Abstract
Bacterial resistance, a global public health concern prioritized by the World Health Organization, is particularly alarming in Staphylococcus aureus and Escherichia coli. Urgently addressing this, the search for new antibiotics has turned to plant essential oils. Our study focused essential oils derived from Colombian plants Croton killipianus, Croton smithianus, Croton leptostachyus, Croton hondensis, and Croton gossypiifolius. We performed antimicrobial susceptibility tests targeting Staphylococcus aureus, methicillin-resistant Staphylococus aureus, sensitive Escherichia coli, and ampicillin- and streptomycin-resistant Escherichia coli. Simultaneosly, citotoxic assays and chemical analysis were carried out. The essential oil derived from C. hondensis demonstrated superior inhibitory efficacy, effectively targeting methicillin-resistant S. aureus, susceptible S. aureus, and both sensitive and ampicillin- and streptomycin-resistant strains of E. coli. Furthermore, it exhibited notable potential for protective activity in Chinese hamster ovary cells. C. killipianus manifested inhibitory effects against MRSA and susceptible S. aureus, whereas C. smithianus specifically affected susceptible strains of S. aureus. Chemical analysis of the essential oils revealed rich content in phenolic compounds, flavonoids, tannins, and steroids. Gas-coupled mass spectrometry identified key compounds like γ-muurolene, α-humulene, (E)-caryophyllene, α-copaene, curcumene, and (E)-nerolidol. These findings underscore C. hondensis, C. killipianus, and C. smithianus as potential natural sources for antibacterial agent development.
Similar content being viewed by others
Introduction
The World Health Organisation (WHO) has warned of the global threat of bacterial resistance to antibiotics and its major impact on health1. By 2019, an estimated 4.95 million deaths worldwide were associated with bacterial resistance, marking this phenomenon as the third primary cause of mortality within level 3 of the Global disease burden under the no-infection counter-hypothesis2. Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli) predominate among the top six pathogens exhibiting significant resistance rates, contributing to 73.4% of fatalities attributed to antibiotic resistance in the year 20191,2.
S. aureus is a gram-positive pathogen of importance in both human and animal health, causing infections ranging from mild to life-threatening3. Vancomycin remains the preferred therapeutic option for methicillin-resistant Staphylococcus aureus (MRSA) strains, despite its notable adverse effects, including nephrotoxicity, ototoxicity, hypotension, and phlebitis4,5. In 2019 alone, MRSA was estimated to be responsible for 121,000 global deaths2. Certain strains of S. aureus, in addition to their resistance to methicillin, exhibit resistance to vancomycin, thereby practically nullifying the prospects of a successful treatment4,5,6. Consequently, the imperative to develop novel antibiotics for MRSA is paramount in addressing the urgent threat posed by resistant bacteria [3.6].
On the other hand, a study published in 2019, undertaken by the SENTRY Antimicrobial Surveillance Program across more than 200 health centers in 45 countries, identified gram-negative bacterium E. coli as the second most prevalent pathogen, a trend observed since 20057. Similarly, other multinational studies have indicated a troubling evolution of resistance in E. coli to third-generation cephalosporins. These studies estimated that such bacteria might have contributed to between 50,000 and 100,000 deaths worldwide in 20192,7. A study from China published in 2021, reported 80 E. coli strains exhibiting resistance rates above 60% for penicillins, first- and second-generation cephalosporins, and fluoroquinolones. Additionally, 58.8% of these strains produced extended-spectrum beta-lactamases, while one strain produced carbapenemases8. Likewise, a recent 2021 study conducted at several diagnostic centers in Bangladesh revealed that 98% of clinical isolates of E. coli were multidrug-resistant9.
Given the concerning landscape of antibacterial resistance, prompt actions are essential through cross-sectoral initiatives to align with the objectives of the Sustainable Development Goals (SDGs)10. Ensuring continued investment in research and development of new antibiotics becomes imperative2. In this sense, the bioprospecting of medicinal plants is a valuable tool in the search for new antibacterial agents. Throughout history, the use of plants possessing medicinal properties has been extensively documented across various cultures and civilizations globally. Recognized since ancient times for their therapeutic benefits, these plants have constituted a fundamental component of traditional medicine11,12.
