Abstract
Climate models predict an increase in extent, frequency, and duration of marine hypoxia events in the twenty first century. A better understanding of organismal responses to hypoxia in individual species is a crucial step for predicting ecosystem responses. We experimentally subjected a common invertebrate, the bearded fireworm (Hermodice carunculata) to two levels of chronic hypoxia and, in a separate experiment, to intermittent hypoxia. We found components of the conserved hypoxia-inducible factor (HIF) pathway and show a modulated response to hypoxia depending on the severity of hypoxic stress: under mild hypoxia, only the HIF-1α subunit is upregulated, while expression of the other subunit, aryl hydrocarbon nuclear translator, only increases significantly at more severe hypoxia levels. The chronic trials revealed down-regulation of genes related to cell adhesion, transport, development and heme-binding, and up-regulation of genes related to glycolysis, oxygen binding, cell differentiation, digestive and reproductive function. The intermittent hypoxia trials revealed an upregulation of heme transporter activity during hypoxia, and our time series analysis characterized nine clusters of genes with similar expression patterns. Our findings suggest that H. carunculata is likely to tolerate, and be resilient to, predicted future hypoxia conditions.
Similar content being viewed by others
Introduction
Low dissolved oxygen (DO), or hypoxia, events are increasing in area, frequency and duration as the oceans continue to warm and anthropogenic nutrient runoff to coastal ecosystems continues1,2,3. Hypoxia has recently come into focus as a stressor in coral reef ecosystems1,4,5,6 which are already severely decimated by coral disease, sedimentation, reduced herbivory, ocean acidification and rising temperatures7,8. Understanding how marine species respond to environmental stressors, including hypoxia, is crucial for predicting ecosystem-level responses (e.g.9,10,11). When marine hypoxia events occur, size and mobility of animals play important roles in their response12. Large mobile organisms may simply move towards normoxia (> 5 mg O2 L−1) when DO is lowered (e.g.10,13,14,15), while small sediment-dwelling organisms may crawl to the surface (e.g.16,17). Sessile or slow-moving animals have limited options to endure or escape hypoxia18,19,20,21 and their survival depends on physiological, and sometimes morphological responses, to cope with low oxygen levels.
Hypoxia is often defined as dissolved oxygen (DO) levels below 2 ml O2 L−122,23 but this threshold is arbitrary, as different organisms exhibit different sensitivities to diminishing O2 and even slightly reduced oxygen levels can negatively impact some organisms24. The term “hypoxic” is context-specific and can either refer to the level at which an organism responds to low oxygen conditions or to the environment in which the organism resides. For the purpose of this study, we characterize DO concentrations of 4.5 mg O2 L−1 as Mild hypoxia25,26, 2.5 mg O2 L−1 as Moderate hypoxia, and 1 mg O2 L−1 as Severe hypoxia. Our previous studies indicate that previous exposure to the Moderate hypoxia increases H. carunculata’s metabolic rate in the long-term27.
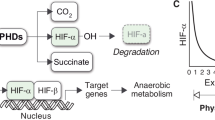
Throughout metazoans, hypoxia responses are triggered by the conserved hypoxia inducible factor (HIF) pathway28,29 which activates downstream processes (e.g. blood vessel formation, metabolic depression, etc.)21,30,31,32. The HIF transcription factor consists of two constitutively expressed subunits known as HIF-1α and aryl hydrocarbon nuclear translator (ARNT) or HIF-1β (Fig. 1a). Under normal oxygen conditions, HIF-1α is unstable and a functional transcription factor cannot form. HIF-1α is hydroxylated at one of two proline residues by the oxygen-dependent enzymes proline hydroxylase (PHD) or factor inhibiting HIF-1α hydroxylation (FIH)31. The Van-Hippel-Landau protein (VHL) binds to the hydroxylation site and leads to the addition of a poly-ubiquitin tail to HIF-1α, marking it for proteasomal degradation. Under hypoxic conditions, HIF-1α is not hydroxylated and forms a heterodimer with the ARNT and relocates from the cytoplasm to the nucleus as a functional transcription factor to trigger downstream responses31.
(a) HIF-1 pathway under normoxic (left) and hypoxic conditions (right). HSP90 is a heat shock protein that stabilizes HIF1-α until (under normoxic conditions) O2 facilitates the hydroxylation of HIF-1α at two proline residues with proline hydroxylase (PHD) and one asparagine residue with factor inhibiting HIF-1α hydroxylation (FIH) (created and modified from Liu and Semenza 2007). (b) Anterior end of the bearded fireworm (Hermodice carunculata) showing the primary tissues for oxygen-uptake which are the red branching branchia (similar to gills or lungs). Photo by CJ Grimes.
Hermodice carunculata (Pallas, 1766) is an amphinomid annelid (Fig. 1b) which is abundant and widespread throughout the tropical and subtropical Atlantic. It exhibits high tolerance to environmental extremes (reviewed in33) and is usually regarded as a nuisance species in coral reefs, as it feeds on live corals, leaving distinctive feeding scars34,35 and hindering coral reef restoration efforts36,37. Although the bearded fireworm is motile, its slow speed and limited movement range are likely to impede its escape from a large-area hypoxic event. In captivity, this amphinomid climbs toward the surface of the water when DO is lowered, either by crawling up the wall of aquaria or onto rock surfaces38,39. Recently, Yáñez-Rivera and Salazar-Vallejo34 proposed the existence of two species: H. carunculata in the Western Atlantic and H. nigrolineata Iin the Eastern Atlantic, and the distinction between the two was based primarily on the number of branchial filaments40. However, based on molecular evidence41 and phenotypic plasticity of branchiae in response to DO conditions38,39, H. nigrolineata is now considered a junior synonym to H. carunculata.
