Abstract
An efficient [4 + 1] annulation between α-bromooximes and sulfur ylides via in situ generation of nitrosoalkenes under mild basic reaction conditions has been developed, providing an expeditious and scalable approach to synthesize biologically interesting isoxazoline derivatives with good to excellent yields.
Similar content being viewed by others
Introduction
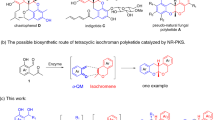
Nitrosoalkenes are considered as highly reactive and versatile synthetic intermediates in organic synthesis1,2,3,4,5. This is mainly due to easy access from available precursors: the base-mediated dehydrohalogenation of α-halooximes. As shown in Fig. 1, the most important synthetic application of this valuable intermediate is their use as an electron-deficient heterodiene to participate in [4 + 2] cycloaddition with electron-rich heterocycles or nucleophilic olefins (Fig. 1a)6,7 and as Michael-type acceptors in conjugate addition reactions (Fig. 1b)8, which are effective synthetic methodologies to 1,2-oxazines and open-chain oximes9,10.
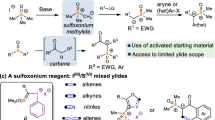
However, despite the impressive advances that have been achieved, to the best of our knowledge, the formation of biologically interesting five-membered heterocycles11 from the reaction of nitrosoalkene intermediates is much less studied12. In addition, [4 + 1] annulation strategy represents a straightforward protocol for the construction of five-membered rings13,14,15. Sulfur ylides are vital C1 units that have been widely applied for the construction of five-membered rings via [4 + 1] annulation reactions16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33. For example, the groups of Tang and Xiao independently reported the [4 + 1] annulation of nitroolefines and stable sulfur ylides to give isoxazoline derivatives17,18. Based on these elegant results, we imagined that if nitrosoalkenes could be a good annulation partner with sulfur ylides, since it can be easily generated in situ via the dehydrohalogenation of α-halooximes. In this regard, Bolm’s group recently reported the first example of copper-catalyzed asymmetric formal [4 + 1] cyclization of in situ generated azoalkenes with stable sulfur ylides to synthesize chiral dihydropyrazoles19,29. Subsequently, Xiao and co-workers have described highly efficient indole and indoline synthesis that relies on [4 + 1] reactions of stable sulfur ylides and aza-o-quinodimethane intermediate generated in situ20,21. As a part of our interest in the reactions with sulfur ylide14,25 and developing efficient synthetic protcols towards the construction of biologically interesting heterocycles34,35,36,37,38,39,40,41, we envision that sulfur ylides would be the perfect C1 units to react with nitrosoalkene intermediates generated from α-bromooximes to afford isoxazoline derivatives (Fig. 1c), which are significant motifs existing in pharmaceutical and medicinal chemistry42,43,44,45,46,47,48,49,50.
Results and discussion
We test our hypothesis with the easily prepared α-bromooxime 1a and the phenylacyl-substituted sulfur ylide 2a as model substrates. The initial experiment was carried out with potassium carbonate (1.2 equiv.) as base in CH2Cl2 at room temperature. Gratifyingly, the model reaction successfully took place and the desired product 3aa was obtained in a good yield of 71% (Table 1, entry 1). Intrigued by this preliminary result, we examined a variety of reaction parameters to improve product yield and the related key results are illustrated in Table 1. Firstly, the effect of the solvent for this transformation was investigated, and it was shown that CHCl3 was identified as an ideal medium for the generation of 3aa (Table 1, entries 1–5). Once an efficient solvent was found, crucial for the success of this reaction is the choice of a suitable base, which could promote the in situ generation of the nitrosoalkene intermediates. Therefore, a brief screening of the base was conducted (Table 1, entries 6–9). Fortunately, the yield was improved to 89% by use of sodium carbonate (Table 1, entry 6). Further adjusting the amount of the base and the substrate ratio increased the yield to 94% (Table 1, entries 10 and 11). Finally, we have tried to change the leaving group of the oxime but unfortunately, no satisfactory result was obtained (Table 1, entry 12).
