight, receiving help at school, age at scan, and IQ, based on the 14 term-born participants and 12 preterm/VLBW participants who met for assessment and were excluded from this analysis.
Longitudinal analyses of changes in cortical morphometry from timepoint 1 to 2 were run in Matlab 2015b (MATLAB and Statistics Toolbox Release 2015b. The MathWorks, Inc., Natick, Massachusetts, USA) by adapting the linear mixed effects module in FreeSurfer 5.3.064. A linear mixed effects model was fitted in each location (vertex) across the reconstructed cortical surface, with cortical area or cortical thickness as the dependent variable; intercept, time from baseline, age at baseline, group, sex, and interaction (group × time) as independent variables; and intercept as random factor. Using these variables, contrast vectors were set in order to test for an interaction effect between group and time and for each of the executive function tests and IQ. Effects of time were assessed within each group for each of the cognitive scores.
General linear models were fitted in Matlab 2015b for cross-sectional cortical thickness and surface area analyses for both timepoints, controlled for age at scan and sex. General linear models were also fitted to assess relationships between cortical surface area or thickness with IQ and executive function scores, between and within groups, at each timepoint. General linear models for both cortical surface area and thickness were also fitted at timepoint 2 with cortical measure (area or thickness) as the dependent variable and group as the independent variable, co-varying for sex, age at scan, and retinopathy of prematurity. General linear models in SPSS were fitted for cross-sectional group comparisons of subcortical brain structure volumes, controlled for ICV, age at scan, and sex; analysis of ICV controlled only for age at scan and sex.
To correct for multiple comparisons, the p-maps from left and right hemisphere were combined and thresholded to yield an expected false discovery rate (FDR) of 5% across both hemispheres. In order to generate effect size maps that are comparable across the morphometry variables to investigate annualized rate of change, cortical area and cortical thickness were log-transformed prior to fitting the model to the data; the beta value for time, resulting from the model fit, was back-transformed and multiplied by 100 in order to obtain percent change per unit of time26. Brain figures display MRI data overlaid on the FreeSurfer fsaverage white surface.
Holm–Bonferroni step-down65 was used to correct for multiple comparisons for tests of group differences and correlations in subcortical volumes based on 24 structures compared and α = 0.05. Partial correlation tests, controlled for age at scan, sex, and ICV were used to investigate the relationships between subcortical volumes and cognitive and perinatal data. Raw cognitive test scores were adjusted for age.
Ethics
The Regional Committee for Medical Research Ethics approved the study protocol (project number 2010/2359), and written, informed consent was obtained from the parents/guardians of all participants. The study was performed in accordance with relevant guidelines and regulations.
Results
Clinical profile
Demographic and clinical characteristics of the two groups are presented in Table 1. VLBW participants had lower IQ by approximately 1 SD, were more likely to be receiving or plan to receive help at school or preschool based on parent report (36.6% vs 7.3%), and were younger than term-born peers by approximately 6 months at both scans. There was no statistically significant association between socioeconomic status and group (χ2(1) = 7.39, p = 0.12). Average time between scans was 14.3 months for VLBW participants and 16.0 months for term-born peers. Drop-out analysis did not reveal any significant differences within either group in terms of gestational age, birth weight, receiving help at school, age at scan, or IQ.
Executive function
Scores on the two executive function tests are presented in Table 2 with significance testing and at each timepoint and for longitudinal group × time effects. WMS-III Spatial Span was significantly worse in the VLBW group at timepoint 2 (d = −0.62, p = 0.005).
Subcortical volumes
Group differences: longitudinal and cross-sectional
Group differences in subcortical gray matter structures, corpus callosum volume, and ICV are presented in Supplemental Table 1, and effect sizes for structures showing significant group differences in Figure 1. Longitudinal analyses of subcortical brain structure volumes did not identify any group × time effects. Post-hoc removal of ICV as a covariate also did not identify any longitudinal group differences. Corpus callosum (central, mid-posterior, and posterior segmentations, and total corpus callosum volume), right globus pallidus, and right thalamus were significantly smaller in the VLBW group compared to controls at both timepoints. The ventricular system was significantly larger in the VLBW group at both timepoints. In addition, group differences in bilateral hippocampus, left thalamus, and corpus callosum mid-anterior subsegmentation size were significant at timepoint 1, with the VLBW group showing smaller volumes.
Subcortical structures showing significant group differences (indicated by *) at timepoint 1 and timepoint 2, in right (R) and left (L) hemispheres, shown with effect size of VLBW group compared to controls. Volumes adjusted for sex, age at scan, and ICV (ICV itself only adjusted for sex and age at scan). Abbreviations: CC: corpus callosum; ICV: intracranial volume; VLBW: very low birth weight.
Volume-cognition relationships
No group × score interactions (for executive function scores, IQ, or receiving help at school) were found for any of the subcortical volumes after correction for multiple comparisons. Several associations were found within the groups. In the VLBW group at timepoint 2, larger corpus callosum volume (total volume and posterior, mid-posterior, and central subsegmentations) was negatively associated with receiving help/special education at school, although only the posterior segment structure-function relationship was significant after correction for multiple comparisons (p = 0.00089). Among controls, ventricular system volume was significantly correlated with receiving/planning to receive help at school at timepoint 1 (R = 0.45, p < 0.001) and timepoint 2 (R = 0.31, p = 0.0011), and left nucleus accumbens volume was correlated with NEPSY statue score at timepoint 1 (R = 0.33, p = 0.001).
Volume-perinatal health data relationships in VLBW group
Birth weight was positively correlated with left thalamus volume at timepoint 1 (R = 0.67, p = 0.002). Right hippocampus volume at timepoint 2 was negatively associated with Apgar 5 minute score (R = −0.71, p = 0.00091).
Group differences in cortical structure
Longitudinal changes
No evidence was found for longitudinal group × time interactions in either cortical thickness or surface area development between the two scanning timepoints (approximately 16 months apart).
Cross-sectional findings
Cross-sectional group differences in cortical thickness and surface area were widespread across the cortical mantle at timepoint 2 (Table 3). Surface area group differences were more global than those for cortical thickness. Cortical thickness was increased in the VLBW group frontally and decreased in parietal and temporal regions. Similar morphometry findings at timepoint 1 were previously reported using cross-sectional processing in an overlapping sample in this cohort35.
Surface area
Group differences in surface area were widespread, shown in Figure 2 and Table 3 for timepoint 2, and were more widespread than group differences in cortical thickness. There were no cortical areas with larger surface area in the VLBW group, as indicated by Cohen’s d values (Figure 2B, range: −1.37 > d < −0.054 on left; −1.50 > d < −0.0075 on right). Cortical surface area results from timepoint 1 in this study using the longitudinal processing stream are presented in Supplementary Figure 1.