Among marine invertebrates, gene expression in response to hypoxia has been particularly well studied in crustaceans (e.g.42,43,44,45) and bivalves (e.g.46,47,48,49) due to their ecological and economic importance. In the Pacific oyster, Crassostrea gigas (Thunberg 1793), hypoxia exposure led to increased stress response, glutamine synthetase and metallothionein concentrations50, and an overexpression of genes related to respiration, metabolism, and immune system51. To date, few gene expression studies in response to hypoxia have been performed on annelids. Recently, Ogino and Toyohara17 showed that the sediment-dwelling Capitella teleta expresses TRP1A, a hypoxia-sensor which identifies the severity of hypoxic conditions to determine whether or not to trigger a behavioral response. The hydrothermal vent-inhabiting Paralvinella sp. up-regulates extracellular hemoglobin but down-regulates intracellular hemoglobin under hypoxia52,53. An increased production of extracellular hemoglobin may provide a more efficient way to bind and transport the limited oxygen than intracellular hemoglobin which appears to be more important for longer-term oxygen storage.
Intermittent hypoxia occurs in common habitats of H. carunculata, such as tide pools54,55 and on coral reefs1,56, while chronic hypoxia is less common in their typical habitat due to algae and coral symbiont primary productivity during the day4. However, based on climate change models, chronic hypoxic events are more likely in the future1,2,3,57. In addition, due to unique physiological and behavioral responses at different levels of hypoxia25,26, gene expression associated with chronic Mild and Moderate DO should be investigated to understand organismal response. While it has been suggested that amphinomids may thrive in lowered DO conditions 58,59,60, no studies have yet determined direct gene expression responses to this stressor.
Previous work has focused on morphological changes and oxygen uptake in H. carunculata in response to hypoxia27,38,39. Most notably, branchial surface area increases in response to both intermittent38 and chronic39 hypoxia. While the effect on oxygen uptake rates during hypoxia is small27,38, hypoxia exposure results in a long-term elevation of the metabolic rate, potentially leading to increased feeding activity27. Differential gene expression will help detect additional physiological processes that affect overall organismal functioning and find explanations for the environmental hardiness of H. carunculata. The objectives of this study were to characterize gene expression responses in H. carunculata (1) under chronic Mild and Moderate hypoxia (as defined above) and (2) under severe intermittent hypoxia. Under chronic hypoxia, we hypothesize that hypoxic response genes (e.g. HIF1-α, ARNT, VHL) and metabolic genes associated with glycolysis will be up-regulated as it is an anaerobic process32,48. Given documented physiological responses to chronic vs intermittent hypoxia, we can expect differences in gene expression patterns as well especially with regards to oxidative stress61,62. When organisms undergo intermittent hypoxia, they must be able to handle times of low oxygen and high oxygen when the reactive oxygen species increase which threaten cell health and structure which can result from the formation of reactive oxygen species under hypoxia or upon reoxygenation61,62. For intermittent hypoxia, we predict an up-regulation of aerobic metabolism genes after normoxic timepoints and behavioral response genes (such as TRP1A) as in Capitella teleta17 after hypoxic timepoints.
Methods
Experimental animals
Bearded fireworms were collected in October of 2016 and November 2017, respectively, from Riviera Beach, Florida (26.783703, − 80.044643). The animals were kept at their native environmental conditions (35 ppt, 20–23 °C, and 6.80–7.25 mg O2 L−1) in the Sea Life Facility (SLF) at Texas A&M University at Galveston prior to the experiments. They were fed 0.5 g of Loligo spp. (squid) or penaeid shrimp by hand once per week and their consumption was observed. No significant difference in weights was observed between treatments for the chronic (2.7–8.2 g) or intermittent (2.3–12 g) trials. All worms survived the experimental trials. We additionally used 2 samples from Praia do Forte, Bahia, Brazil (− 12.559757, − 37.991274) and 1 from the Florida location (26.783703, − 80.044643) to assemble the super-transcriptome. These field-collected samples consisted of the amputated posterior ends (~ 20 segments) preserved in RNAlater and kept refrigerated until they could be transferred to a − 80 °C freezer.
Hypoxia trials
Throughout the trials, the worms were not fed to reduce ammonia accumulation and decomposition. DO levels were controlled by the influx of nitrogen gas (Radnor nitrogen from Airgas Welding Supplies, TX, USA) and controlled with Neptune System’s Apex Jr. Controller. See 33 for more detailed descriptions and a diagram of the experimental setup. For the chronic hypoxia exposure, the DO was dropped over 48 h to desired levels for Mild and Moderate DO conditions (4.5 ± 0.25 mg O2 L−1 [64% of normoxia level] and 2.5 ± 0.25 mg O2 L−1 [36% of normoxia level], respectively). The temperature in the aquaria tanks were maintained at 20.1 ± 1.5 °C. Normoxic conditions were monitored and held steady (7.0 ± 0.25 mg O2 L−1) with a YSI Model 550A. Fireworms (n = 3 for each treatment) were sampled at the end of the 7-day trials by amputation of the posterior ~ 20 segments which were stored in RNAlater for transcriptome sequencing as described below. For the intermittent hypoxia exposure, the DO was dropped 1 mg O2 L−1 per 45 min intervals until severe hypoxia (1 mg O2 L−1 or 14% of normoxia level) was reached (Fig. 2). This DO level was then held stable for 6 h to mimic DO levels in tide pools. Fireworms (n = 3) were sampled at each time point (time point 1: 6 h [hypoxic]; time point 2: 18 h [normoxic]; time point 3: 24 h [hypoxic]; time point 4: 42 h [normoxic]) then flash frozen for later extractions and sequencing.
RNA isolation and RNA-seq
The posterior ends from the chronic hypoxia exposure and field-collected samples were stored in RNAlater in − 80 °C until further analysis. For total RNA extraction, the tissues (n = 3 per treatment and timepoint) were homogenized with the TissueRuptor II, followed by extraction with the Qiagen RNeasy kit (Qiagen: Catalog no 74104). Tissues from the intermittent hypoxia experiments were flash frozen. For all samples, RNA was extracted from the entire homogenized fragment, without regard for potential differences among different tissue types. Total RNA was extracted using the TRIzol protocol (Life Technologies: Cat No. 15596026), followed by the Qiagen RNeasy clean up. Total RNA was quantified and quality-checked with Qubit RNA High Sensitivity (HS) Assay and SYBR Gold Nucleic Acid Gel Stain, respectively. Illumina TruSeq RNA double-stranded libraries were prepared and sequenced at Texas A&M University’s Agrilife Research Center on the Illumina NovaSeq 6000 (College Station, Texas) with paired end- 150 bp quality controlled with Fragment Analyzer through PROSize 2.0.