With the optimal reaction conditions established (Table 1, entry 11), we started to examine the generality of this [4 + 1] annulation reaction (Fig. 2). Firstly, we explored the tolerance of the functional substitution of substrates 1. A broad range of α-bromooximes 1 underwent the annulation reaction to furnish products 3 in 72–99% yields. For instance, α-bromooximes substituted by either an electron-donating or -withdrawing group at the 4-position of benzene ring were well tolerated in this transformation, affording products with 72–94% yields (3aa-3fa). Substituents at the ortho or meta position on the benzene ring also react well (3ga, 90% yield and 3ha, 99% yield). Significantly, α-bromooximes with two CF3 substituents on the benzene ring also gave a good yield of 3ia. It is also worth noting that heteroaromatic.
α-bromooxime, such as furyl-substituted, was successfully applicable to the desired product 3ja. Furthermore, the scope of α-bromooximes could be smoothly extended to alkyl- and ester-substituted α-bromooximes to provide the corresponding products 3ka and 3la in 99% and 89% yield, respectively. Additionally, introduction of tetrahydronaphthalene in the α-bromooximes could proceed efficiently in the reaction to afford the tricyclic product 3ma in high yield and excellent diastereoselectivity.
Of equal significance is the observation that structural modification of the sulfur ylide substrate is possible. As depicted in Fig. 3, substrates with electron-donating groups (4-Me, 4-OMe) and inductive electron-deficient groups (4-Cl, 4-Br, 3-Br) on the benzene ring were proven to react smoothly with 1a, delivering the corresponding products 3ab-3af in generally high to excellent yields. Moreover, naphthol-derived and heteroaromatic acyl sulfur ylides were also applicable as appropriate substrates for this transformation as products 3ag and 3ah were provided in 94% yield and 76% yield, respectively. In addition, the reaction with alkylacyl-substituted sulfur ylide 2j proceeded successfully to afford the desired product in 51% yield.
Based on previous works about enantioselective [4 + 1] annulation reactions of (R)-BINOL derived chiral sulfur ylide13,16,21, we applied this strategy to examine the asymmetric version in our process. To our delight, the enantioselective reaction indeed worked and product 3ac was isolated with 47% ee, which could not be achieved with the corresponding precursor: (R)-BINOL derived chiral sulfonium salts due to the harsh reaction condition for the transformation from the related (R)-BINOL derived chiral sulfonium salts to sulfur ylide15,21. In an attempt to show the value of 2-isoxazolines as building blocks in synthesis, we tested a follow-up reduction reaction with Sodium borohydride in MeOH at room temperature. The desired product was obtained in 90% yield with 3:2 dr value after 20 min (Fig. 4a). A plausible mechanism for this [4 + 1] annulation reaction based on our experimental results and previous reports5,19,20,21 is illustrated in Fig. 4b. First, the nitrosoalkenes are generated in situ via a 1,4-elimination reaction under basic conditions. Then, a Michael addition occurs between the sulfur ylides 2 and the nitroalkenes, followed by an intramolecular O alkylation with loss of dimethylsulfide, delivering the final product 3.
Conclusion
In conclusion, we have reported an facile [4 + 1] annulation reaction between in situ formed nitrosoalkene intermediates and sulfur ylides via a Michael/intramolecular O alkylation sequence. The protocol provides a direct and efficient entry into valuable 2-isoxazoline derivatives in good to excellent yields. The reaction takes place under relatively mild and simple experimental conditions.
Methods
General procedure for the synthesis of isoxazoline derivatives 3: To a solution of sulfur ylide 2 (0.52 mmol) in trichloromethane (4.0 mL), Na2CO3 (0.52 mmol) was added and stirred at room temperature for 15 min. Then α-bromooxime 1 (0.40 mmol) was added at room temperature. The reaction was monitored via TLC (petroleum ether/ether = 10:3). Upon consumption of the starting materials, the reaction mixture was purified by flash chromatography on silica gel (petroleum ether/ether = 10:1) to give the desired product 3.