Colombia, recognized as a mega-diverse country, is home to an extensive array of plants with therapeutic and pharmacological properties, including significant natural compounds such as essential oils12,13. These oils represent intricate blends comprising over 100 components, predominantly consisting of low molecular weight aliphatic compounds, monoterpenes, sesquiterpenes, and phenylpropanes. Owing to their chemical diversity, these compounds have found widespread application in diverse industries, including but not limited to food, agriculture, cosmetics, and medicine14,15.
Among the plants that have garnered significant medical interest are those belonging to the class MAGNOLIOPSIDA, family Euphorbiaceae, and the genus Croton, encompassing approximately 1300 species distributed across tropical and subtropical regions16. In Colombia, the genus Croton exhibits a widespread distribution throughout the national territory and has been traditionally employed by indigenous populations. Notably, the red-colored plant extract known as "Sangre de Drago" has been historically utilized, attributing various properties such as anti-inflammatory, immunomodulatory, antioxidant, antiparasitic, antiviral, and antibacterial effects, among others17,18.
In response to the growing demand for novel antibiotics, recent research has explored extracts derived from Croton sp. In Kenya (2016), researchers assessed the extracts of Croton macrostachyus stem bark, traditionally employed in medicine, revealing their antibacterial activity against both S. aureus and E. coli19. Subsequently, a study conducted in Nigeria (2018) identified antimicrobial effects against S. aureus in the extract of Croton zambesicus muell20. In Cuba (2019), researchers observed the activity of an extract from Croton linearis Jacq. against S. aureus21. A study in India (2021) determined the antimicrobial activity against five human pathogens in Croton dichogamusse Pax, reporting that all extracts demonstrated activity against S. aureus, while showing no activity against E. coli strains22. In Brazil (2021), studies involving Croton heliotropiifolius Kunth reported antifungal and antioxidant properties with low cytotoxicity23. Most recently, a study in Peru (2023) evaluated the antimicrobial activity of three species—Croton adipatus Kunth, Croton thurifer Kunth, and Croton collinus Kunth—revealing antimicrobial activity against B. subtilis, C. albicans, and S. aureus24. These studies affirm the medicinal value of this plant, providing results of significance for the development of new drugs and the production of novel antibiotics.
In South America and Colombia, numerous species of the Croton genus, commonly known as "Sangre de Drago" (Dragon's Blood), are employed in traditional medicine24. Several Colombian native species, including Croton leptostachyus Kunth, Croton gossypiifolius Vahl, Croton killipianus Croizat, Croton hondensis (H. Karst) G.L. Webster, and Croton smithianus Croizat, hold promise for further research due to their limited scientific exploration despite traditional usage.
The ethanolic extract derived from the aerial parts of C. leptostachyus has demonstrated antimalarial activity, aligning with its traditional use for treating fever, headaches, and various illnesses25,26. Similarly, C. gossypiifolius latex exhibits antimicrobial and potential antibiofilm properties27. Traditional applications of other species include C. killipianus for digestive disorders, fever, and skin ailments, and C. hondensis for skin conditions and wounds28. Furthermore, the extracts from C. smithianus is used in folk medicine to address leishmaniasis lesions and promote wound healing29.
Considering the imperative need for novel therapeutic interventions against antibiotic-resistant bacteria, particularly those prioritized by the WHO, and acknowledging the promising alternative presented by essential oils extracted from plants of the genus Croton, the aim of this study was to assess the antibacterial activity against S. aureus and E. coli, as well as the chemical composition of essential oils obtained from five plants within the genus Croton from three sub-regions of Antioquia (Colombia).