Transcriptome assembly, annotation, and gene-level estimates
To account for all of the possible transcripts that could have been expressed under varying levels of DO, we assembled and annotated a “super” transcriptome with the sequences (N = 11) from the Florida collections, Brazilian collections, and the chronic hypoxia studies with default Trinity63 parameters and following the Trinotate pipeline (version 3.0.2)64, respectively. Proteins were predicted with TransDecoder (version 3.0.1) using default parameters65 and compared to Swiss-Prot database using NCBI BLAST + (version 2.7.1). BLASTX were conducted on contigs of the super transcriptome against non-redundant nucleotide database from UniProt (downloaded 17 June 2017). The sequences were pseudoaligned with kallisto (version 0.46.1) which identifies k-mer (given set of nucleotides) compatibility between the samples (instead of true nucleotide alignment) compared to the reference transcriptome which has been used to classify transcripts from k-mer associations in the de Bruijn graph66. Then, kallisto produces transcript counts for each sample which were then converted to gene counts with tximport (version 1.10.1)67 and analyzed for differential gene expression with edgeR (version 3.24.3)68. We used the TMM (trimmed-mean of M values) method for normalization of reads and CPM (counts per million) filter based on library sizes according to the edgeR protocol68. We also analyzed the transcripts abundances in edgeR (according to the same protocol) for comparison of genes with transcripts due to the issues with detecting true isoforms from chimeras and gene fragments when utilizing a reference transcriptome instead of a reference genome. Genes and transcripts were analyzed for differential expression with the Generalized Linear Model statistical test which is robust to low replicate numbers. Log fold change (FC) values ≥ |1| were identified as differentially expressed features (DEFs) (genes [DEGs] and transcripts [DETs]) with a false discovery rate (FDR) p value < 0.05. The intermittent hypoxia samples were analyzed for time course expression analysis at 4 timepoints (time point 1: 6 h [hypoxic]; time point 2: 18 h [normoxic]; time point 3: 24 h [hypoxic]; time point 4: 42 h [normoxic]) using maSigPro69,70,71 which clusters differentially expressed transcripts/genes into 9 groups that have similar expression profiles across the time points. This program uses a two-step regression analysis to (1) select differentially expressed genes with a global model then (2) identify statistically significant differences in expression between treatments (p < 0.05 and R > 0.7)69. Data available on FigShare at https://figshare.com/s/700e408ff5beae60225e.
Results
Preliminary investigation of H. carunculata’s transcriptome revealed homologs necessary for hypoxic response (such as HIF-1α, ARNT, VHL, TRP1A, PHD, etc.). The super transcriptome was analyzed for estimated completeness (94.7%) with a BUSCO analysis (Eukarya—S: 61.7%, D: 33.0%, F: 5.3%, M: 0.0%, n: 303). The number of total Trinity transcripts and subsequent genes are shown in Table 1 from 23 samples of H. carunculata with 3 biological replicates for per treatment (except Normal during chronic hypoxia = 2). Contigs with BLAST hits corresponding to Viruses, Bacteria, or Archaea were removed from this analysis. During chronic hypoxia, 122 DEGs and 587 DETs were down-regulated while 112 DEGs and 405 DETs were up-regulated (Fig. 3a–d).
Differentially expressed genes (a, b) and transcripts (c, d) from chronic hypoxia treatments. On the left, we compared the chronic hypoxia treatments (a, c), and on the right, (b, d) those worms in captivity for the chronic hypoxia trial were compared to those sampled from the natural environment (Brazil and Florida).
Chronic hypoxia trials
The highest number of DEGs (89 genes) occurred in the Mild vs. Moderate comparison, followed by Normal vs Moderate (32 genes). A unique set of 5 genes were differentially expressed in the Normal vs. Mild comparison (Fig. 3a). When comparing fireworms from the lab to field-collected worms, the number of DEGs progressed with increasing levels of hypoxia (Normal [6] < Mild [64] < Moderate [70]) (Fig. 3b). The DEFs between organisms in laboratory trials and those from the field largely consisted of metabolism-associated features and unclassified sequences.
Anaerobic metabolism genes associated with glycolysis were up-regulated in the chronic hypoxia treatments (Tables 2, 3) as opposed to those associated with cell adhesion, transport, and development which were down-regulated. There was also a significant up-regulation of HIF-1α in the Mild DO treatment compared to Normal and Moderate DO treatments, and an up-regulation of ARNT in the Moderate DO treatment compared to Normal (Tables 3, 4, Fig. 5). Heme-binding protein (CP3AD) was down-regulated in the Moderate treatment while oxygen-binding (extracellular hemoglobin) was up-regulated. However, there was a decrease in the expression of metabolic genes and an increase in those associated with cell differentiation, digestive tract, and reproductive system development.
Intermittent hypoxia trials
The inclusion of timepoints in the pairwise differential expression resulted in 0 DEGs and 65 DETs up-regulated compared with 3 DEGs and 67 DETs down-regulated (Fig. 4). There was a distinction between hypoxia and normoxia conditions, but timepoints within treatments were not separated in the dendrogram (Fig. 4, top). The majority of DEFs between conditions serve metabolic functions, nucleotide binding, GTPase, and polymerase activity. However, heme-transporter activity (proton-coupled folate transporter) was down-regulated at the hypoxic timepoints and protein ubiquitination was down-regulated at the normoxic timepoints.
Heatmap of the top 44 differentially expressed genes for each sample after the intermittent hypoxia trial with the treatments and timepoints. Values within the heatmap are counts per million (CPM) that that have been selected as differentially expressed based on log fold-change (FC) values with a ± 1 cutoff. Time point 1: 6 h (hypoxic); time point 2: 18 h (normoxic); time point 3: 24 h (hypoxic); time point 4: 42 h (normoxic).