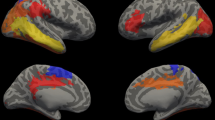
Cortical surface area group differences between VLBW and control groups at timepoint 2. Row A shows p-maps and row B shows effect size. The p-maps were produced from GLM models fitted at each location (vertex) across the cortical surface, with cortical area as the dependent variable and group as the independent variable, co-varying for sex and age at scan. The p-maps were thresholded to yield an expected 5% FDR across both hemispheres. In the effect size maps, blue represents areas of reduced surface area in the VLBW. Abbreviations: d: Cohen’s d; FDR: false discovery rate; VLBW, very low birth weight.
Cortical thickness
The groups showed significantly different cortical thickness in areas in all cortical lobes at timepoint 2, as shown in Figure 3 and Table 3. Group differences in cortical thickness were much less widespread than those for surface area (Table 3). Thicker occipital cortex and medial frontal cortex was found bilaterally in the VLBW group, while thinning was seen in posterior temporal lobe, particularly on the left side. Left and right hemispheres showed generally the same extent of group differences, with some variation in location. Cohen’s d values (Figure 3D, range: −0.91 > d < 1.36 on left; −0.81 > d < 1.57 on right) indicated areas of large effect sizes in group differences. Cortical thickness results from timepoint 1 in this study using the longitudinal processing stream are presented in Supplementary Figure 2.
Cortical thickness group differences between VLBW and control groups at timepoint 2. Row C shows p-maps and row D shows effect size. The p-maps were produced from GLM models fitted at each vertex across the cortical surface, with cortical thickness as the dependent variable and group as the independent variable, co-varying for sex and age at scan. The p-maps were thresholded to yield an expected 5% FDR across both hemispheres. In the effect size maps, red-yellow color represents areas of increased thickness in the VLBW, while blue represents cortical thinning. Abbreviations: d: Cohen’s d; FDR: false discovery rate; VLBW, very low birth weight.
Figure 4 summarizes cortical regions demonstrating significant group differences in cortical thickness, surface area, and both. The majority of areas that differed significantly in cortical thickness also differed in surface area (overlap shown in red). The cortical areas showing group differences in both thickness and surface area were similar bilaterally: superior temporal sulcus, angular gyrus, supramarginal gyrus, anterior cingulate, orbitofrontal cortex, cuneus, and calcarine sulcus; right hemisphere also showed overlap in the posterior cingulate, and left hemisphere showed more overlap in the superior temporal sulcus. Group differences in cortical surface area and thickness when additionally covarying for retinopathy of prematurity (Supplementary Figure 3) showed similar patterns of areas affected to those in Figures 2 and 3.
Cortical morphometry and cognitive performance
Two left hemisphere regions showed a group × IQ interaction in surface area at timepoint 2 (Figure 5). Significant group × IQ interactions were seen at the border of the left parietal and occipital lobes (superior occipital and transverse occipital sulci, middle occipital gyrus, angular gyrus, intraparietal sulcus in the left hemisphere) and to a lesser extent in the left inferior temporal cortex (middle temporal gyrus towards the frontal pole). Greater surface area in these regions was associated with higher IQ scores in the VLBW group (r2 ≤ 0.16). These areas also showed group × IQ interaction at timepoint 1, as previously reported35.
Effect size map (r2, bottom row) and p-map (top row) showing group × IQ interaction at timepoint 2, indicating parieto-occipital and inferior temporal regions where greater surface area was associated with higher IQ scores in the VLBW group. Left, left hemisphere lateral view; right, left hemisphere posterior view.
No significant group × score interactions for the executive functions were found for surface area or cortical thickness in the longitudinal or cross-sectional analyses after FDR correction.
In the control group only, longitudinal surface area changes showed a structure-function relationship with change in NEPSY statue scores, with higher scores associated with increased surface area in right superior precentral sulcus, left planum temporale, transverse temporal sulcus, posterior segment of the lateral sulcus, and cingulate gyrus posterior-ventral. For spatial span, higher scores were associated with reduced surface area in right subparietal sulcus and the marginal part of the cingulate sulcus. See Supplementary Figure 3.
Discussion
No group × time interactions were seen in brain growth between approximately 8 and 9.3 years of age, while significant group differences in subcortical volumes and, cortical surface area, and to a lesser extent cortical thickness, persisted from timepoint 1 to 2. However, within the VLBW group, higher IQ was associated with greater surface area in left hemisphere regions of parieto-occipital and inferior temporal cortex, and perinatal health markers were to a limited extent associated with reduced volumes in right hippocampus and left thalamus. We found no group × time longitudinal interactions in morphometry for the executive function scores. The longitudinal results suggest a similar trajectory of cortical and subcortical development between preterm and term-born peers in this middle childhood window, in conjunction with evidence of altered cognitive networks in preterm-born children. This result among preterm children born in the 2000s extends our findings from a cohort born in the late 1980s, which demonstrated that VLBW preterm-born individuals and term-born peers did not show divergent developmental trajectories for cortical thickness, surface area26, or subcortical volumes23 from 15 to 20 years of age.
Executive function and IQ interaction with morphometric development
Lower IQ was associated with reduced surface area in two cortical regions within the VLBW group (Figure 5). In the area in parieto-occipital cortex, the preterm group showed both significantly reduced surface area, as well as an added effect of IQ on surface area in the preterm group compared to term-born (i.e., increased surface area and higher IQ). This finding points to a specific region within a lateral parieto-temporal module reported to show stronger association between IQ and gray matter volume in very preterm-born adolescents/adults22. This area constitutes part of the proposed dorsal stream66,67, which is believed to be involved in visuomotor control of actions68. The dorsal stream is reported to be impacted in the preterm population69, among other clinical groups, and may be related to impairments in attention and executive function70, a potential explanation for its relationship to IQ scores in this study. This interesting finding may indicate specific cortical regions where surface area development reflects compensatory mechanisms used for general cognitive abilities in place by early school age.
This study did not find group × executive function score interactions for morphometry measures in early school age. These findings were somewhat unexpected, as altered morphometry has been frequently associated with differences in executive function, visual-motor skills, and cognition in long-term follow-up of preterm birth survivors71,72,73,74,75,76. It is possible that the relatively high functioning of the preterm-born sample in this study limited the ability to identify different structure-score associations between groups, making it difficult to comment on reorganization or alternate cortical development among preterm survivors with more reduced executive function.