Materials and methods
Plant material collection
Specimens of Croton killipianus and Croton smithianus were collected in the municipality of El Retiro, situated at an altitude of 2,500 m above sea level. Croton hondensis and Croton leptostachyus were sourced from the dry forest of the Cauca River canyon, located in the municipality of Sopetrán, at an altitude of 670 m above sea level. Additionally, Croton gossypiifolius was collected in the Porce River canyon, municipality of Amalfi, at an altitude of 1,800 m above sea level. The leaves were meticulously separated, selected, and subjected to drying in forced-convection ovens at 40 °C for 48–72 h. Control specimens were classified and deposited at the University of Antioquia’s Herbarium (HUA), as detailed in Table 1.
Extract preparation
The dried leaves of each plant were processed using a blade mill with a No. 40 sieve, followed by percolation with 30% ethanol, in a plant material-to-solvent ratio of 1:3. The filtered extracts underwent concentration using a rotary evaporator and were stored at 4 °C until utilization.
Extraction of essential oils
The dried samples were subjected to a hydro-distillation process using indirect steam distillation equipment for 2–3 h. The hydro-distillate was treated with a saturated sodium chloride solution and extracted with dichloromethane (CH2Cl2) to separate the essential oil from water. The organic phase was concentrated using a rotary evaporator at 30 °C. Subsequently, the final volume of essential oil obtained was quantified. The solvents used were HPLC or analytical grade (Merck Chemicals, Darmstadt, Germany). Deionized water was prepared with a Milli-Q water purification kit (Millipore, Bedford, MA, USA).
Antibacterial activity
Bacterial strains
The reference microorganisms used were Staphylococcus aureus subsp. aureus Rosenbach (ATCC 29213), methicillin and oxacillin-resistant Staphylococcus aureus subsp. aureus Rosenbach (MRSA, ATCC 43300), Escherichia coli (Migula) Castellani and Chalmers (ATCC 25922), and ampicillin and streptomycin-resistant Escherichia coli (Migula) Castellani and Chalmers (ATCC 700891).
Preparation of the bacterial inoculum
The microorganisms were cultured under sterile conditions on blood agar (ICMT, Instituto Colombiano de Medicina Tropical, Colombia) and incubated at 37 °C for 18–20 h. An inoculum was prepared by selecting 3–5 isolated colonies until achieving a turbidity equivalent to the 0.5 McFarland standard (1 × 108 CFU/mL). A 1:20 dilution was subsequently performed on this bacterial suspension to attain a concentration of 5 × 105 CFU/mL. Subsequently, 0.01 mL was added to each plate well, containing the culture medium and essential oil extract. This ensured a final volume of 0.1 mL with a bacterial concentration of 5 × 104 CFU/mL, in adherence to the guidelines set by the Clinical and Laboratory Standards Institute (CLSI)30.
Control of inoculum
A control was performed to validate that the final concentration of the bacterial inoculum cultured in each well corresponded to 5 × 104 CFU/mL. Post-inoculation, 0.01 mL of the growth control was promptly extracted and diluted in 10 mL of sterile saline solution. Subsequently, 0.1 mL of this dilution was seeded on a solid culture medium (blood agar for S. aureus and MacConkey agar for E. coli) and incubated at 37 °C for 24 h. The concentration was considered adequate when approximately 50 CFU/mL were counted.
Minimum inhibitory concentration (MIC)
Antibacterial activity was determined using the serial microdilution method, following the recommendations established by the Clinical and Laboratory Standards Institute (CLSI)30, and subsequently confirmed using the colorimetric method proposed by Abate et al.31. The microdilution method was performed in 96-well U-bottom plates, each well containing a final volume of 0.1 mL. The composition included 80% Müeller–Hinton culture medium, 10% of the essential oil, and 10% of the bacterial inoculum at a concentration of 5 × 104 CFU/mL.
