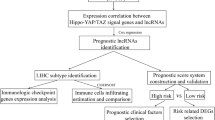
Abstract
Pyroptosis is an inflammatory form of cell death triggered by certain inflammasomes. However, research concerning pyroptosis-related lncRNAs in hepatocellular carcinoma (HCC) remains scarce. This study aims to explore the prognostic pyroptosis-related long non-coding RNAs (lncRNAs) of HCC patients. Data of 373 HCC patients were obtained from the TCGA database. The entire cohort was randomly divided into a training cohort and a validation cohort in a 2:1 ratio. Pyroptosis-related lncRNAs were identified by the Pearson correlation analysis with reported pyroptosis-related genes. LASSO Cox regression was used to construct the signature. A prognostic signature consisting of nine pyroptosis-related lncRNAs was identified, and patients with lower risk scores had a better prognosis than those with higher risk scores. Multivariate Cox regression analysis showed that the signature was an independent risk factor for prognosis in both the training and validation cohorts. In the training cohort, the area under the signature curve reached 0.8043 at 1-year, 0.7878 at 2-year, and 0.8118 at 3-year; in the validation cohort, it reached 0.7315 at 1-year, 0.7372 at 2-year, and 0.7222 at 3-year. Gene set enrichment analysis (GSEA) suggested associations between the signature and several immune-related pathways. The expression of multiple immune checkpoints was also increased in the high-risk group, including PD-1, PD-L1, CTLA4, B7-H3, VSIR, LAG3, and TIGIT. A novel pyroptosis-related lncRNA signature, which may be associated with tumor immunity and potentially serve as an indicator for immunotherapy, has been identified to precisely predict the prognosis of HCC patients.
Similar content being viewed by others
Introduction
Hepatocellular carcinoma (HCC), one of the leading causes of cancer-related death, is the most common liver cancer, followed by intrahepatic cholangiocarcinoma1,2. Although early-stage HCC can be cured by surgical intervention, many challenges remain in treating the advanced HCC, leading to a poor prognosis, high economic costs, and heavy disease burden3. Thus, exploring reliable prognostic factors is vital to preferable individualized management and treatment.
First mentioned in 1992, pyroptosis is an inflammatory form of cell death triggered by certain inflammasomes4. Extensive studies have focused on the association between pyroptosis and human diseases, revealing that pyroptosis is related to not only inflammatory diseases but also various cancers, including HCC5,6. Additionally, pyroptosis-related genes were previously investigated as well. Lozano-Ruiz et al. described that the absent in melanoma 2 (AIM2) could trigger pyroptosis by activating the inflammasome cascade in HCC7.
With advances in sequencing technology, long non-coding RNAs (lncRNAs), a class of RNAs with more than 200 nucleotides, have been found to be functional in most biological and pathological processes8. Emerging evidence has suggested the crucial role of lncRNAs in the tumorigenesis and progression of HCC9. However, the role of pyroptosis-related lncRNAs in HCC pathogenesis and immune regulation remains underappreciated. Thus, this study was performed to recognize the prognostic pyroptosis-related lncRNAs in HCC, thus providing a better understanding of the prognosis prediction and selection of immunotherapy patients.
Materials and methods
Data acquisition
Transcriptome and clinical data of HCC patients, including 373 tumor tissues and 50 normal tissues, were retrieved from the LIHC project of TCGA database (http://cancergenome.nih.gov/). Patients without adequate clinical data were excluded from the analysis. Expression data were normalized to the values of transcripts per kilobase million (TPM) for further analysis.
Identification of pyroptosis-related lncRNAs
Thirty-three pyroptosis-related genes were obtained from Ye’s reports (Supplementary Material 1)10. Pearson correlation test was used to calculate the correlations between lncRNAs and pyroptosis-related genes. Based on the cut-off criteria of Pearson correlation coefficient > 0.3, these lncRNAs were considered as candidate pyroptosis-related lncRNAs.
Construction and validation of the prognostic pyroptosis-related lncRNAs signature
The cohort was randomly divided into a training cohort and a validation cohort in a 2:1 ratio. The data from the training cohort were used to construct the prognostic pyroptosis-related lncRNAs signature, while the other cohort was used for validation. Univariate Cox regression analysis was used to identify the prognostic pyroptosis-related lncRNAs. Subsequently, the least absolute shrinkage and selection operator (LASSO) Cox regression was used to construct the signature by R packages (glmnet and survival) as follows: risk score = expression of lncRNA1 × β1lncRNA1 + expression of lncRNA2 × β2lncRNA2 + …expression of lncRNAn × βnlncRNAn. The two cohorts were further divided into the low-risk and high-risk groups, respectively. Survival analysis and time-dependent ROC curves were performed to investigate the prognostic value. Moreover, multivariate Cox regression of available patient characteristics was performed to reconfirm the prognostic value of the signature. Additionally, a nomogram was constructed to predict the patient prognosis more precisely.