Executive function encompasses encompass working memory, cognitive control, and inhibitory control/reward processing77,78. Prematurity can lead to a cascade of downstream impairments on cognitive performance, beginning with slower processing speed, poorer executive functioning and working memory, and finally lower achievement in math and reading79. Consistent with the hypothesis of Rose, et al.79, preterm-born children in this sample were indeed more likely than term-born peers to receive special services in school.
It is possible that differential trajectories linking executive function and cortical development occur earlier in life, prior to the age window assessed in this study. Rathbone et al.80 found a positive correlation between perinatal growth rate of cortical surface area and NEPSY summary score at age 6 and speculated that genetic and environmental influences during infancy are related to the development in childhood of executive function, attention, and planning capacities. Term-born and preterm-born children did not show significantly different trajectories in this study between the ages of 8 and 9.3, similar to findings of Edgin, et al.10 in children between the ages of 2 and 4. Very preterm-born children in the Edgin, et al.10 study had similar executive function performance as term-born peers, while those with white matter abnormalities showed persistent cognitive inflexibility and poor inhibitory control, underscoring the value of neuroimaging for identifying individuals at highest risk for cognitive control difficulties already by age two.
Functional neuroimaging studies have shown evidence for differences in verbal, learning, and memory cortical circuits in the preterm population81,82,83,84,85,86,87,88,89,90. Further multimodal analysis in this preterm/MoBa cohort combining white matter imaging with morphometry and functional imaging may be able identify specific risks for those with poorer white matter development.
Persistent differences in cortical thickness and surface area
Both cortical thickness and surface area showed widespread cross-sectional group differences in this study as expected, with significant overlap (Figure 4), which held when taking retinopathy of prematurity into account (Supplementary Figure 2). Frontal, occipital, and temporo-parietal regions implicated in this study have previously shown the greatest cortical thickness deviation among the most immature preterm survivors (birth weight ≤ 1250 g or gestational age ≤ 28 weeks) in adolescence73.
This study’s findings of cortical and subcortical deficits may be due to epigenetic effects of immature birth on genes controlling growth, or the same effects due to fetal growth restriction caused by placental pathology. Preterm/VLBW-related insults to the brain may be first and foremost limited to the perinatal period, a highly plastic and vulnerable period for the immature brain91,92,93,94,95,96. Compared to fetuses of comparable age, preterm/VLBW infants who live ex utero for their “third trimester” show decreased brain growth – even in the absence of severe brain injury – suggesting that their course of brain development leading up to term age is altered97. Disruption in this window of plasticity may initiate or require reorganization of neural connections29,91.
The ages assessed in this study encompass periods of peak cortical thickness and surface area98,99,100, which are followed by pruning of experience-expectant synapses and plasticity through adolescence101, especially in white matter. It is likely that both neural growth and pruning mechanisms are disturbed in the preterm population, leading to the constellation of preterm brain features including both thinning and thickening of cortex and widespread surface area reduction.
The structural differences in cortex among preterms in this study may be caused by a cascading mechanism from white matter damage. While the most severe lesions including cystic periventricular leukomalacia have declined following advances in neonatal care, periventricular white matter injury is still common in the preterm-born population15,102. Vollmer et al.103 speculate that early disturbance of growth in white matter pathways, rather than reduced structural volumes, contribute to worse cognitive function in the preterm-born population104,105.
Reduced subcortical structure volumes
The VLBW group showed persistently smaller volumes of corpus callosum, right globus pallidus, and right thalamus. Growth rates for subcortical structures (Table 2) did not differ between groups whether or not ICV was used as a covariate. Corpus callosum, hippocampus, and thalamus are particularly vulnerable in this group, and alterations in their development in the VLBW population have been linked to cognitive deficits or psychiatric symptoms23,106,107,108. Corpus callosum volume in this clinical population has been linked to IQ17 and executive functions11,109,110.
Moreover, volume associations with cognitive and perinatal markers in the preterm group were predominant in structures critical for learning, memory, and cognition. Volumes of right hippocampus and left thalamus were related to perinatal health measures in the VLBW group. Smaller corpus callosum subsegmentation volumes, especially posteriorly, showed strong correlations to receiving help at school. Posterior corpus callosum is often affected in long-term follow-up of preterm survivors, and perinatal brain injury to its connectivity may affect visual and perceptual skills111. Formation of deep gray matter structures, in particular the thalamus, occurs at the same time as preterm birth and is linked to development of white matter and cortex112. Thalamocortical fibers and projections from sensory and associative thalamus can be affected by both focal and diffuse lesions in prematurity, related to vulnerability of subplate neurons in the second and third trimesters113,114. These functional outcomes likely share common mechanisms with structural alterations and/or reflect cascading cognitive effects.
It is difficult to discern whether or to what extent these structural changes are compensating for impaired function in the preterm brain, or are altered for physiological reasons as part of the so-called encephalopathy of prematurity20,115. Moreover, reduced brain volumes may not be caused by prematurity itself, but rather postnatal events and risk factors, such as the need for prolonged supplementary oxygen, which may exert an environmental influence on brain growth116.
Clinical and Classroom Implications
These results suggest that improvements in neonatal medical care and other early childhood services have buffered the neurodevelopmental impact of preterm birth with VLBW and that children born in the 2000s show more similar structure-function relationships to their term-born peers than in previous decades36. For example, intubation for extremely preterm neonates has been increasingly replaced with less-invasive methods of ventilation, such as increased surfactant use and early continuous positive airway pressure102,117.
The preterm behavioral phenotype has been described as anxious and inattentive, rather than hyperactive or disruptive, which may also mean that their cognitive difficulties may not be as readily visible in a classroom setting20,118,119. Promisingly, working memory training interventions have shown learning gains in preterm-born preschoolers and adolescents120,121. Identifying sensitive windows for “catch up” in brain function is critical for survivors of preterm birth with VLBW, given their well-documented cognitive challenges.
Strengths and Limitations
Structural MRI can detect variation in early brain development and serve as a reference point for functional differences122,123. Longitudinal imaging is the only way to accurately measure structural growth and maturation and determine links between cognitive development and brain growth124. A strength of this study is the use of a robust longitudinal image registration protocol55 and a statistical analysis approach that allows for explicit modeling and analysis of within- and across-subject sources of variability in temporal covariance64. Following the same individuals longitudinally is a challenge for researchers, and this study includes MRI data from 2 timepoints from 120 children, with no significant difference in socioeconomic status between groups. This study included a subset of participants in the Norwegian MoBa Study, which in total recruited 108,000 births from across Norway in an extensive prospective study of health and development38. Future study designs could do more to incentivize continued participation to ensure a robust and representative sample.