To ensure miscibility, essential oils stock solutions were prepared aseptically in dimethyl sulfoxide (DMSO 5%) and sonicated. The essential oils were tested at six concentrations with each bacterial strain: 0.17, 0.085, 0.0425, 0.0213, 0.0106 and 0.0053 µL/mL. Four controls were integrated into the experiment: a negative or growth control (culture medium + bacterial inoculum), a positive or inhibition control (culture medium + bacterial inoculum + 10 µL of Lincomycin 630 mg / 2 mL or 10 µL of gentamicin 160 mg/2 mL for S. aureus and E. coli strains, respectively), a solvent toxicity control (culture medium + bacterial inoculum + dimethyl sulfoxide (DMSO)), and a contamination control (culture medium only). The plates were incubated at 37 °C for 16–20 h.
To confirm the results of the microdilution method, 10 μL of MTT (3-(4,5-dimethyltiazol-2-yl)-2,5-diphenyltetrazol bromide) at a concentration of 8 mg/mL were added to each well. The plate was then incubated at 37 °C under stirring and darkness for 1 h. The interpretation was conducted based on color changes, where yellow indicated inhibition of bacterial growth, while blue indicated the presence of bacteria. MIC was determined as the lowest concentration demonstrating inhibition of bacterial growth.
Minimum bactericidal concentration (MBC)
To determine the Minimum Bactericidal Concentration (MBC), 25 μL from each well of the microdilution test plates were seeded on solid agar and subsequently incubated at 37 °C for 24 h32. The MBC was defined as the lowest concentration of the essential oil that induced a complete bactericidal effect, evident by the absence of bacterial growth on the agar.
Cytotoxicity assessment
The Chinese hamster ovary (CHO)-K1 cell line (ATCCⓇ, CCL-61) was cultured in RPMI 1640 medium (Gibco), supplemented with 10% fetal bovine serum (FBS, Gibco) and 1% glutamine (Gibco), within T-25 cell culture flasks, for 18 h, at 37 °C, CO2 5% and 95% relative humidity. Once the cells reached the exponential phase, with a confluence of not less than 80% and viability ≥ 95%, 10,000 cells/well were cultured in 96-well plates, with a final volume of 0.1 mL for 24 h. Following this, the supernatant was removed, the culture medium was replaced, and the essential oils were added to each well. Negative control wells contained cells with culture medium, while positive control wells included cells with 0.01 mL DMSO. The cells were then reincubated for 24 h under the previously stated conditions. Subsequently, 0.01 mL of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) at a concentration of 5.0 mg/mL (resulting in a final well concentration of 0.5 mg/mL) was added, and the cells were incubated for an additional 4 h at 37 °C, with 5% CO2 and 95% relative humidity. The culture medium was then removed and 0.1 mL of DMSO was added. The dish was wrapped in aluminium foil and left to stir for 30 min. Finally, spectrophotometer readings were taken at 570 nm. The cell viability of each well was calculated by normalizing the absorbance of the negative control to 100% viability.
Phytochemical analysis of crude extracts
Qualitative determination of secondary metabolites in the extracts was conducted through precipitation and/or staining reactions. The assessed secondary metabolites included phenolic compounds (PC), tannins (TA), flavonoids (FL), leucoanthocyanidins (LE), cardiotonic compounds, α-β-unsaturated lactones (CA), saponins (SA), steroids (TE), quinones (QU), and alkaloids (AL).
Test for phenolic compounds (PC)
For qualitative phenolic compounds determination, two drops of Ferric chloride (FeCl3 2%) reagent were added to 2 mL of extract. The formation of a dark blue, violet, purple or dark green color was considered positive33.
Test for tannin (TA)
For qualitative tannin analysis, a few drops 1% gelatine solution, with 10% sodium chloride, were added to 2 mL of extract. A precipitate formation was considered a positive test for tannin33.
Test for flavonoids (FL)—Shinoda test
The crude extract, 2 mL, were mixed with a few small pieces of magnesium ribbon and then concentrated Hydrochloric acid was added to it drop by drop. After a few minutes, the appearance of pink to red color indicates the availability of flavonoids in the sample33.
Test for leucoanthocyanidins (LE)
The presence of leucoanthocyanin was tested with 2 mL of plant extract, it was add with 1 mL of Isoamyl alcohol. A red color in organic layer indicated the absence of leucoanthocyanins in the extracts33.