Our timepoint analysis for intermittent hypoxia revealed 9 DEF clusters with characteristic expression profiles (Table 5). In clusters 3, 4, and 7, expression patterns followed similar trends for the normoxic and hypoxic timepoints (line trends in Table 5); however, DEFs in cluster 4 had higher expression levels under normoxia, whereas DEFs in clusters 3 and 7 were more expressed under normoxia. Cluster 1 contained genes associated with epithelial cell morphogenesis and metabolism and were expressed at higher levels after the first timepoint of hypoxia but decreased to around normal levels after the second 6-h hypoxia block (timepoint 3). Cluster 2 genes, consisting primarily of heme-binding, metabolism, and translation features, increased after both hypoxic and normoxic timepoints; however, the increase at hypoxia timepoints was more drastic. Genes in cluster 3 were expressed at higher levels in hypoxic timepoints consisting of increased heme-binding affinity and increased production of red blood cells. We did not see any differential expression of HIFs in intermittent hypoxia; however, we did see an initial decrease in expression of Usp19 (Ubiquitin carboxyl-terminal hydrolase 19) after hypoxia timepoint 1 compared to normoxia timepoint 2 but equally expressed after timepoints 3 and 4 (Cluster 5). Genes associated with embryonic development, cell proliferation, and angiogenesis were up-regulated during hypoxia (Cluster 7). The largest slope increase and decrease in expression trends occurred in cluster 8 for hypoxic and normoxic timepoints, respectively. The large positive slope associated with cluster 8 under hypoxia, nearly doubles in expression between to the two timepoints. Cluster 8 genes had inverse expression trends between hypoxic and normoxic timepoints and contained genes associated with epidermal growth factor-, actin-, and Notch-binding.
Discussion
Our chronic trials revealed down-regulation of genes related to cell adhesion, transport, development and heme-binding, and up-regulation of genes related to glycolysis, oxygen binding, cell differentiation, digestive, and reproductive function under hypoxic conditions. The overall up-regulation of DEFs associated with metabolic functions in the Moderate DO fireworms indicates a stress response through shifted metabolism in response to chronic hypoxia from aerobic to anaerobic (Tables 2, 3). We found differential expression of metabolic genes between Mild and Moderate treatments indicating modulated responses (e.g. down-regulation of aerobic metabolic pathways to hypoxic exposure based on level of severity) in Table 4. For example, we saw a down-regulation of ATP synthase and cytochrome c oxidase, key members of the electron transport (Table 4). This response has been shown in several invertebrate species and shows that fireworms have a similar physiological response to hypoxia as the Pacific oyster, Crassostrea gigas, and the water flea, Daphnia magna42,48,51.
The two subunits of the HIF transcription factor, HIF-1α and ARNT, are constitutively expressed, even under normoxic conditions, but a functional transcription factor only forms under hypoxia when the oxygen-dependent degradation of HIF-1α is disrupted. Once dimerized, HIF triggers downstream hypoxia responses in the organism21,30,31,32. However, few studies have examined the differential expression of the two HIF subunits in response to different levels of hypoxia. We found that both HIF-1α and ARNT are upregulated under Mild hypoxia (Fig. 5), although the upregulation was only significant for HIF-1α. Under Moderate hypoxia, only ARNT is significantly upregulated whereas HIF-1α expression decreases. The increase in ARNT expression from Normal to Mild to Moderate chronic hypoxia (Fig. 5) further indicates a modulated response to chronic hypoxia exposure dependent upon DO level. This implies that downstream hypoxia responses may not only be triggered through oxygen-dependent degradation of HIF-1α, but possibly also through a modulation of expression levels depending on DO levels. The higher expression of HIF-1α after Mild chronic hypoxia but return to normal expression at moderate levels could indicate yet unknown underlying acclimatory responses to this chronic condition.
As in Paralvinella sp.52,53, extracellular hemoglobin was up-regulated in the fireworms after Moderate chronic hypoxia compared to Mild hypoxia (Table 4). Another invertebrate blood pigment, hemocyanin, has putatively been found in the transcriptomes of a closely related fireworm, Paramphinome jeffreysii72, and was down-regulated in the bearded fireworm after Moderate chronic hypoxia compared to normoxic conditions. The increased production of extracellular hemoglobin and decreased production of hemocyanin in Moderate hypoxia suggests that the two pigments are optimized for different DO levels in the blood; however, a more targeted study is necessary to understand this relationship.
Our control DO levels were similar to prevailing conditions around the time of capture of the field-collected specimens; consequently, the fewest DEFs were found between the Normal treatment and the field-collected worms. Also, the down-regulation of certain metabolic genes associated with aerobic metabolism when comparing the animals exposed to lowered DO conditions to those of their natural habitat indicates the overall metabolic depression of worms exposed to chronic hypoxia. These results therefore support a previous study demonstrating lowered oxygen uptake in chronic hypoxic fireworms27.
When analyzing the top DEFs during the intermittent hypoxia trials, no clear separation of timepoints within the hypoxic treatment was obvious, but there was a distinction between the normoxic timepoints (Fig. 4, top left and right, respectively). While most of the differentially expressed genes in the Fig. 4 remained unclassified, a few in the upper right section (expressed more after normoxic timepoints) were putatively characterized as genes related to aerobic metabolism and cell adhesion, such as Cytochrome c oxidase subunit 1 (COX1) and periostin. However, the time course cluster analysis revealed some expression trends depending on time points (Table 5). The worms used in this study had likely never been exposed to hypoxia before, so it is not surprising that we see different expression trends at timepoint 1 and 3. The increase in expression of genes related to epithelial morphogenesis after 6 h (Table 5) may be the first sign of increasing branchial filament formation in this species to cope with hypoxic conditions, which occurs both under intermittent38 and chronic hypoxia39. Although fireworms most likely cannot increase their branchial surface area within six hours, this short time may be sufficient to trigger the onset of branchial tissue formation.