The mRNA-lncRNA co-expression network
In order to better demonstrate the associations between pyroptosis-related genes and pyroptosis-related lncRNAs, a co-expression network was constructed. A Sankey diagram was used to illustrate the mRNA-lncRNA relationships.
Gene set enrichment analysis (GSEA) and subsequent functional enrichment analysis
The tumor tissues were divided into the low-risk group and high-risk group based on risk scores. Differentially expressed genes between groups were identified by the “DEseq2” package with cut-off criteria of false discovery rate < 0.05 and |log2foldchange| > 1. After that, these differentially expressed genes were uploaded for GSEA analysis (http://www.broadinstitute.org/gsea)11.
Immunological analysis
The abundance of tumor-infiltrating immune cells in HCC tissues was investigated using the Tumor IMmune Estimation Resource (TIMER) database (https://cistrome.shinyapps.io/timer/)12.
Statistical analysis
R 3.3.0 and Statistical Package for Social Sciences 23.0 (SPSS Inc., Chicago, IL, United States) were used for statistical analysis. One-way analysis of variance (ANOVA) with homogeneous variances was used to analyze differences in immune cell components between the normal and HCC tissues, while Welch’s ANOVA was applied when variances were heterogeneous. Kaplan–Meier curve was used for survival analysis, where a Log-rank test was adopted for comparison between groups.
Ethics approval and consent to participate
Extra informed consent is not essential for the data were all obtained from public database. The authors cannot assess to any identifying characteristics, which do not distort scientific meaning.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Results
Cohort studies of the training cohort and validation cohort
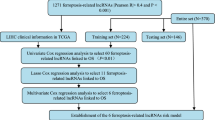
A total of 373 HCC patients were enrolled. However, two patients were excluded from this study due to incomplete clinical data (Fig. 1a). Thus, 371 patients were finally included in this study and randomly divided into the training cohort and the validation cohort in a 2:1 ratio. The characteristics of HCC patients in the two cohorts are shown in Table 1, with no statistically significant differences in all characteristics.
Identification of the pyroptosis-associated lncRNAs in HCC patients. (a) The flow chart of constructing the pyroptosis-related lncRNAs signature. (b) The heatmap of 9 prognostic pyroptosis-related lncRNAs in HCC tissues and adjacent normal tissues. (c) The barplots comparing the 9 prognostic pyroptosis-related lncRNAs between HCC tissues and adjacent normal tissues. HCC, hepatocellular carcinoma. *P < 0.05; **P < 0.01; ***P < 0.001.
Identification of prognostic pyroptosis-related lncRNAs
Pearson correlation test was performed between lncRNAs and 33 pyroptosis-related genes, and then 5372 lncRNAs were preliminarily identified with PCA analysis in supplement material 2. Subsequently, in the training cohort, the prognostic value was examined by the univariate Cox regression, and 1030 prognostic pyroptosis-related lncRNAs were chosen for further analysis. Using LASSO Cox regression analysis, 9 lncRNAs (AC019080.5, AP003392.4, MKLN1-AS, AL031985.3, PCCA-DT, AC007128.1, LNCSRLR, AL445228.3, AC023157.2) were screened according to lambda.min, and a prognostic signature of the 9 lncRNAs was calculated as follows: risk score = (0.218*AC019080.5 expression) + (0.219*AP003392.4 expression) + (0.103*MKLN1-AS expression) + (0.163*AL031985.3 expression) + (0.003*PCCA-DT expression) + (0.484*AC007128.1 expression) + (0.236*LNCSRLR expression) + (0.161*AL445228.3 expression) + (0.060*AC023157.2 expression). The expression patterns of the 9 lncRNAs in HCC are shown by heatmaps in Fig. 1b and Supplementary Material 3. The 9 lncRNAs were all significantly up-regulated in HCC tissues compared with adjacent normal tissues (Fig. 1c). Their prognostic values are shown in Fig. 2a–i with Kaplan-Miere curves and Fig. 2j with the univariate Cox regression. As shown in Fig. 2a–i, high levels of these candidate lncRNAs are correlated with poor prognosis in HCC patients.