Test for cardiotonic compounds, α-β-unsaturated lactones (CA) -Kedde’s test
Qualitative analysis of cardiac glycosides in the extracts was performed with the Kedde reactive. To five milliliters of the extract were editioned 2 mL of alcoholic 3,5- dinitrobenzoic acid reagent (10%) and 1 ml of KOH solution (2 N in methanol). The reaction mixture immediately turns purple-violet33.
Test for alkaloid (AL)
For alkaloid determination, a few drops of Meyer's reagent were added to 1 mL of extract. The formation of a precipitate was considered positive for the alkaloid33.
Test for saponin (SA)
To detect the presence of saponin, 5 mL of extract in a test tube were vigorously agitated for 5 min. The formation of a foam column that did not disappear within the following 10 min were considered positive33.
Test for steroids (TE)—Liebermann–Burchard test
Five millilitres of the extract were dried, then, 2 mL of chloroform was added, and few drops of concentrated acetic anhydride was also added side by side. The evolution of red to violet colour in the lower chloroform layer directs the presence of steroids33.
Test for quinones (QU)—Borntrager’s test
A liquid–liquid extraction was made with 3 mL of extracts and 3 mL of chloroform. The chloroform layer was isolated. To this phase, five drops of 5% potassium hydroxide dissolution were added. Occurrence of red color in alkaline phase indicated the presence of quinones33.
Gas chromatographic analysis of essential oils
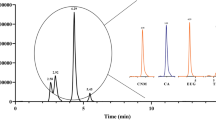
The essential oils were analyzed utilizing an Agilent Technologies 6890 Plus gas chromatograph coupled with an Agilent Technologies MSD 5973 mass selective detector. The system featured a split/splitless injection port (250 °C, 1:30 split ratio) and an Agilent 7863 automatic injector. A fused silica capillary column, HP-5MS (50 m × 0.25 mm DI), with a 5% phenyl-poly(methylsiloxane) stationary phase was employed, and helium (99.995%) served as the carrier gas at a velocity of 35 cm/s. The oven temperature was programmed from 40 °C for 15 min to 250 °C for 15 min, with a heating rate of 5 °C/min. Mass spectra were generated by electron impact, utilizing an ionization energy of 70 eV, and compound identification in the essential oils was accomplished using the NIST 98 database.
Statistical analysis
Normality tests were performed to determine the distribution of the data. The nonparametric Kruskal–Wallis test, followed by Post Hoc Dunn’s analysis, was employed for comparing multiple groups. The statistical analyses were performed using GraphPad Prism software, version 9.0 (GraphPad Software, Inc, San Diego, CA, USA.) Statistical significance was set at p < 0.05.
IUCN policy statement
The species included in this study do not have a diagnosis of vulnerability in terms of their conservation, according to the IUCN red list. Three of the species have been diagnosed as least-concern (LC) and the remaining two have not been evaluated (NE):
Croton smithianus LC.
Croton killipianus LC.
Croton hondensis NE.
Croton leptostachys NE.
Croton gossypiifolius LC.
Results and discussion
Yield of essential oils
Each of the five Croton sp. species exhibited distinct yields, ranging from 0.04 to 0.08 V/W. The highest yield was obtained by C. leptostachyus, followed by C. gossypiifolius (Table 2).
Yields of essential oils vary based on plant species and genera; however, these differences may be attributed to various factors, including pollinator activity, seasonal variations, and plant stressors, among others34. Sampaio de Souza et al. demonstrated that the essential oil yield from three Croton sp. species (Croton argyrophylloides Muell. Arg., Croton jacobinensis Baill, and Croton sincorensis Mart. Ex Muell. Arg.) was influenced by solar radiation, the season, and time of day35. Even though this study did not specifically focus on these factors, it is pertinent to consider that they could play a crucial role in the variability of results obtained in future research.