Although group × time analyses did not show significant interactions for any of the brain structures, it is still possible that the growth rates in the two groups do differ, but either at a different age, or at a rate that was not detectable in this study. A challenge with the longitudinal cognitive assessment portion was the possibility of practice effects when administering the same test twice125. Moreover, the NEPSY Statue subtest is typically administered to children three to six years old but was used here with older children as part of the longitudinal design and to increase standardization across the entire age span of participants. Finally, it is possible that the preterm-born children with the most compromised executive function were not included in the study due to incomplete testing and/or poor quality neuroimaging, which would skew overall performance in the preterm group upwards.
Basis for multimodal analysis
A natural extension of this project would be to move from correlational analysis to developing a predictive model that integrates multimodal MRI features (such as cortical thickness, surface area, subcortical volumes, white matter properties, and activation patterns) and with neuropsychological and cognitive follow-up results. Differences in the development of brain structures can parallel differences in cognitive skills and behavior126,127. Predictive modeling using functional and morphometric data has shown potential in estimating cognitive skills later in childhood128 and may be useful for the VLBW community129. As more neurodevelopmental predictive models using MRI are created and tested, they may prove useful for identifying children with greater need for follow-up services and education interventions130 or to predict potential variance in treatment outcomes131,132. As preterm birth and VLBW remain global health challenges, increasing our knowledge linking subtle changes in brain structures with measurable deficits in cognitive performance is an important step forward.
A future step would be to study gray and white matter changes longitudinally in the same cohort to better link white matter damage with cortical development and maturation, and evaluate how this relationship evolves starting in the neonatal period, since morphometric and diffusion parameters have been shown to follow different developmental paths111,133,134,135. While this study does not provide evidence for catch-up growth in brain structures among preterm-born individuals, it is possible that there is a critical window open earlier in childhood during which targeted interventions can stimulate cognitive development, especially in the most at-risk babies born preterm with VLBW.
Data Availability
Inquiries about and requests for access to data generated and analyzed during this study should be directed to the corresponding author.
References
Allin, M. et al. Effects of very low birthweight on brain structure in adulthood. Developmental medicine and child neurology 46, 46–53 (2004).
Løhaugen, G. C. et al. Cognitive profile in young adults born preterm at very low birthweight. Dev Med Child Neurol 52, 1133–1138, https://doi.org/10.1111/j.1469-8749.2010.03743.x (2010).
Martinussen, M. et al. Cerebral cortex thickness in 15-year-old adolescents with low birth weight measured by an automated MRI-based method. Brain 128, 2588–2596, https://doi.org/10.1093/brain/awh610 (2005).
Martinussen, M. et al. Segmental brain volumes and cognitive and perceptual correlates in 15-year-old adolescents with low birth weight. The Journal of pediatrics 155, 848–853 e841, https://doi.org/10.1016/j.jpeds.2009.06.015 (2009).
Skranes, J. S. et al. Cerebral MRI findings in very-low-birth-weight and small-for-gestational-age children at 15 years of age. Pediatric radiology 35, 758–765, https://doi.org/10.1007/s00247-005-1446-2 (2005).
Aarnoudse-Moens, C. S., Weisglas-Kuperus, N., Duivenvoorden, H. J., van Goudoever, J. B. & Oosterlaan, J. Executive function and IQ predict mathematical and attention problems in very preterm children. PloS one 8, e55994, https://doi.org/10.1371/journal.pone.0055994 (2013).
Ross, G., Boatright, S., Auld, P. A. & Nass, R. Specific cognitive abilities in 2-year-old children with subependymal and mild intraventricular hemorrhage. Brain Cogn 32, 1–13, https://doi.org/10.1006/brcg.1996.0054 (1996).
Taylor, H. G. & Clark, C. A. Executive function in children born preterm: Risk factors and implications for outcome. Semin Perinatol 40, 520–529, https://doi.org/10.1053/j.semperi.2016.09.004 (2016).
van de Weijer-Bergsma, E. V., Wijnroks, L. & Jongmans, M. J. Attention development in infants and preschool children born preterm: A review. Infant Behav Dev 31, 333–351, https://doi.org/10.1016/j.infbeh.2007.12.003 (2008).
Edgin, J. O. et al. Executive functioning in preschool children born very preterm: Relationship with early white matter pathology. J Int Neuropsych Soc 14, 90–101, https://doi.org/10.1017/S1355617708080053 (2008).
Aanes, S., Bjuland, K. J., Skranes, J. & Lohaugen, G. C. Memory function and hippocampal volumes in preterm born very-low-birth-weight (VLBW) young adults. Neuroimage 105, 76–83, https://doi.org/10.1016/j.neuroimage.2014.10.023 (2015).
Mulder, H., Pitchford, N. J., Hagger, M. S. & Marlow, N. Development of executive function and attention in preterm children: a systematic review. Dev Neuropsychol 34, 393–421, https://doi.org/10.1080/87565640902964524 (2009).
Skranes, J. Executive function deficits in preterm subjects are a combination of social risk factors and brain maldevelopment. Acta paediatrica 106, 1380–1382, https://doi.org/10.1111/apa.13955 (2017).
Skranes, J. & Lohaugen, G. C. Reduction in general intelligence and executive function persists into adulthood among very preterm or very low birthweight children. Evid Based Ment Health 19, e28, https://doi.org/10.1136/eb-2015-102249 (2016).
Back, S. A., Riddle, A. & McClure, M. M. Maturation-dependent vulnerability of perinatal white matter in premature birth. Stroke 38, 724–730, https://doi.org/10.1161/01.STR.0000254729.27386.05 (2007).
Thomason, M. E. et al. Weak functional connectivity in the human fetal brain prior to preterm birth. Sci Rep-Uk 7, ARTN 39286 10.1038/srep39286 (2017).
Allin, M. et al. Growth of the corpus callosum in adolescents born preterm. Arch Pediat Adol Med 161, 1183–1189, https://doi.org/10.1001/archpedi.161.12.1183 (2007).
Parker, J. et al. Cerebellar growth and behavioural & neuropsychological outcome in preterm adolescents. Brain 131, 1344–1351, https://doi.org/10.1093/brain/awn062 (2008).
Nam, K. W. et al. Alterations in cortical thickness development in preterm-born individuals: Implications for high-order cognitive functions. Neuroimage 115, 64–75, https://doi.org/10.1016/j.neuroimage.2015.04.015 (2015).
Boardman, J. P. et al. A common neonatal image phenotype predicts adverse neurodevelopmental outcome in children born preterm. Neuroimage 52, 409–414, https://doi.org/10.1016/j.neuroimage.2010.04.261 (2010).
Nosarti, C. et al. Preterm birth and structural brain alterations in early adulthood. Neuroimage Clin 6, 180–191, https://doi.org/10.1016/j.nicl.2014.08.005 (2014).