The prognostic value of pyroptosis-associated lncRNAs in HCC patients. (a–i) Kaplan-Meire curve of AC019080.5 (a), AP003392.4 (b), MKLN1-AS (c), AL031985.3 (d), PCCA-DT (e), AC007128.1 (f), LNCSRLR (g), AL445228.3 (h), AC023157.2 (i) on overall survival of HCC patients. (j) Univariate Cox regression of 9 prognostic pyroptosis-related lncRNAs in the training cohort.
Then, a co-expression network between the pyroptosis-related genes and pyroptosis-related lncRNAs was constructed to confirm their relationships (Supplementary Material 4). As shown in Fig. 3a and supplement material 5, 24 pyroptosis-related genes and 9 pyroptosis-related lncRNAs are included in the network. It is worth noting that MKLN1-AS and AL031985.3 might be the most likely pyroptosis-related lncRNAs. In addition, the close correlation between pyroptosis-related genes and pyroptosis-related lncRNAs is indicated by the Sankey diagram (Fig. 3b). These findings suggest that the 9 pyroptosis-related lncRNAs might play important roles in HCC.
Validation of the prognostic pyroptosis-related lncRNA signature
The prognostic signature was subsequently validated in the training cohort and validation cohort. The patients in the two cohorts were divided into the low-risk group and high-risk group based on the median risk score. The characteristics of the two groups in the training cohort are compared in Table 2. The high-risk group is proved to be associated with a poorer T stage (p = 0.031), pathologic stage (p = 0.013), and histologic grade (p < 0.001). Figure 4a–d shows that the probability of death is higher in the high-risk group than in the low-risk group in both cohorts. Kaplan–Meier curves of OS show consistent results in both cohorts (Fig. 4e, f), indicating that the survival of HCC-TCGA patients in the high-risk group is significantly worse than that in the low-risk group. A time-dependent receiver operating characteristic (ROC) was performed to investigate values in predicting the patient prognosis (Fig. 4g, h). The area under the curve (AUC) reached 0.8043 at 1-year, 0.7878 at 2-year, and 0.8118 at 3-year in the training cohort, while 0.7315 at 1-year, 0.7372 at 2-year, and 0.7222 at 3-year in the validation cohort. In order to further verify the prognostic value of the signature, multivariate Cox regression analysis was performed in the training cohort (Table 3) and the validation cohort (Table 4). The results show that the risk score could be an independent factor to predict the patient prognosis in the two cohorts.
Prognostic analysis of pyroptosis-related lncRNA signature in the training cohort and validation cohort. (a) The distribution of the risk scores in the training cohort. (b) The distribution of the risk scores in the validation cohort. (c) The distributions of overall survival status, overall survival, and risk score in the training cohort. (d) The distributions of overall survival status, overall survival, and risk score in the validation cohort. (e) Kaplan–Meier curves for the overall survival of patients in the high- and low-risk groups in the training cohort. (f) Kaplan–Meier curves for the overall survival of patients in the high- and low-risk groups in the validation cohort. (g) AUC of time-dependent ROC curves verified the prognostic accuracy of the risk score in the training cohort. (h) AUC of time-dependent ROC curves verified the prognostic accuracy of the risk score in the validation cohort.
Nomogram based on the signature for predicting the OS of HCC patients
In order to develop a more accurate model for prognosis prediction, univariate Cox regression analysis was performed in both cohorts (Fig. 5a). The pyroptosis-related lncRNA signature (hazard ratio 2.013, 95%; confidence interval 1.731–2.341), hepatitis B (hazard ratio 0.357, 95% confidence interval 0.221–0.576), and tumor stage (hazard ratio 2.448, 95% confidence interval 1.689–3.548) were risk factors for the prognosis of HCC patients. Proportional hazard assumption analysis was subsequently performed, and the results showed the variables did not violate the proportional hazard assumption (Supplementary Material 6). Then, a nomogram, including hepatitis B, tumor stage, and risk scores, was constructed to explore the probability of the lncRNA signature in predicting 1-, 3-, and 5-year survival in HCC patients with a C-index of 0.721. In this combined nomogram, the risk score model exerts the most excellent weight in predicting the 1-year, 3-year, and 5-year survival probability with a C-index of 0.711 (Fig. 5b). The findings identify that this novel pyroptosis-related lncRNA signature can act as a promising prognostic model for HCC patients.