The consistent higher expression of hematopoietic prostaglandin D synthase (Cluster 3) after the hypoxic timepoints suggests physiological responses related to vasodilation and inhibition of clotting so that blood transporting oxygen can flow more freely73. This short-term response to intermittent hypoxic stress may increase blood flow and oxygen transport to maintain necessary metabolic functions during times of lowered oxygen conditions.
Ubiquitin-specific protease 19 (Usp19) protects HIF-1α from degradation and is required for typical hypoxia response in vertebrates74,75. If Usp19 is absent under oxygen depletion, HIF-1α will follow an oxygen-independent pathway to degradation not shown in Fig. 1a75,76. Usp19 interacts with the N-terminus of HIF-1α which is also where the chaperone, Heat shock protein 90 (Hsp90), associates to stabilize HIF-1α (Fig. 1a). In our experiment, Usp19 was initially less expressed after hypoxic timepoint 1 compared to normoxia (Cluster 5 in Table 5), possibly indicating the destabilization of the HIF-1α chaperone which may allow it to dimerize with ARNT more rapidly. However, Usp19’s similar expression level at timepoints 3 and 4 suggests it has other roles under normoxic conditions as well. Typically, under hypoxic conditions, HIF-1α is protected from proteasomal degradation by Usp19, but the expression trends (higher expression after first hypoxic exposure) here suggest there may be another protective mechanism for HIF-1α in the fireworm and potentially other animals.
Upon comparison of the intermittent and chronic studies, we found consistent regulation of a TRP1A homolog across all hypoxia and normoxic conditions and timepoints supporting its role in oxygen sensing in annelids17. Heme-binding proteins were more highly expressed after the hypoxia timepoints during intermittent hypoxia and portrayed a positive slope as time progressed indicating increased expression after repeated hypoxic exposure. Compared with chronic hypoxia, heme-binding proteins were up-regulated after Mild hypoxic exposure compared with Normal, followed by a down-regulation in severe compared with Mild. Such a result may entail several stages of hypoxic response in this species depending on DO level, exposure time, and frequency of exposure. It may be necessary to reach a certain level of lowered DO (4.5 mg O2 L−1) before heme-binding protein genes are up-regulated in response to hypoxia, but then they may be down-regulated if the production of the protein is too energetically expensive. However, more research would need to be done to further support this suggestion.
Filamin A (FLNA) is a structural protein associated with actin-binding that has been described as important for cancer tumor growth in the lungs77, and we saw it up-regulated in the Moderate chronic hypoxia treatment compared to Normal. In addition, in the intermittent trials, FLNA was consistently more expressed after hypoxic timepoints indicating a similar response to chronic vs intermittent hypoxia. Since this protein is involved with new growth of cancerous tumors and other cell types (such as blood vessels78), the higher expression after chronic Moderate and intermittent hypoxia supports the idea that lowered oxygen levels triggers growth in specific cell types.
The different expression patterns of hematopoietic prostaglandin D synthase during intermittent hypoxia, but absence during chronic hypoxia indicates that this may be purely a short-term reaction to hypoxic stress. After the initial stress, the organism seems to shift to morphological and longer-term responses (such as metabolic depression and development of new blood vessels) to cope with hypoxia. H. carunculata has previously been shown to exhibit plasticity in its branchial morphology to cope with hypoxic conditions38,39,41, but the underlying molecular mechanisms for these changes have not previously been described. Our study shows that hypoxia responses in H. carunculata can be modulated depending on the type (intermittent vs. chronic) and the severity of hypoxia exposure.
Our chronic hypoxia experiments showed that Notch-binding was up-regulated in the Moderate treatment compared to the Mild but was down-regulated in the Mild treatment compared to Normal indicating a differential response that is dependent on DO levels. We also saw differential expression of Notch associated proteins between the chronic and intermittent hypoxia trials. The up-regulation of Notch in Moderate hypoxia compared to Mild was similar to the drastic increase in regulation between timepoints 1 and 3 in the intermittent trials. The up-regulation of Notch proteins, associated with development (especially that of the heart) under hypoxia conditions has been described in zebrafish, Danio rerio32. Likewise, hypoxia-triggered up-regulation of Notch-binding proteins has been shown to increase tracheal formation in insects79. Our findings suggest that these three divergent phyla, representing the three major branches of the Bilateria (Deuterostomia, Ecdysozoa, Spiralia/Lophotrochozoa) may utilize similar signaling pathways to alter their vastly different oxygen-delivery systems. The role of Notch-binding in oxygen delivery in lophotrochozoans requires further study. In addition, Notch signaling has been shown to increase expression of HIF-1α in hypoxic conditions80, so its importance in development and response to hypoxia has been exemplified here again.
Fibropellin-1, related to Notch protein-binding, was recently shown to have drastically increased in expression post chronic hypoxia in the clam Ruditapes philippinarum81. In the current study, we see a similar expression pattern in the intermittent but not chronic hypoxia trial. We saw an increase in expression of Fibropellin-1 in the bearded fireworm after the second hypoxic timepoint in the intermittent hypoxia trial (over 18 h after initial hypoxic exposure) which corresponds well with the clam’s drastic increase in this gene after 2 days of chronic hypoxia. However, it is important to note that a significant decrease in expression of Fibropellin-1 was seen after 5 and 8 days in the clam81. During our chronic hypoxia exposure experiment, we were only able to sample after 7 days, so we may have missed this spike in expression, but our intermittent experiment allows us to see a similar response.
Although our biological replicates were limited to 2–3 individuals per treatment, our gene expression analyses discerned statistically significant differences and trends between groups exposed to different types (chronic vs. intermittent) and levels of hypoxia. Many of the gene expression responses that we observed are consistent with previous observations. For example, the higher expression levels of genes related to epithelial morphogenesis are consistent with the increase in surface branchial surface area39; likewise, expression patterns of metabolism-related genes correspond with our observation of oxygen uptake27. Other observed trends, such as the expression patterns of genes in the HIF or Notch pathways, can guide future biochemical and physiological studies in H. carunculata or other species.