Karolis, V. R. et al. Volumetric grey matter alterations in adolescents and adults born very preterm suggest accelerated brain maturation. Neuroimage 163, 379–389, https://doi.org/10.1016/j.neuroimage.2017.09.039 (2017).
Bjuland, K. J., Rimol, L. M., Lohaugen, G. C. & Skranes, J. Brain volumes and cognitive function in very-low-birth-weight (VLBW) young adults. Eur J Paediatr Neurol 18, 578–590, https://doi.org/10.1016/j.ejpn.2014.04.004 (2014).
de Kieviet, J. F., Zoetebier, L., Van Elburg, R. M., Vermeulen, R. J. & Oosterlaan, J. Brain development of very preterm and very low-birthweight children in childhood and adolescence: a meta-analysis. Developmental Medicine and Child Neurology 54, 313–323, https://doi.org/10.1111/j.1469-8749.2011.04216.x (2012).
Giedd, J. N. et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience 2, 861–863, https://doi.org/10.1038/13158 (1999).
Rimol, L. M. et al. Cortical trajectories during adolescence in preterm born teenagers with very low birthweight. Cortex; a journal devoted to the study of the nervous system and behavior 75, 120–131, https://doi.org/10.1016/j.cortex.2015.12.001 (2016).
Nosarti, C. et al. Grey and white matter distribution in very preterm adolescents mediates neurodevelopmental outcome. Brain 131, 205–217, https://doi.org/10.1093/brain/awm282 (2008).
Ball, G. et al. The effect of preterm birth on thalamic and cortical development. Cereb Cortex 22, 1016–1024, https://doi.org/10.1093/cercor/bhr176 (2012).
Fischi-Gomez, E. et al. Structural Brain Connectivity in School-Age Preterm Infants Provides Evidence for Impaired Networks Relevant for Higher Order Cognitive Skills and Social Cognition. Cereb Cortex 25, 2793–2805, https://doi.org/10.1093/cercor/bhu073 (2015).
Franke, K., Luders, E., May, A., Wilke, M. & Gaser, C. Brain maturation: Predicting individual BrainAGE in children and adolescents using structural MRI. Neuroimage 63, 1305–1312, https://doi.org/10.1016/j.neuroimage.2012.08.001 (2012).
Alduncin, N., Huffman, L. C., Feldman, H. M. & Loe, I. M. Executive function is associated with social competence in preschool-aged children born preterm or full term. Early Human Development 90, 299–306, https://doi.org/10.1016/j.earlhumdev.2014.02.011 (2014).
Loe, I. M. et al. Oculomotor assessments of executive function in preterm children. J Pediatr 161, 427–433 e421, https://doi.org/10.1016/j.jpeds.2012.02.037 (2012).
Luu, T. M., Vohr, B. R., Allan, W., Schneider, K. C. & Ment, L. R. Evidence for Catch-up in Cognition and Receptive Vocabulary Among Adolescents Born Very Preterm. Pediatrics 128, 313–322, https://doi.org/10.1542/peds.2010-2655 (2011).
Shanmugan, S. & Satterthwaite, T. D. Neural Markers of the Development of Executive Function: Relevance for Education. Curr Opin Behav Sci 10, 7–13, https://doi.org/10.1016/j.cobeha.2016.04.007 (2016).
Sølsnes, A. E. et al. Cortical morphometry and IQ in VLBW children without cerebral palsy born in 2003-2007. Neuroimage-Clin 8, 193–201, https://doi.org/10.1016/j.nicl.2015.04.004 (2015).
Sølsnes, A. E., Sripada, K. et al. Limited microstructural and connectivity deficits despite subcortical volume reductions in school-aged children born preterm with very low birth weight. Neuroimage 130, 24–34, https://doi.org/10.1016/j.neuroimage.2015.12.029 (2016).
Diamond, A. In Lifespan cognition: Mechanisms of change (eds Bialystok, E. and Craik, F. I. M.) 70–95 (Oxford University Press, 2006).
Magnus, P. et al. Cohort Profile Update: The Norwegian Mother and Child Cohort Study (MoBa). Int J Epidemiol 45, 382–388, https://doi.org/10.1093/ije/dyw029 (2016).
Magnus, P. et al. Cohort profile: The Norwegian Mother and Child Cohort Study (MoBa). Int J Epidemiol 35, 1146–1150, https://doi.org/10.1093/ije/dyl170 (2006).
Dale, A. M. & Sereno, M. I. Improved Localizadon of Cortical Activity by Combining EEG and MEG with MRI Cortical Surface Reconstruction: A Linear Approach. J Cogn Neurosci 5, 162–176, https://doi.org/10.1162/jocn.1993.5.2.162 (1993).
Dale, A. M., Fischl, B. & Sereno, M. I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 9, 179–194, https://doi.org/10.1006/nimg.1998.0395 (1999).
Fischl, B., Sereno, M. I. & Dale, A. M. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage 9, 195–207, https://doi.org/10.1006/nimg.1998.0396 (1999).
Fischl, B., Sereno, M. I., Tootell, R. B. & Dale, A. M. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp 8, 272–284 (1999).
Fischl, B. & Dale, A. M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 97, 11050–11055, https://doi.org/10.1073/pnas.200033797 (2000).
Fischl, B., Liu, A. & Dale, A. M. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging 20, 70–80, https://doi.org/10.1109/42.906426 (2001).
Fischl, B. et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33, 341–355 (2002).
Fischl, B. et al. Sequence-independent segmentation of magnetic resonance images. Neuroimage 23(Suppl 1), S69–84, https://doi.org/10.1016/j.neuroimage.2004.07.016 (2004).
Fischl, B. et al. Automatically parcellating the human cerebral cortex. Cereb Cortex 14, 11–22 (2004).
Segonne, F. et al. A hybrid approach to the skull stripping problem in MRI. Neuroimage 22, 1060–1075, https://doi.org/10.1016/j.neuroimage.2004.03.032 (2004).
Desikan, R. S. et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980, https://doi.org/10.1016/j.neuroimage.2006.01.021 (2006).
Han, X. et al. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage 32, 180–194, https://doi.org/10.1016/j.neuroimage.2006.02.051 (2006).
Jovicich, J. et al. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage 30, 436–443, https://doi.org/10.1016/j.neuroimage.2005.09.046 (2006).
Destrieux, C., Fischl, B., Dale, A. & Halgren, E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage 53, 1–15, https://doi.org/10.1016/j.neuroimage.2010.06.010 (2010).
Reuter, M., Rosas, H. D. & Fischl, B. Highly accurate inverse consistent registration: a robust approach. Neuroimage 53, 1181–1196, https://doi.org/10.1016/j.neuroimage.2010.07.020 (2010).