References
Gilchrist, T. L. Nitroso-alkenes & nitroso-alkynes. Chem. Soc. Rev. 12, 53–73 (1983).
Lyapkalo, I. M. & Ioffe, S. L. Conjugated nitrosoalkenes. Russ. Chem. Rev. 67, 467–484 (1998).
Reissig, H. U. & Zimmer, R. 1-Nitrosoalkenes. In Science of Synthesis Vol. 33 (ed. Molander, G. A.) 371–389 (Georg Thieme Verlag, Stuttgart, 2007).
Boyko, Y. D., Dorokhov, V. S., Sukhorukov, A. Y. & Ioffe, S. L. Conjugated nitrosoalkenes as Michael acceptors in carbon–carbon bond forming reactions: a review and perspective. Beilstein. J. Org. Chem. 13, 2214–2234 (2017).
Lopes, S. M. M., Cardoso, A. L., Lemos, A. & Pinho e Melo, T. M. V. D. Recent advances in the chemistry of conjugated nitrosoalkenes and azoalkenes. Chem. Rev. 118, 11324–11352 (2018).
Blond, G., Gulea, M. & Mamane, V. Recent contributions to hetero diels-alder reactions. Curr. Org. Chem. 20, 2161–2210 (2016).
Brachet, E. & Belmont, P. Inverse electron demand diels-alder (IEDDA) reactions: synthesis of heterocycles and natural products along with bioorthogonal and material sciences applications. Curr. Org. Chem. 20, 2136–2160 (2016).
Naumovich, Y. A., Sukhorukov, A. Y. & Ioffe, S. L. Michael addition of P-nucleophiles to conjugated nitrosoalkenes. J. Org. Chem. 84, 7244–7254 (2019).
Sukhorukov, A. Y. & Ioffe, S. L. Chemistry of six-membered cyclic oxime ethers. application in the synthesis of bioactive compounds. Chem. Rev. 111, 5004–5041 (2011).
Gilchrist, T. L. & Wood, J. E. 1,2-Oxazines and their benzo derivatives. In Comprehensive Heterocyclic Chemistry Vol. 6 (eds Rees, C. W. & Scriven, E. F. V.) 279–299 (Springer, Oxford, 1996) (Module 6.04).
Liu, X. et al. Recent developments in the synthesis of nitrogen-containing heterocycles through C–H/N–h bond functionalizations and oxidative cyclization. Synlett 30, 1026–1036 (2019).
Khairnar, P. V. & Lin, W. W. An intramolecular wittig approach toward heteroarenes: synthesis of pyrazoles, isoxazoles, and chromenone-oximes. Org. Lett. 21, 4219–4223 (2019).
Chen, J. R., Hu, X. Q., Lu, L. Q. & Xiao, W. J. Formal [4+1] annulation reactions in the synthesis of carbocyclic and heterocyclic systems. Chem. Rev. 115, 5301–5365 (2015).
Hua, T. B., Xiao, C., Yang, Q. Q. & Chen, J. R. Recent advances in asymmetric synthesis of 2-substituted indoline derivatives. Chin. Chem. Lett. 31, 311–323 (2020).
Zhao, S. et al. An efficient synthesis of 2-isoxazolines from α-haloketone oximes and dimethyl sulfonium salts. Tetrahedron Lett. 60, 382–385 (2019).
Lu, L. Q., Li, T. R., Wang, Q. & Xiao, W. J. Beyond sulfide-centric catalysis: recent advances in the catalytic cyclization reactions of sulfur ylides. Chem. Soc. Rev. 46, 4135–4149 (2017).
Zhu, C. Y., Deng, X. M., Sun, X. L., Zheng, J. C. & Tang, Y. Highly enantioselective synthesis of isoxazoline N-oxides. Chem. Commun. 6, 738–740 (2008).
Lu, L. Q. et al. A new entry to cascade organocatalysis: reactions of stable sulfur ylides and nitroolefins sequentially catalyzed by thiourea and DMAP. J. Am. Chem. Soc. 130, 6946–6948 (2008).