This study showed that six hours of hypoxia exposure is sufficient to initiate molecular pathways for epithelial morphogenesis and blood vessel remodeling, providing further support that morphological changes of respiratory structures are not reliable taxonomic characters in amphinomids (or even possibly in annelids in general). Our data strongly support the notion that H. carunculata can swiftly initiate physiological and morphological responses to hypoxia38,41 even if a behavioral response (avoidance) is not an option. This study supports our previous prediction33 that H. carunculata will thrive under changing ocean conditions and may experience population increases that could potentially have damaging effects on coral reefs and other sensitive marine habitats in its distribution range.
References
Altieri, A. H. et al. Tropical dead zones and mass mortalities on coral reefs. Proc. Natl. Acad. Sci. 114, 3660–3665 (2017).
Lehrter, J. C., Ko, D. S., Lowe, L. L. & Penta, B. Predicted effects of climate change on northern Gulf of Mexico hypoxia. In Modeling coastal hypoxia 173–214 (Springer, 2017).
Breitburg, D. et al. Declining oxygen in the global ocean and coastal waters. Science 359, eaam7240 (2018).
Nelson, H. R. & Altieri, A. H. Oxygen: The universal currency on coral reefs. Coral Reefs 38, 177–198 (2019).
Hughes, D. J. et al. Coral reef survival under accelerating ocean deoxygenation. Nat. Clim. Change 10, 1–12 (2020).
Murphy, J. W. & Richmond, R. H. Changes to coral health and metabolic activity under oxygen deprivation. PeerJ 4, e1956 (2016).
Harborne, A. R., Rogers, A., Bozec, Y.-M. & Mumby, P. J. Multiple stressors and the functioning of coral reefs. Ann. Rev. Mar. Sci. 9, 5.1-5.24 (2017).
Van Oppen, M. J. et al. Shifting paradigms in restoration of the world’s coral reefs. Glob. Change Biol. 23, 3437–3448 (2017).
Montagna, P. A. & Ritter, C. Direct and indirect effects of hypoxia on benthos in Corpus Christi Bay, Texas, USA. J. Exp. Mar. Biol. Ecol. 330, 119–131 (2006).
Pollock, M., Clarke, L. & Dubé, M. The effects of hypoxia on fishes: from ecological relevance to physiological effects. Environ. Rev. 15, 1–14 (2007).
Seitz, R. D., Dauer, D. M., Llansó, R. J. & Long, W. C. Broad-scale effects of hypoxia on benthic community structure in Chesapeake Bay, USA. J. Exp. Mar. Biol. Ecol. 381, S4–S12 (2009).
Diaz, R. J. & Rosenberg, R. Marine benthic hypoxia: A review of its ecological effects and the behavioural responses of benthic macrofauna. Oceanogr. Mar. Biol. Ann. Rev. 33, 245–203 (1995).
Dean, T. L. & Richardson, J. Responses of seven species of native freshwater fish and a shrimp to low levels of dissolved oxygen. NZ J. Mar. Freshw. Res. 33, 99–106 (1999).
Wannamaker, C. M. & Rice, J. A. Effects of hypoxia on movements and behavior of selected estuarine organisms from the southeastern United States. J. Exp. Mar. Biol. Ecol. 249, 145–163 (2000).
Richardson, J., Williams, E. K. & Hickey, C. W. Avoidance behaviour of freshwater fish and shrimp exposed to ammonia and low dissolved oxygen separately and in combination. NZ J. Mar. Freshwat. Res. 35, 625–633 (2001).
McAllen, R., Davenport, J., Bredendieck, K. & Dunne, D. Seasonal structuring of a benthic community exposed to regular hypoxic events. J. Exp. Mar. Biol. Ecol. 368, 67–74 (2009).
Ogino, T. & Toyohara, H. Identification of possible hypoxia sensor for behavioral responses in a marine annelid. Capitella teleta. Biol. Open 8, bio37630 (2019).
Lenihan, H. S. & Peterson, C. H. How habitat degradation through fishery disturbance enhances impacts of hypoxia on oyster reefs. Ecol. Appl. 8, 128–140 (1998).
Li, F.-G., Chen, J., Jiang, X.-Y. & Zou, S.-M. Transcriptome analysis of blunt snout bream (Megalobrama amblycephala) reveals putative differential expression genes related to growth and hypoxia. PLoS ONE 10, e0142801 (2015).
Sahlmann, A., Wolf, R., Holth, T. F., Titelman, J. & Hylland, K. Baseline and oxidative DNA damage in marine invertebrates. J. Toxicol. Environ. Health A 80, 807–819 (2017).
Zoccola, D. et al. Structural and functional analysis of coral Hypoxia Inducible Factor. PLoS ONE 12, e0186262 (2017).
Díaz, R. J. & Rosenberg, R. Marine benthic hypoxia: A review of its ecological effects and the behavioural responses of benthic macrofauna. Oceanogr. Mar. Biol. Annu. Rev. 33, 245–303 (1995).
Bodamer, B. L. & Bridgeman, T. B. Experimental dead zones: two designs for creating oxygen gradients in aquatic ecological studies. Limnol. Oceanogr. Methods 12, 441–454 (2014).
Vaquer-Sunyer, R. & Duarte, C. M. Thresholds of hypoxia for marine biodiversity. Proc. Natl. Acad. Sci. 105, 15452–15457. https://doi.org/10.1073/pnas.0803833105 (2008).
Branco, P. et al. Potamodromous fish movements under multiple stressors: Connectivity reduction and oxygen depletion. Sci. Total Environ. 572, 520–525 (2016).
Hayes, D. S., Branco, P., Santos, J. M. & Ferreira, T. Oxygen depletion affects kinematics and shoaling cohesion of cyprinid fish. Water 11, 642 (2019).
Grimes, C. J., Capps, C., Petersen, L. H. & Schulze, A. Oxygen consumption during and post hypoxia exposure in bearded fireworms (Annelida: Amphinomidae). J. Comp. Physiol. B 190, 681–689 (2020).
Semenza, G. L. Hypoxia-inducible factor 1 (HIF-1) pathway. Sci. Stke 407, 1–3 (2007).