GSEA and functional enrichment analysis
GSEA analysis was performed to explore the potential biological functions involved in the signature, using the differentially expressed genes between the low- and high-risk groups (Supplementary Material 7). Figure 6a–i shows the top 9 immune-associated signaling pathways, including the CTLA4 pathway, antigen presentation folding assembly and peptide loading of class I MHC, autoimmune thyroid disease, inflammatory pathway, antigen processing and presentation, IL5 pathway, cytokines and inflammatory response, TCR signaling, and TH1TH2 pathway. The results show that the pyroptosis-related lncRNA signature may be associated with multiple immune-related pathways.
Gene set enrichment analysis (GSEA) about the pyroptosis-related lncRNA prognostic signature. (a) CTLA4 pathway. (b) antigen presentation folding assembly and peptide loading of class I MHC. (c) autoimmune thyroid disease. (d) inflam pathway. (e) antigen processing and presentation. (f) IL5 pathway. (g) cytokines and inflammatory response. (h) TCR signaling. (i) TH1TH2 pathway.
Associations between the signature and immune infiltration
In order to explore the roles of the pyroptosis-related lncRNA signature in the immune microenvironment of HCC patients, the associations between the signature and immune infiltration cells were further investigated using the ESTIMATE algorithm (Supplementary Material 8). The proportions of various immune cells are shown in Fig. 7a, b, and the correlations between these immune cells in the low-risk and high-risk groups are shown in Fig. 7c, d. Among the immune cells, the CD4 + memory activated T cells, regulatory T cells (Tregs), M0 macrophages, and neutrophils were all increased in the high-risk group, while the CD4 + memory resting T cells, monocytes, and M2 macrophages were decreased in the high-risk group compared with the low-risk group (Fig. 7e). In addition, the associations between the signature and other immune checkpoints, including PD-1, PD-L1, CTLA4, B7-H3, VSIR, LAG3, and TIGIT, were also investigated. As shown in Fig. 7f, the immune checkpoints were significantly up-regulated in the high-risk group compared with the low-risk group. To conclude, the results indicate that the pyroptosis-related lncRNA signature may be associated with immune-related mechanisms and the response of HCC patients to immunotherapy.
The interactions between pyroptosis-related lncRNA signature and immune regulation in HCC patients. (a) The barplot of the tumor-infiltrating cell proportions. (b) The heatmap of the tumor-infiltrating cell proportions. (c) Correlation matrix of immune cell proportions in the low-risk group. (d) Correlation matrix of immune cell proportions in the high-risk group. (e) Comparisons of immune cell proportions between the low-risk group and the high-risk group. (f) Comparisons of multiple immune checkpoints between the low-risk group and the high-risk group, including PD-1, PD-L1, CTLA4, B7-H3, VSIR, LAG3, TIGIT.
Discussion
In this study, prognostic pyroptosis-related lncRNAs in HCC were comprehensively investigated. A novel prognostic signature consisting of 9 pyroptosis-related lncRNAs (AC019080.5, AP003392.4, MKLN1-AS, AL031985.3, PCCA-DT, AC007128.1, LNCSRLR, AL445228.3, AC023157.2) was developed using LASSO Cox regression analysis and validated by the validation cohort. The subsequent functional analysis confirms the associations between the signature and multiple immune-related pathways. The results suggest that the identified signature has potential values in predicting the patient prognosis and managing immunotherapy for HCC patients.
Several studies have investigated the roles of lncRNAs in pyroptosis13. It is reported that lncRNA KCNQ1OT1 inhibits the miR-21 expression and subsequently promotes the caspase-1-dependent pyroptosis during cataract formation14. It is found that LncRNA MALAT1 increases the level of NLRP3 by up-regulating ELAVL1 expression, thus leading to cell pyroptosis in diabetic nephropathy15. It has been proven that LncRNA ANRIL up-regulates NLRP3 and IL-1β by increasing the expression of BRCC3, thus activating pyroptosis in uric acid nephropathy16. Furthermore, a recent study by Zhang et al. has demonstrated that lncRNANeat1 can promote the assembly of the NLRP3 and AIM2 inflammasome by directly binding to pro-caspase-1, thus accelerating caspase-1-dependent pyroptosis17. In terms of cancer, lncRNA RP1‑85F18.6 has been identified to promote pyroptosis by cleaving GSDMD in colorectal cancer18; LncRNA ADAMTS9-AS2 can activate NLRP3-mediated pyroptosis via sponging miR-223-3p in gastric cancer19. Another recent study has reported that SNHG7 inhibits NLRP3-dependent pyroptosis by targeting the miR-34a/SIRT1 axis in HCC20. At present, detailed knowledge about the roles of lncRNAs in regulating pyroptosis in HCC remains limited.