Antibacterial activity of essential oils
Antibacterial activity was identified in three of the five assessed Croton sp. species: C. killipianus, C. smithianus and C. hondensis. Notably, the essential oil derived from C. hondensis exhibited the highest activity, demonstrating both bacteriostatic and bactericidal effects against the susceptible strain of S. aureus. Additionally, it displayed a bacteriostatic effect against MRSA and both susceptible and resistant strains of E. coli (Fig. 1). Conversely, while the essential oil of C. killipianus demonstrated a bacteriostatic effect against both susceptible and resistant strains of S. aureus, no antibacterial activity was observed against the E. coli strains. Finally, the essential oil from C. smithianus exhibited activity solely against the susceptible strain of S. aureus (Table 3).
Representation of results for determination of MIC and MBC of C. hondensis essential oils against the four bacterial strains under study. (a) Representation of results for determination of MIC from essential oil of C. hondensis against the four studied bacterial strains. (a1) Map of MIC, (a2) sensitive S. aureus, (a3) Resistant S. aureus, (a4) Sensitive E. coli, (a5) Resistant E. coli, (a6) Controls: C + (inhibition control): culture medium + bacterial inoculum + 10 µL of Lincomycin 630 mg/2 mL or 10 µL of gentamicin 160 mg/2 mL for S. aureus and E. coli strains, respectively; C− (growth control): culture medium + bacterial inoculum. (b) Representation of results for MBC determination from essential oil of C. hondensis against S. aureus ATCC 29213. (b1–b4) Determination of bacterial inhibition or MBC by different essential oil concentrations. (b5) Inoculum control, to validate the final bacterial density inoculated for well in the MIC determinations. (b6) Growth control to validate the viability of bacteria in essential oil absence. The image for the growth control was obtained under a different background setting compared to the remaining figures. This variation does not impact the interpretation of the experimental results. All tests were conducted in triplicate.
Previous research has documented the antibacterial activity of essential oils from Croton cajura Benth36 and Croton conduplicatus Benth37 against MRSA. To our knowledge, our study contributes novel findings by revealing, for the first time, the antibacterial activity of essential oils derived from C. killipianus and C. hondensis against susceptible S. aureus strains and MRSA. Existing literature presents conflicting results concerning the impact of essential oils from various Croton sp. species on E. coli. Vieira da Costa et al. reported the activity of C. rhamnifolioides Pax&Hoffm. oil against an antibiotic-sensitive E. coli strain without evaluating such activity in resistant strains38. In contrast, other authors have reported a low inhibitory capacity of oils from different Croton sp. species against E. Coli39, and in some cases, no activity against this bacterium has been observed21,37,40. These findings underscore the species-dependent variability in the antibacterial activity of Croton sp. Notably, in this study, the C. hondensis essential oil was effective against both sensitive and ampicillin- and streptomycin-resistant E. coli strains and showed activity against MRSA. The ability of C. hondensis to act on both gram-negative and gram-positive bacteria and susceptible and resistant bacteria demonstrates the great potential of this species in pursuit of novel therapeutic alternatives.
Cytotoxic assessment of the essential oil of C. hondensis
Considering the exceptional antibacterial efficacy of C. hondensis essential oil against gram-negative and gram-positive strains, including resistant ones, we conducted a cytotoxicity assessment on the CHO-K1 cell line. The viability of cells exposed to essential oil at concentrations of 0.17, 0.085, and 0.0425 µL/mL was comparable to the negative control, exhibiting no statistically significant differences (p = 0.729, p = 0.277, and p = 0.052, respectively). These three concentrations are higher than MIC values found for the four bacterial strains. Interestingly, cell viability displayed a direct correlation with essential oil doses, demonstrating that lower oil concentrations resulted in reduced cell viability. This observation suggests that, in addition to its antibacterial properties, C. hondensis essential oil may have a stimulatory or protective impact on CHO cells at the mentioned concentrations (Fig. 2). The beneficial effect on CHO cell viability could be attributed to the presence of antioxidants, such as phenolic compounds (PC). Prior investigations demonstrated the notable antioxidant capacity of C. conduplicatus Kunth essential oils, evidenced by their significant inhibition of synthetic free radicals41.