Reuter, M., Schmansky, N. J., Rosas, H. D. & Fischl, B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage 61, 1402–1418, https://doi.org/10.1016/j.neuroimage.2012.02.084 (2012).
Hansen, T. I., Brezova, V., Eikenes, L., Håberg, A. & Vangberg, T. R. How Does the Accuracy of Intracranial Volume Measurements Affect Normalized Brain Volumes? Sample Size Estimates Based on 966 Subjects from the HUNT MRI Cohort. AJNR. American journal of neuroradiology 36, 1450–1456, https://doi.org/10.3174/ajnr.A4299 (2015).
Wechsler, D. Wechsler Intelligence Scale for Children, fourth edition (Norwegian version, 2009 edition). (Pearson, 2003).
Wechsler, D. Wechsler Preschool and Primary Scale of Intelligence, third edition (Norwegian version, 2008 edition). (Pearson, 2002).
Wechsler, D. Wechsler Abbreviated Scale of Intelligence. (Pearson, 1999).
Korkman M, K. U., Kemp S.. A Developmental NEuroPSYchological Assessment, Norwegian version (NEPSY): Norwegian version., (Hogrefe Psykologiförlaget AB, 2002).
Hermansen, T. K., Roysamb, E., Augusti, E. M. & Melinder, A. Behavior and inhibitory control in children with prenatal exposure to antidepressants and medically untreated depression. Psychopharmacology 233, 1523–1535, https://doi.org/10.1007/s00213-016-4248-3 (2016).
Wechsler, D. Wechsler Memory Scale, third edition. (The Psychological Corporation, 1997).
Hollingshead, A. B. Two factor index of social position. (1957).
Bernal-Rusiel, J. L. et al. Statistical analysis of longitudinal neuroimage data with Linear Mixed Effects models. Neuroimage 66, 249–260, https://doi.org/10.1016/j.neuroimage.2012.10.065 (2013).
Holm, S. A Simple Sequentially Rejective Multiple Test Procedure. Scand J Stat 6, 65–70 (1979).
Goodale, M. A. & Milner, A. D. Two visual pathways - Where have they taken us and where will they lead in future? Cortex; a journal devoted to the study of the nervous system and behavior 98, 283–292, https://doi.org/10.1016/j.cortex.2017.12.002 (2018).
Goodale, M. A. & Milner, A. D. Separate visual pathways for perception and action. Trends Neurosci 15, 20–25 (1992).
Hutchison, R. M. & Gallivan, J. P. Functional coupling between frontoparietal and occipitotemporal pathways during action and perception. Cortex; a journal devoted to the study of the nervous system and behavior 98, 8–27, https://doi.org/10.1016/j.cortex.2016.10.020 (2018).
Agyei, S. B., van der Weel, F. R. & van der Meer, A. L. Longitudinal study of preterm and full-term infants: High-density EEG analyses of cortical activity in response to visual motion. Neuropsychologia 84, 89–104, https://doi.org/10.1016/j.neuropsychologia.2016.02.001 (2016).
Atkinson, J. & Braddick, O. In Handbook of Growth and Growth Monitoring in Health and Disease (ed. Victor R. Preedy) 543-565 (Springer New York, 2012).
Skranes, J. et al. Cortical surface area and IQ in very-low-birth-weight (VLBW) young adults. Cortex; a journal devoted to the study of the nervous system and behavior 49, 2264–2271, https://doi.org/10.1016/j.cortex.2013.06.001 (2013).
Sripada, K. et al. Visual-motor deficits relate to altered gray and white matter in young adults born preterm with very low birth weight. Neuroimage 109, 493–504, https://doi.org/10.1016/j.neuroimage.2015.01.019 (2015).
Bjuland, K. J., Løhaugen, G. C., Martinussen, M. & Skranes, J. Cortical thickness and cognition in very-low-birth-weight late teenagers. Early Hum Dev 89, 371–380, https://doi.org/10.1016/j.earlhumdev.2012.12.003 (2013).
Løhaugen, G. C. et al. Small for gestational age and intrauterine growth restriction decreases cognitive function in young adults. J Pediatr 163, 447–453, https://doi.org/10.1016/j.jpeds.2013.01.060 (2013).
Østgård, H. F. et al. Executive function relates to surface area of frontal and temporal cortex in very-low-birth-weight late teenagers. Early Hum Dev 95, 47–53, https://doi.org/10.1016/j.earlhumdev.2016.01.023 (2016).
Skranes, J. et al. Entorhinal cortical thinning affects perceptual and cognitive functions in adolescents born preterm with very low birth weight (VLBW). Early Hum Dev 88, 103–109, https://doi.org/10.1016/j.earlhumdev.2011.07.017 (2012).
Farah, M. J. et al. Childhood poverty: Specific associations with neurocognitive development. Brain Research 1110, 166–174, https://doi.org/10.1016/j.brainres.2006.06.072 (2006).
Barkley, R. A. Executive functions: what they are, how they work, and why they evolved. (Guilford Press, 2012).
Rose, S. A., Feldman, J. F. & Jankowski, J. J. Modeling a cascade of effects: the role of speed and executive functioning in preterm/full-term differences in academic achievement. Developmental Sci 14, 1161–1175, https://doi.org/10.1111/j.1467-7687.2011.01068.x (2011).
Rathbone, R. et al. Perinatal cortical growth and childhood neurocognitive abilities. Neurology 77, 1510–1517, https://doi.org/10.1212/WNL.0b013e318233b215 (2011).
Brittain, P. J. et al. Neural compensation in adulthood following very preterm birth demonstrated during a visual paired associates learning task. Neuroimage Clin 6, 54–63, https://doi.org/10.1016/j.nicl.2014.08.009 (2014).
Everts, R. et al. Language lateralization correlates with verbal memory performance in children with focal epilepsy. Epilepsia 51, 627–638, https://doi.org/10.1111/j.1528-1167.2009.02406.x (2010).
Gimenez, M. et al. Hippocampal functional magnetic resonance imaging during a face-name learning task in adolescents with antecedents of prematurity. Neuroimage 25, 561–569, https://doi.org/10.1016/j.neuroimage.2004.10.046 (2005).
Gozzo, Y. et al. Alterations in neural connectivity in preterm children at school age. Neuroimage 48, 458–463, https://doi.org/10.1016/j.neuroimage.2009.06.046 (2009).
Lawrence, E. J. et al. The very preterm brain in young adulthood: the neural correlates of verbal paired associate learning. The Journal of pediatrics 156, 889–895, https://doi.org/10.1016/j.jpeds.2010.01.017 (2010).