In the co-expression network, MKLN1-AS and AL031985.3 might be the most likely pyroptosis-related lncRNAs in the TCGA-HCC cohort. Studies have suggested that MKLN1-AS aggravates the progression of HCC by sponging miR-654-3p21. However, no studies have reported the potential role of AL031985.3 in HCC. Because of the great significance of MKLN1-AS, AL031985.3, and 7 other lncRNAs in HCC, future studies are encouraged to reveal the underlying mechanisms of these candidate lncRNAs in HCC biology, especially cell pyroptosis.
Our study shows significant relationships between the signature and many immune-associated signaling pathways. Patients with higher risk scores display a higher expression of multiple immune checkpoints, including PD-1, PD-L1, CTLA4, B7-H3, VSIR, LAG3, and TIGIT. Additionally, PD-1, a member of the B7/CD28 costimulatory receptor family, is expressed on multiple immune cells, including activated T cells, B cells, Tregs, and monocytes. Moreover, PD-L1 functions as the main ligand of PD-122. Immunotherapy targetings PD-1 and PD-L1 have been developed, such as PD-1 inhibitors nivolumab and pembrolizumab targeting and PD-L1 inhibitors durvalumab and avelumab23. CTLA-4, a member of the immunoglobulin superfamily homologous to CD28, can inhibit T cells activity by competing with CD2824. Drugs targeting CTLA-4, such as ipilimumab and tremelimumab, have been developed and have displayed promising anti-tumor effects25. Here, this study suggests that patients with higher risk scores may benefit more from immunotherapy than those with lower risk scores.
However, this study has certain limitations. Firstly, cohorts in this study were mainly based on the TCGA database. Thus, real-world data are needed for further validations of the pyroptosis-related lncRNA signature in HCC patients. Secondly, this study failed to investigate the underlying immune-related mechanisms of the identified lncRNAs. Moreover, further studies should focus on the potential of the pyroptosis-related lncRNA signature as an indicator of immunotherapy.
Conclusion
In summary, a novel pyroptosis-related lncRNA signature has been identified to precisely predict the prognosis of HCC patients. The signature is robustly associated with the tumor immunity, providing a personalized prediction model for the prognosis and immunotherapeutic response of HCC patients.
Data availability
All data generated or analyzed during this study are included in this published article and supplement materials.
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- lncRNAs:
-
Long non-coding RNAs
- TPM:
-
Transcripts per kilobase million
- LASSO:
-
Least absolute shrinkage and selection operator
- GSEA:
-
Gene set enrichment analysis
- TIMER:
-
Tumor IMmune Estimation Resource
- ANOVA:
-
One-way analysis of variance
- AUC:
-
Area under curve
References
Feng, D. et al. Prognostic value of the albumin-bilirubin grade in patients with hepatocellular carcinoma and other liver diseases. Ann. Transl. Med. 8(8), 553 (2020).
Huang, X., Qin, F., Meng, Q. & Dong, M. Protein tyrosine phosphatase receptor type D (PTPRD)-mediated signaling pathways for the potential treatment of hepatocellular carcinoma: a narrative review. Ann. Transl. Med. 8(18), 1192 (2020).
Laube, R. et al. Palliative care in hepatocellular carcinoma. J. Gastroenterol. Hepatol. 36(3), 618–628 (2021).
Shi, C. et al. Diabetes induces hepatocyte pyroptosis by promoting oxidative stress-mediated NLRP3 inflammasome activation during liver ischaemia and reperfusion injury. Ann. Transl. Med. 8(12), 739 (2020).
Fang, Y. et al. Pyroptosis: A new frontier in cancer. Biomed. Pharmacother. 121, 109595 (2020).
Li, L., Dai, B., Sun, Y. & Zhang, T. The activation of IL-17 signaling pathway promotes pyroptosis in pneumonia-induced sepsis. Ann. Transl. Med. 8(11), 674 (2020).
Lozano-Ruiz, B. & González-Navajas, J. M. The emerging relevance of AIM2 in liver disease. Int. J. Mol. Sci. 21(18), 6535 (2020).
Chen, W., Yang, J., Fang, H., Li, L. & Sun, J. Relevance function of Linc-ROR in the pathogenesis of cancer. Front. Cell Dev. Biol. 8, 696 (2020).