CHO cells viability. C +: Positive control (cells with culture medium and 0.01 mL DMSO). C−: Negative control (cells with culture medium). *p < 0.05. ns: Non-significant difference. The viability of cells at concentrations 0.0213, 0.0106 and 0.0053 µL/mL was statistically different to negative control (p < 0.05), indicating a not admissible viability. All tests were conducted in triplicate.
Phytochemical screening of extracts
This study identified five secondary metabolites in the leaf extracts of analyzed Croton sp. species, including phenolic compounds (PC), tannins (TA), flavonoids (FL), steroids (TE), and quinones. However, we confirmed the absence of leucoanthocyanidins, cardiotonic compounds-α-β-unsaturated lactones, saponins, and alkaloids (Table 4). Morais et al. reported not finding phenolic compounds in four species of Croton sp. they evaluated42. This finding contrasts with our results, where all analyzed Croton sp. species exhibited the presence of phenolic compounds, known for their significant antioxidant activity. It could be hypothesized that these compounds are associated with the viability observed in CHO cells exposed to the highest concentrations of C. hondensis. Additionally, this study found the presence of flavonoids in this plant. Previous reports have associated these compounds with bacteriostatic, bactericidal, and free-radical scavenging activities43.
Analysis of essential oils through gas-coupled mass spectrometry
The essential oils from the plants investigated in this study exhibited distinct qualitative chemical compositions. While certain components were common across all species, others were unique to specific ones, creating variability in their overall profiles. The compounds γ-muurolene, α-humulene, (E)-caryophyllene, and curcumene were identified in all five species of Croton sp. On the contrary, α-copaene and (E)-nerolidol were not detected in C. smithianus and C. killipianus, respectively. γ-Muurolene stood out as the primary component in four out of the five Croton sp. species, except for C. smithianus, where α-humulene predominated as the major constituent (Table 5).
Our study results diverge from those reported by De Souza et al., where (E)-caryophyllene was identified as the most abundant element in certain Croton sp. species35. However, in alignment with our findings, another study identified the presence of γ-muurolene in the essential oil of C. heliotropiifolius Kunth, linking this compound to observed antibacterial activity against S. aureus44. γ-Muurolene was the predominant component in the C. hondensis essential oil. Remarkably, this specie was the sole plant among the five evaluated that displayed bactericidal activity and show bacteriostatic activity against ampicillin- and streptomycin-resistant E. coli and MRSA.
This study has limitations. While C. hondensis exhibited clear antibacterial activity, this effect was not observed in the essential oils of C. leptostachyus and C. gossypiifolius. Additionally, C. killipianus and C. smithianus demonstrated moderate antibacterial activity, exhibiting only bacteriostatic effects against S. aureus (both sensitive and methicillin-resistant) or sensitive S. aureus alone, respectively. However, the low concentrations of essential oils used in this study necessitate further investigation. The presence of potentially antibacterial compounds, such as γ-muurolene, and the observed effect at low concentrations of oils suggest that higher concentrations might demonstrate enhanced antibacterial activity. Furthermore, the significant antibacterial activity exhibited by C. hondensis at these low concentrations, coupled with its potential stimulating effect on eukaryotic cells, underscores its promising biological potential.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
WHO—World Health Organization. Report signals increasing resistance to antibiotics in bacterial infections in humans and need for better data. Report signals increasing resistance to antibiotics in bacterial infections in humans and need for better data https://www.who.int/news/item/09-12-2022-report-signals-increasing-resistance-to-antibiotics-in-bacterial-infections-in-humans-and-need-for-better-data (2022).
Murray, C. J. L. et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. The Lancet 399, 629–655 (2022).
Cheung, G. Y. C., Bae, J. S. & Otto, M. Pathogenicity and virulence of Staphylococcus aureus. Virulence 12, 547–569 (2021).