Chen, J. R. et al. Enantioselective synthesis of dihydropyrazoles by formal [4+1] cycloaddition of in situ-derived azoalkenes and sulfur ylides. J. Am. Chem. Soc. 134, 6924–6927 (2012).
Yang, Q. Q. et al. Synthesis of indoles through highly efficient cascade reactions of sulfur ylides and N-(ortho-chloromethyl)aryl amides. Angew. Chem. Int. Ed. 51, 9137–9140 (2012).
Yang, Q. Q. et al. Construction of optically active indolines by formal [4+1] annulation of sulfur ylides and N-(ortho-chloromethyl)aryl amides. Chem. Eur. J. 19, 8401–8404 (2013).
Rousseau, O. et al. Formal asymmetric [4+1] annulation reaction between sulfur ylides and 1,3-dienes. Chem. Eur. J. 21, 12899–12902 (2015).
Wang, Q. et al. Catalytic asymmetric [4+1] annulation of sulfur ylides with copper-allenylidene intermediates. J. Am. Chem. Soc. 138, 8360–8365 (2016).
Wang, Q. et al. Iron-catalyzed decarboxylative [4+1] cycloadditions: exploiting the reactivity of ambident iron-stabilized intermediates. Angew. Chem. Int. Ed. 55, 2840–2844 (2016).
Yang, Q. Q. & Xiao, W. J. Catalytic asymmetric synthesis of chiral dihydrobenzofurans through a formal [4+1] annulation reaction of sulfur ylides and in situ generated ortho-quinone methides. Eur. J. Org. Chem. 2017, 233–236 (2017).
Li, C., Jiang, K., Liu, T. Y. & Chen, Y. C. Asymmetric [4+1] cycloadditions of N-thioacylimines and sulfur ylides. Adv. Synth. Catal. 359, 2530–2534 (2017).
Cheng, B. Y. & Lu, L. Q. Synthesis of polysubstituted pyrroles through a formal [4+1] cycloaddition/E1cb elimination/aromatization sequence of sulfur ylides and α, β-unsaturated imines. J. Org. Chem. 82, 12134–12140 (2017).
Liu, Y. Y. & Chen, J. R. Visible light-driven aza-ortho-quinone methide generation enables a multicomponent reaction. Angew. Chem. Int. Ed. 56, 9527–9531 (2017).
Wang, Z. Y., Yang, Y. Z., Gao, F., Luo, Q. & Fang, L. Synthesis of 5-(trifluoromethyl)pyrazolines by formal [4 + 1]-annulation of fluorinated sulfur ylides and azoalkenes. Org. Lett. 20, 934–937 (2018).
Clergue, S. et al. Asymmetric sulfur-ylide-mediated formal [4+1]-annulation reaction: scope and mechanism. Chem. Eur. J. 24, 11417–11425 (2018).
Zhi, Y., Zhao, K., Essen, C. V., Rissanen, K. & Enders, D. Synthesis of trans -disubstituted-2,3-dihydrobenzofurans by a formal [4 + 1] annulation between para-quinone methides and sulfonium salts. Org. Chem. Front. 5, 1348–1351 (2018).
Zhou, F., Chen, J. R., Cheng, Y., Liu, X. P. & Xiao, W. J. A visible light photoredox catalyzed carbon radical-mediated generation of ortho-quinone methides for 2,3-dihydrobenzofuran synthesis. Chem. Commun. 55, 3117–3120 (2019).
He, X. W. et al. Catalyst-free synthesis of 2,3-dihydrobenzofurans via a formal [4+1] annulation of propargylamines with sulfur ylides. J. Org. Chem. 84, 11623–11638 (2019).
Liu, M. G., Liu, N., Xu, W. H. & Wang, L. Tandem reaction strategy of the Passerini/Wittig reaction based on the in situ capture of isocyanides: one-pot synthesis of heterocycles. Tetrahedron 75, 2748–2754 (2019).
Liu, N. et al. Odorless isocyanide chemistry: one-pot synthesis of heterocycles via the Passerini and postmodification tandem reaction based on the in situ capture of isocyanides. J. Org. Chem. 84, 2366–2371 (2019).