Ment, L. R. & Constable, R. T. Injury and recovery in the developing brain: evidence from functional MRI studies of prematurely born children. Nat Clin Pract Neurol 3, 558–571, https://doi.org/10.1038/ncpneuro0616 (2007).
Murner-Lavanchy, I. et al. Visuospatial working memory in very preterm and term born children–impact of age and performance. Dev Cogn Neurosci 9, 106–116, https://doi.org/10.1016/j.dcn.2014.02.004 (2014).
Narberhaus, A. et al. Neural substrates of visual paired associates in young adults with a history of very preterm birth: alterations in fronto-parieto-occipital networks and caudate nucleus. Neuroimage 47, 1884–1893, https://doi.org/10.1016/j.neuroimage.2009.04.036 (2009).
Salvan, P. et al. Road work on memory lane–functional and structural alterations to the learning and memory circuit in adults born very preterm. Neuroimage 102(Pt 1), 152–161, https://doi.org/10.1016/j.neuroimage.2013.12.031 (2014).
Schafer, R. J. et al. Alterations in functional connectivity for language in prematurely born adolescents. Brain 132, 661–670, https://doi.org/10.1093/brain/awn353 (2009).
Fischi-Gomez, E. et al. Brain network characterization of high-risk preterm-born school-age children. Neuroimage Clin 11, 195–209, https://doi.org/10.1016/j.nicl.2016.02.001 (2016).
Pandit, A. S. et al. Whole-brain mapping of structural connectivity in infants reveals altered connection strength associated with growth and preterm birth. Cereb Cortex 24, 2324–2333, https://doi.org/10.1093/cercor/bht086 (2014).
Sur, M. & Rubenstein, J. L. R. Patterning and plasticity of the cerebral cortex. Science 310, 805–810, https://doi.org/10.1126/science.1112070 (2005).
Walhovd, K. B. et al. Neurodevelopmental origins of lifespan changes in brain and cognition. P Natl Acad Sci USA 113, 9357–9362, https://doi.org/10.1073/pnas.1524259113 (2016).
Rees, S., Harding, R. & Walker, D. The biological basis of injury and neuroprotection in the fetal and neonatal brain. Int J Dev Neurosci 29, 551–563, https://doi.org/10.1016/j.ijdevneu.2011.04.004 (2011).
Volpe, J. J. Neurology of the newborn. 5th edn, (Saunders/Elsevier, 2008).
Bouyssi-Kobar, M. et al. Third Trimester Brain Growth in Preterm Infants Compared With In Utero Healthy Fetuses. Pediatrics 138, https://doi.org/10.1542/peds.2016-1640 (2016).
Huttenlocher, P. R. & Dabholkar, A. S. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol 387, 167–178 (1997).
Shaw, P. et al. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci 28, 3586–3594, https://doi.org/10.1523/JNEUROSCI.5309-07.2008 (2008).
Wierenga, L. M., Langen, M., Oranje, B. & Durston, S. Unique developmental trajectories of cortical thickness and surface area. Neuroimage 87, 120–126, https://doi.org/10.1016/j.neuroimage.2013.11.010 (2014).
Hensch, T. K. Critical period plasticity in local cortical circuits. Nat Rev Neurosci 6, 877–888, https://doi.org/10.1038/nrn1787 (2005).
Stoll, B. J. et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993-2012. Jama-J Am Med Assoc 314, 1039–1051, https://doi.org/10.1001/jama.2015.10244 (2015).
Vollmer, B. et al. Correlation between white matter microstructure and executive functions suggests early developmental influence on long fibre tracts in preterm born adolescents. PloS one 12, ARTN e0178893 10.1371/journal.pone.0178893 (2017).
Hagberg, H. et al. The role of inflammation in perinatal brain injury. Nat Rev Neurol 11, 192–208, https://doi.org/10.1038/nrneurol.2015.13 (2015).
Volpe, J. J., Kinney, H. C., Jensen, F. E. & Rosenberg, P. A. The developing oligodendrocyte: key cellular target in brain injury in the premature infant. Int J Dev Neurosci 29, 423–440, https://doi.org/10.1016/j.ijdevneu.2011.02.012 (2011).
Cole, J. H. et al. Subregional Hippocampal Morphology and Psychiatric Outcome in Adolescents Who Were Born Very Preterm and at Term. PloS one 10, ARTN e0130094 10.1371/journal.pone.0130094 (2015).
Rogers, C. E. et al. Altered gray matter volume and school age anxiety in children born late preterm. The Journal of pediatrics 165, 928–935, https://doi.org/10.1016/j.jpeds.2014.06.063 (2014).
Thompson, D. K. et al. Longitudinal growth and morphology of the hippocampus through childhood: Impact of prematurity and implications for memory and learning. Human brain mapping 35, 4129–4139, https://doi.org/10.1002/hbm.22464 (2014).
Narberhaus, A. et al. Corpus callosum and prefrontal functions in adolescents with history of very preterm birth. Neuropsychologia 46, 111–116, https://doi.org/10.1016/j.neuropsychologia.2007.08.004 (2008).
Nosarti, C. et al. Corpus callosum size and very preterm birth: relationship to neuropsychological outcome. Brain 127, 2080–2089, https://doi.org/10.1093/brain/awh230 (2004).
Skranes, J. et al. Clinical findings and white matter abnormalities seen on diffusion tensor imaging in adolescents with very low birth weight. Brain 130, 654–666, https://doi.org/10.1093/brain/awm001 (2007).
Smyser, C. D. et al. Longitudinal analysis of neural network development in preterm infants. Cereb Cortex 20, 2852–2862, https://doi.org/10.1093/cercor/bhq035 (2010).
Kostovic, I. & Rakic, P. Developmental history of the transient subplate zone in the visual and somatosensory cortex of the macaque monkey and human brain. J Comp Neurol 297, 441–470, https://doi.org/10.1002/cne.902970309 (1990).
Kostovic, I., Kostovic-Srzentic, M., Benjak, V., Jovanov-Milosevic, N. & Rados, M. Developmental dynamics of radial vulnerability in the cerebral compartments in preterm infants and neonates. Front Neurol 5, 139, https://doi.org/10.3389/fneur.2014.00139 (2014).
Volpe, J. J. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol 8, 110–124, https://doi.org/10.1016/S1474-4422(08)70294-1 (2009).
Boardman, J. P. et al. Early growth in brain volume is preserved in the majority of preterm infants. Annals of Neurology 62, 185–192, https://doi.org/10.1002/ana.21171 (2007).
Grytten, J. et al. Saving Newborn Babies - the Benefits of Interventions in Neonatal Care in Norway over More Than 40 Years. Health Econ 26, 352–370, https://doi.org/10.1002/hec.3314 (2017).