Lou, W., Ding, B. & Fu, P. Pseudogene-derived lncRNAs and their miRNA sponging mechanism in human cancer. Front. Cell Dev. Biol. 8, 85 (2020).
Ye, Y., Dai, Q. & Qi, H. A novel defined pyroptosis-related gene signature for predicting the prognosis of ovarian cancer. Cell Death Discov. 7(1), 71 (2021).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 102(43), 15545–15550 (2005).
Li, T. et al. TIMER: A web server for comprehensive analysis of tumor-infiltrating immune cells. Cancer Res. 77(21), e108–e110 (2017).
He, D., Zheng, J., Hu, J., Chen, J. & Wei, X. Long non-coding RNAs and pyroptosis. Clin. Chim. Acta Int. J. Clin. Chem. 504, 201–208 (2020).
Jin, X., Jin, H., Shi, Y., Guo, Y. & Zhang, H. Long non-coding RNA KCNQ1OT1 promotes cataractogenesis via miR-214 and activation of the caspase-1 pathway. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 42(1), 295–305 (2017).
Li, X. et al. Long noncoding RNA MALAT1 regulates renal tubular epithelial pyroptosis by modulated miR-23c targeting of ELAVL1 in diabetic nephropathy. Exp. Cell Res. 350(2), 327–335 (2017).
Hu, J., Wu, H., Wang, D., Yang, Z. & Dong, J. LncRNA ANRIL promotes NLRP3 inflammasome activation in uric acid nephropathy through miR-122-5p/BRCC3 axis. Biochimie 157, 102–110 (2019).
Zhang, P., Cao, L., Zhou, R., Yang, X. & Wu, M. The lncRNA Neat1 promotes activation of inflammasomes in macrophages. Nat. Commun. 10(1), 1495 (2019).
Ma, Y., Chen, Y., Lin, C. & Hu, G. Biological functions and clinical significance of the newly identified long non-coding RNA RP1-85F18.6 in colorectal cancer. Oncol. Rep. 40(5), 2648–2658 (2018).
Ren, N. et al. LncRNA ADAMTS9-AS2 inhibits gastric cancer (GC) development and sensitizes chemoresistant GC cells to cisplatin by regulating miR-223-3p/NLRP3 axis. Aging 12(11), 11025–11041 (2020).
Chen, Z., He, M., Chen, J., Li, C. & Zhang, Q. Long non-coding RNA SNHG7 inhibits NLRP3-dependent pyroptosis by targeting the miR-34a/SIRT1 axis in liver cancer. Oncol. Lett. 20(1), 893–901 (2020).
Gao, W., Chen, X., Chi, W. & Xue, M. Long non-coding RNA MKLN1-AS aggravates hepatocellular carcinoma progression by functioning as a molecular sponge for miR-654-3p, thereby promoting hepatoma-derived growth factor expression. Int. J. Mol. Med. 46(5), 1743–1754 (2020).
Hui, E. et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science (New York, N.Y.). 355(6332), 1428–1433 (2017).
Llovet, J. M. et al. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 7(1), 6 (2021).
Collins, A. V. et al. The interaction properties of costimulatory molecules revisited. Immunity 17(2), 201–210 (2002).
Zongyi, Y. & Xiaowu, L. Immunotherapy for hepatocellular carcinoma. Cancer Lett. 470, 8–17 (2020).
Funding
This study is supported by grants from China Postdoctoral Science Foundation (2021T140754, 2020M672521), the Natural Science Foundation of Hunan Province (2019JJ50932, 2020JJ5934), the Postdoctoral Science Foundation of Central South University (248485), the Hunan Development and Reform Investment (2019, No. 412), the Natural Science Foundation of Hunan Province (2021JJ30904), and the horizontal project (143010100).
Author information
Authors and Affiliations
Contributions
Z.Y.Z., F.D.X. contributed to data analysis, drafting the article. F.H.K., J.B.L. prepared figures, while W.Q.Z., Q.H.H. prepared tables. Z.J.X., J.W.P. revised the article. All the authors gave final approval of the version to be published, agreed to the submitted journal, and agree to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, Z., Xia, F., Xu, Z. et al. Identification and validation of a novel pyroptosis-related lncRNAs signature associated with prognosis and immune regulation of hepatocellular carcinoma. Sci Rep 12, 8886 (2022). https://doi.org/10.1038/s41598-022-13046-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-13046-y