Taylor, C. T. & McElwain, J. C. Ancient atmospheres and the evolution of oxygen sensing via the hypoxia-inducible factor in metazoans. Physiology 25, 272–279 (2010).
Wang, G. L., Jiang, B.-H., Rue, E. A. & Semenza, G. L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. 92, 5510–5514 (1995).
Kaelin, W. G. Jr. & Ratcliffe, P. J. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol. Cell 30, 393–402 (2008).
Marques, I. J. et al. Transcriptome analysis of the response to chronic constant hypoxia in zebrafish hearts. J. Comp. Physiol. B. 178, 77–92 (2008).
Schulze, A., Grimes, C. J. & Rudek, T. E. Tough, armed and omnivorous: Hermodice carunculata (Annelida: Amphinomidae) is prepared for ecological challenges. J. Mar. Biol. Assoc. UK. 97,1–6 (2017).
Witman, J. D. Effects of predation by the fireworm Hermodice carunculata on milleporid hydrocorals. Bull. Mar. Sci. 42, 446–458 (1988).
Vreeland, H. & Lasker, H. Selective feeding of the polychaete Hermodice carunculata Pallas on Caribbean gorgonians. J. Exp. Mar. Biol. Ecol. 129, 265–277 (1989).
Vargas-Ángel, B., Thomas, J. D. & Hoke, S. M. High-latitude Acropora cervicornis thickets off Fort Lauderdale, Florida, USA. Coral Reefs 22, 465–473 (2003).
Miller, M., Marmet, C., Cameron, C. & Williams, D. Prevalence, consequences, and mitigation of fireworm predation on endangered staghorn coral. Mar. Ecol. Prog. Ser. 516, 187–194 (2014).
Lucey, N. M., Collins, M. & Collin, R. Oxygen‐mediated plasticity confers hypoxia tolerance in a corallivorous polychaete. Ecol. Evol. 10, 1145–1157 (2019).
Grimes, C. J., Paiva, P. C., Petersen, L. H. & Schulze, A. Rapid plastic responses to chronic hypoxia in the bearded fireworm, Hermodice carunculata (Annelida: Amphinomidae). Mar. Biol. https://doi.org/10.1007/s00227-020-03756-0 (2020).
Yáñez-Rivera, B. & Salazar-Vallejo, S. I. Revision of Hermodice Kinberg, 1857 (Polychaeta: Amphinomidae). Sci. Mar. 75, 251–262 (2011).
Ahrens, J. B. et al. The curious case of Hermodice carunculata (Annelida: Amphinomidae): Evidence for genetic homogeneity throughout the Atlantic Ocean and adjacent basins. Mol. Ecol. 22, 2280–2291 (2013).
Gorr, T. A., Cahn, J. D., Yamagata, H. & Bunn, H. F. Hypoxia-induced synthesis of hemoglobin in the crustacean Daphnia magna is hypoxia-inducible factor-dependent. J. Biol. Chem. 279, 36038–36047 (2004).
Li, T. & Brouwer, M. Hypoxia-inducible factor, gsHIF, of the grass shrimp Palaemonetes pugio: Molecular characterization and response to hypoxia. Comp. Biochem. Physiol. B: Biochem. Mol. Biol. 147, 11–19 (2007).
Soñanez-Organis, J. G. et al. Molecular characterization of hypoxia inducible factor-1 (HIF-1) from the white shrimp Litopenaeus vannamei and tissue-specific expression under hypoxia. Comp. Biochem. Physiol. C: Toxicol. Pharmacol. 150, 395–405 (2009).
Wei, L. et al. Comparative studies of hemolymph physiology response and HIF-1 expression in different strains of Litopenaeus vannamei under acute hypoxia. Chemosphere 153, 198–204 (2016).
Giannetto, A. et al. Hypoxia-inducible factor α and Hif-prolyl hydroxylase characterization and gene expression in short-time air-exposed Mytilus galloprovincialis. Mar. Biotechnol. 17, 768–781 (2015).
Philipp, E. E. et al. Gene expression and physiological changes of different populations of the long-lived bivalve Arctica islandica under low oxygen conditions. PLoS ONE 7, e44621 (2012).
Sussarellu, R., Fabioux, C., Le Moullac, G., Fleury, E. & Moraga, D. Transcriptomic response of the Pacific oyster Crassostrea gigas to hypoxia. Mar. Genom. 3, 133–143 (2010).
Woo, S. et al. Expressions of oxidative stress-related genes and antioxidant enzyme activities in Mytilus galloprovincialis (Bivalvia, Mollusca) exposed to hypoxia. Zool. Stud. 52, 15 (2013).
Burgeot, T. et al. Oyster summer morality risks associated with environmental stress. Summer Mortality of Pacific Oyster Crassostrea Gigas. The Morest Project. Éd. Ifremer/Quæ, 107–151 (2008).
David, E., Tanguy, A., Pichavant, K. & Moraga, D. Response of the Pacific oyster Crassostrea gigas to hypoxia exposure under experimental conditions. FEBS J. 272, 5635–5652 (2005).
Hourdez, S. et al. Gas transfer system in Alvinella pompejana (Annelida polychaeta, Terebellida): Functional properties of intracellular and extracellular hemoglobins. Physiol. Biochem. Zool. 73, 365–373 (2000).
Boutet, I., Jollivet, D., Shillito, B., Moraga, D. & Tanguy, A. Molecular identification of differentially regulated genes in the hydrothermal-vent species Bathymodiolus thermophilus and Paralvinella pandorae in response to temperature. BMC Genom. 10, 222 (2009).
Eyre, B. D., Andersson, A. J. & Cyronak, T. Benthic coral reef calcium carbonate dissolution in an acidifying ocean. Nat. Clim. Change 4, 969–976 (2014).
Huggett, J. & Griffiths, C. Some relationships between elevation, physico-chemical variables and biota of intertidal rock pools. Mar. Ecol. Prog. Ser. 29, 189–197 (1986).
Kinsey, D. & Kinsey, E. Diurnal changes in oxygen content of the water over the coral reef platform at Heron I. Mar. Freshw. Res. 18, 23–34 (1967).