Johnson, S. & Marlow, N. Early and long-term outcome of infants born extremely preterm. Arch Dis Child 102, 97–102, https://doi.org/10.1136/archdischild-2015-309581 (2017).
Olsen, A. et al. Preterm birth leads to hyper-reactive cognitive control processing and poor white matter organization in adulthood. Neuroimage 167, 419–428, https://doi.org/10.1016/j.neuroimage.2017.11.055 (2017).
Grunewaldt, K. H., Løhaugen, G. C., Austeng, D., Brubakk, A. M. & Skranes, J. Working memory training improves cognitive function in VLBW preschoolers. Pediatrics 131, e747–754, https://doi.org/10.1542/peds.2012-1965 (2013).
Løhaugen, G. C. et al. Computerized working memory training improves function in adolescents born at extremely low birth weight. J Pediatr 158, 555–561 e554, https://doi.org/10.1016/j.jpeds.2010.09.060 (2011).
Mathur, A. M., Neil, J. J. & Inder, T. E. Understanding brain injury and neurodevelopmental disabilities in the preterm infant: the evolving role of advanced magnetic resonance imaging. Semin Perinatol 34, 57–66, https://doi.org/10.1053/j.semperi.2009.10.006 (2010).
Ment, L. R., Hirtz, D. & Huppi, P. S. Imaging biomarkers of outcome in the developing preterm brain. Lancet Neurol 8, 1042–1055, https://doi.org/10.1016/S1474-4422(09)70257-1 (2009).
de Vries, L. S. & Volpe, J. J. Value of sequential MRI in preterm infants. Neurology 81, 2062–2063 (2013).
Brooks, B. L., Sherman, E. M. S. & Strauss, E. Test Review: Nepsy-Ii: A Developmental Neuropsychological Assessment, Second Edition. Child Neuropsychol 16, 80–101, https://doi.org/10.1080/09297040903146966 (2010).
Aylward, G. P. Neurodevelopmental Outcomes of Infants Born Prematurely. Journal of Developmental and Behavioral Pediatrics 35, 394–407, https://doi.org/10.1097/01.DBP.0000452240.39511.d4 (2014).
Volpe, J. J. Systemic inflammation, oligodendroglial maturation, and the encephalopathy of prematurity. Ann Neurol 70, 525–529, https://doi.org/10.1002/ana.22533 (2011).
Ullman, H., Almeida, R. & Klingberg, T. Structural maturation and brain activity predict future working memory capacity during childhood development. J Neurosci 34, 1592–1598, https://doi.org/10.1523/JNEUROSCI.0842-13.2014 (2014).
Woodward, L. J., Anderson, P. J., Austin, N. C., Howard, K. & Inder, T. E. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med 355, 685–694, https://doi.org/10.1056/NEJMoa053792 (2006).
Hoeft, F. et al. Neural systems predicting long-term outcome in dyslexia. Proceedings of the National Academy of Sciences of the United States of America 108, 361–366, https://doi.org/10.1073/pnas.1008950108 (2011).
Gabrieli, J. D., Ghosh, S. S. & Whitfield-Gabrieli, S. Prediction as a humanitarian and pragmatic contribution from human cognitive neuroscience. Neuron 85, 11–26, https://doi.org/10.1016/j.neuron.2014.10.047 (2015).
Yang, Y. et al. Genetic and environmental influences on cortical thickness among 14-year-old twins. Neuroreport 23, 702–706, https://doi.org/10.1097/WNR.0b013e328355a62a (2012).
Tamnes, C. K. et al. Brain maturation in adolescence and young adulthood: regional age-related changes in cortical thickness and white matter volume and microstructure. Cereb Cortex 20, 534–548, https://doi.org/10.1093/cercor/bhp118 (2010).
Vangberg, T. R. et al. Changes in white matter diffusion anisotropy in adolescents born prematurely. Neuroimage 32, 1538–1548, https://doi.org/10.1016/j.neuroimage.2006.04.230 (2006).
Wu, D. et al. Mapping the critical gestational age at birth that alters brain development in preterm-born infants using multi-modal MRI. Neuroimage 149, 33–43, https://doi.org/10.1016/j.neuroimage.2017.01.046 (2017).
Acknowledgements
We are thankful for the generous participation of the children and families in this study. We thank the late Jana Rydland, MD, for clinical radiological evaluation of the MRI scans and for her contributions to our research. We also thank radiographer Bjarte Dagfinn Snekvik for coordinating the imaging, and research assistants Irén Aina Bastholm and Elisabeth Mellemstrand Stavnes. We acknowledge Prof. Kristine B. Walhovd, Prof. Anders M. Fjell, Stine Kleppe Krogsrud, and Stine Bjørløw Dalsøren at the Center for Lifespan Changes in Brain and Cognition, Department of Psychology, University of Oslo (UiO), for their collaboration on this project. The Abel Cluster, owned by UiO and the Norwegian Metacenter for High Performance Computing (NOTUR) and operated by the Department for Research Computing at the UiO Center for Information Technology (http://www.hpc.uio.no), was used for image processing. This project was supported by the Research Council of Norway’s FRIMED program (project number 204935/F20) and the Liaison Committee for Education, Research and Innovation in Central Norway (project number 46056907).
Author information
Authors and Affiliations
Contributions
K.S., K.J.B. and L.M.R. carried out the neuroimaging analyses. K.S., K.J.B., A.E.S. and L.M.R. conducted quality control of neuroimaging and participant data. K.S., L.M.R., and G.C.L. contributed to clinical and cognitive data analysis. A.E.S., G.C.L. and K.H.G. recruited participants and collected data. J.S. and A.K.H. led the study planning and design in Trondheim. K.S. wrote the manuscript with input from all authors.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sripada, K., Bjuland, K.J., Sølsnes, A.E. et al. Trajectories of brain development in school-age children born preterm with very low birth weight. Sci Rep 8, 15553 (2018). https://doi.org/10.1038/s41598-018-33530-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-018-33530-8
Keywords
This article is cited by
-
Heterogeneous, temporally consistent, and plastic brain development after preterm birth
Nature Communications (2025)
-
Amygdala volumes and associations with socio-emotional competencies in preterm youth: cross-sectional and longitudinal data
Pediatric Research (2024)
-
Long-term impact of digital media on brain development in children
Scientific Reports (2024)
-
Association of perinatal factors with suspected developmental delay in urban children aged 1–36 months - a large-scale cross-sectional study in China
BMC Pediatrics (2023)
-
Correlation of NICU anthropometry in extremely preterm infants with brain development and language scores at early school age
Scientific Reports (2023)