He, X. K., Cai, B. G., Yang, Q. Q., Wang, L. & Xuan, J. Visible light-promoted cascade radical cyclization: synthesis of 1,4-diketones containing chroman-one skeletons. Chem. Asian. J. 14, 3269–3273 (2019).
Wang, L. et al. Catalytic aza-Wittig reaction of acid anhydride for the synthesis of 4H-Benzo[d][1,3]oxazin-4-ones and 4-benzylidene-2-aryloxazol-5(4H)-ones. ACS Catal. 6, 4010–4016 (2016).
Hua, T.-B., Yang, Q.-Q. & Zou, Y.-Q. Recent advances in enantioselective photochemical reactions of stabilized diazo compounds. Molecules 24, 3191 (2019).
Yao, W. et al. Retracted: synthesis of 2-arylisoindoline derivatives catalyzed by reusable 1,2,4-triazole iridium on mesoporous silica through a cascade borrowing hydrogen strategy. Chem. Eur. J. 25, 16099–16105 (2019).
Wang, L. et al. Disulfide-directed C–H hydroxylation for synthesis of sulfonyl diphenyl sulfides and 2-(phenylthio)phenols with oxygen as oxidant. Adv. Synth. Catal. 359, 779–785 (2017).
Xie, Y. B. et al. Brønsted-acid-catalyzed multicomponent one-pot reaction: efficient synthesis of polysubstituted 1,2-dihydropyridines. Asian J. Org. Chem. 6, 746–750 (2017).
Kozikowski, A. P. The isoxazoline route to the molecules of nature. Acc. Chem. Res. 17, 410–416 (1984).
Diana, G. D. et al. Isoxazoles with antipicornavirus activity. J. Med. Chem. 28, 748–752 (1985).
Kiran Kumar, A. B. V., Uma Ravi Sankar, A. & Kim, S. H. A simple, efficient one-pot synthesis of 2-isoxazoline derivatives and their antimicrobial activity. J. Heterocycl. Chem. 51, E146–E151 (2014).
Prajapti, S. K. et al. Synthesis and biological evaluation of novel Δ2-isoxazoline fused cyclopentane derivatives as potential antimicrobial and anticancer agents. Med. Chem. Commun. 6, 839–845 (2015).
Antczak, C. et al. A new acivicin prodrug designed for tumor-targeted delivery. Bioorg. Med. Chem. 9, 2843–2848 (2001).
Li, C. L. et al. Palladium-catalyzed synthesis of Δ2-isoxazoline from toluene derivatives enabled by the triple role of silver nitrate. Org. Lett. 17, 5718–5721 (2015).
Geng, C. W. et al. Palladium catalyzed C–H bond acetoxylation: isoxazolinyl as a directing group. RSC Adv. 6, 56971–56976 (2016).
Pan, Z. B. et al. Copper-catalyzed annulation reaction of alkenes and N-alkyl(aryl)-1-(methylthio)-2-nitroethenamine: an approach for the synthesis of isoxazole derivatives. J. Org. Chem. 85, 3364–3373 (2020).
Pavel, Y. U. et al. Synthesis of isoxazolines from nitroalkanes via a [4+1]-annulation strategy. Adv. Synth. Catal. 361, 5322–5327 (2019).
Acknowledgements
We are grateful to China Three Gorges University, the National Natural Science Foundation of China (No. 21702121), Open fund from Hubei Key Laboratory of Natural Products Research and Development of China Three Gorges University (No. NPRD-2018010) and the 111 project (D20015) for support of this research.
Author information
Authors and Affiliations
Contributions
Q.-Q.Y. wrote the main manuscript text. C.-X.L. dealt with the data analysis and T.-B.H. prepared tables and schemes. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hua, TB., Liu, CX., Hu, WM. et al. Mild synthesis of isoxazoline derivatives via an efficient [4 + 1] annulation reaction of transient nitrosoalkenes and sulfur ylides. Sci Rep 11, 2078 (2021). https://doi.org/10.1038/s41598-021-81370-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-81370-w