Helly, J. J. & Levin, L. A. Global distribution of naturally occurring marine hypoxia on continental margins. Deep Sea Res. Part I 51, 1159–1168 (2004).
Levin, L. A., Gage, J. D., Martin, C. & Lamont, P. A. Macrobenthic community structure within and beneath the oxygen minimum zone, NW Arabian Sea. Deep Sea Res. Part II 47, 189–226 (2000).
Gallardo, V. et al. Macrobenthic zonation caused by the oxygen minimum zone on the shelf and slope off central Chile. Deep Sea Res. Part II 51, 2475–2490 (2004).
Gooday, A. et al. Faunal responses to oxygen gradients on the Pakistan margin: a comparison of foraminiferans, macrofauna and megafauna. Deep Sea Res. Part II 56, 488–502 (2009).
Prabhakar, N. R. & Semenza, G. L. Adaptive and maladaptive cardiorespiratory responses to continuous and intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2. Physiol. Rev. 92, 967–1003 (2012).
Du, S. N., Mahalingam, S., Borowiec, B. G. & Scott, G. R. Mitochondrial physiology and reactive oxygen species production are altered by hypoxia acclimation in killifish (Fundulus heteroclitus). J. Exp. Biol. 219, 1130–1138 (2016).
Grabherr, M. G. et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 29, 644 (2011).
Bryant, D. M. et al. A tissue-mapped axolotl de novo transcriptome enables identification of limb regeneration factors. Cell Rep. 18, 762–776 (2017).
Haas, B. J. et al. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat. Protoc. 8, 1494–1512 (2013).
Bray, N. L., Pimentel, H., Melsted, P. & Pachter, L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 34, 525 (2016).
Soneson, C., Love, M. I. & Robinson, M. D. Differential analyses for RNA-seq: transcript-level estimates improve gene-level inferences. F1000Research 4, 1–19 (2015).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Conesa, A., Nueda, M. J., Ferrer, A. & Talón, M. maSigPro: A method to identify significantly differential expression profiles in time-course microarray experiments. Bioinformatics 22, 1096–1102 (2006).
Nueda, M.J., Tarazona, S., & Conesa, A. Next maSigPro: updating maSigPro bioconductor package for RNA-seq time series. Bioinformatics, 30, 2598–2602. https://doi.org/10.1093/bioinformatics/btu333 (2014).
OmicsBox. Bioinformatics Made Easy, BioBam Bioinformatics. https://www.biobam.com/omicsbox (2019).
Costa-Paiva, E. M., Schrago, C. G., Coates, C. J. & Halanych, K. M. Discovery of novel hemocyanin-like genes in Metazoans. Biol. Bull. 235, 134–151 (2018).
Kanaoka, Y. & Urade, Y. Hematopoietic prostaglandin D synthase. Prostaglandins Leukot. Essent. Fatty Acids 69, 163–167 (2003).
Altun, M. et al. Ubiquitin-specific protease 19 (USP19) regulates hypoxia-inducible factor 1α (HIF-1α) during hypoxia. J. Biol. Chem. 287, 1962–1969 (2012).
Ogawa, M. et al. 17β-estradiol represses myogenic differentiation by increasing ubiquitin-specific peptidase 19 through estrogen receptor α. J. Biol. Chem. 286, 41455–41465 (2011).
Isaacs, J. S. et al. Hsp90 regulates a von Hippel Lindau-independent hypoxia-inducible factor-1α-degradative pathway. J. Biol. Chem. 277, 29936–29944 (2002).
Nallapalli, R. K. et al. Targeting filamin A reduces K-RAS–induced lung adenocarcinomas and endothelial response to tumor growth in mice. Mol. Cancer 11, 1–11 (2012).
Feng, Y. et al. Filamin A (FLNA) is required for cell–cell contact in vascular development and cardiac morphogenesis. Proc. Natl. Acad. Sci. 103, 19836–19841 (2006).
Muñoz-Chápuli, R. Evolution of angiogenesis. Int. J. Dev. Biol. 55, 345–351 (2011).
Kim, S., Lee, M. & Choi, Y. K. The role of a neurovascular signaling pathway involving hypoxia-inducible factor and notch in the function of the central nervous system. Biomol. Ther. 28, 45 (2020).
Nie, H., Wang, H., Jiang, K. & Yan, X. Transcriptome analysis reveals differential immune related genes expression in Ruditapes philippinarum under hypoxia stress: potential HIF and NF-κB crosstalk in immune responses in clam. BMC Genom. 21, 1–16 (2020).
Acknowledgements
Many thanks to Justin Hilliard, Katie St. Clair, Sea Life Facility volunteers and workers. Without funding through TAMU-CAPES Collaborative Grant Program (Grant 2015–2016) to conduct the experiments, this would not have been possible. Organisms were collected under the Florida Fish and Wildlife Conservation Commission (FWC) Special Activity License (SAL-17-1946-SR). Paulo C. Paiva and Romulo Barroso supported our collections in Brazil under ICMBIO-SISBIO permit number 10238-2 in the name of Paulo Cesar de Paiva. We would also like to thank Texas A&M’s Agrilife Research Genomics & Bioinformatics Service for sequencing and Texas A&M High Performance Research Computing for providing the servers to analyze the data. CJG was supported by the Marine Biology Department at TAMUG, Erma Lee and Luke Mooney Grant, and Galveston Graduate Student Association at TAMUG for travel to present this research at conferences. We thank the two anonymous reviewers for their constructive feedback on our manuscript.
Author information
Authors and Affiliations
Contributions
Project idea and design: C.J.G., LH.P., and A.S. Conduction of experiment and data acquisition: C.J.G. Data analyses and interpretation: C.J.G. and A.S. Manuscript draft and critical revision of final draft: C.J.G., L.H.P., and A.S. Financial support: A.S.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Grimes, C.J., Petersen, L.H. & Schulze, A. Differential gene expression indicates modulated responses to chronic and intermittent hypoxia in corallivorous fireworms (Hermodice carunculata). Sci Rep 11, 11110 (2021). https://doi.org/10.1038/s41598-021-90540-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-90540-9