Jelassi, M. L., Benlmouden, A., Lefeuvre, S., Mainardi, J.-L. & Billaud, E. M. Niveau de preuve pour le suivi thérapeutique pharmacologique de la vancomycine. Therapies 66, 29–37 (2011).
Bruniera, F. R. et al. The use of vancomycin with its therapeutic and adverse effects: A review. Eur. Rev. Med. Pharmacol. Sci. 19, 694–700 (2015).
Turner, N. A. et al. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Microbiol. 17, 203–218 (2019).
Diekema, D. J. et al. The Microbiology of Bloodstream Infection: 20-Year Trends from the SENTRY Antimicrobial Surveillance Program. Antimicrob. Agents Chemother. 63, (2019).
Xiao, S. et al. Antimicrobial resistance and molecular epidemiology of escherichia coli from bloodstream infection in Shanghai, China, 2016–2019. Front Med. (Lausanne) 8, (2022).
Jain, P. et al. High prevalence of multiple antibiotic resistance in clinical E. coli isolates from Bangladesh and prediction of molecular resistance determinants using WGS of an XDR isolate. Sci. Rep. 11, 22859 (2021).
Charani, E. et al. An analysis of existing national action plans for antimicrobial resistance—gaps and opportunities in strategies optimising antibiotic use in human populations. Lancet Glob. Health 11, e466–e474 (2023).
Petrovska, B. Historical review of medicinal plants′ usage. Pharmacogn. Rev. 6, 1 (2012).
Gómez-Estrada, H. et al. Folk medicine in the northern coast of Colombia: An overview. J. Ethnobiol. Ethnomed. 7, 27 (2011).
Angulo, A. F., Rosero, R. A. & González, M. S. Estudio etnobotánico de las plantas medicinales utilizadas por los habitantes del corregimiento de Genoy, Municipio de Pasto. Colombia. Univ Salud 14, 168–185 (2012).
Bakkali, F., Averbeck, S., Averbeck, D. & Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 46, 446–475 (2008).
Panda, S. et al. Essential oils and their pharmacotherapeutics applications in human diseases. Adv. Trad. Med. 22, 1–15 (2022).
Coy-Barrera, C. A., Gómez, C. & Castiblanco, F. A. Importancia medicinal del género Croton (euphorbiaceae). Revista Cubana de Plantas Medicinales 21, (2016).
Gupta, D. & Gupta, R. K. Bioprotective properties of Dragon’s blood resin: In vitro evaluation of antioxidant activity and antimicrobial activity. BMC Complement Altern. Med. 11, 13 (2011).
Obey, J. K., von Wright, A., Orjala, J., Kauhanen, J. & Tikkanen-Kaukanen, C. Antimicrobial activity of croton macrostachyus stem bark extracts against several human pathogenic bacteria. J. Pathog. 2016, 1–5 (2016).
Ajayi, A. O. & Omomagiowawi, I. E. Antimicrobial Activity of Croton zambesicus on Staphylococcus aureus and Streptococcus species. SYLWAN 160, 114–124 (2018).
García Díaz, J. et al. Antimicrobial activity of leaf extracts and isolated constituents of Croton linearis. J. Ethnopharmacol. 236, 250–257 (2019).
Matara, D. N., Nguta, J. M., Musila, F. M. & Mapenay, I. Phytochemical analysis and investigation of the antimicrobial and cytotoxic activities of croton dichogamus pax crude root extracts. Evid. Based Complement. Altern. Med. 2021, 1–9 (2021).
Fernandes, P. A. de S. et al. Chemical Constituents and Biological Activities of Croton heliotropiifolius Kunth. Antibiotics 10, 1074 (2021).
Cucho-Medrano, J. L. L., Mendoza-Beingolea, S. W., Fuertes-Ruitón, C. M., Salazar-Salvatierra, M. E. & Herrera-Calderon, O. Chemical profile of the volatile constituents and antimicrobial activity of the essential oils from Croton adipatus, Croton thurifer, and Croton collinus. Antibiotics 10, 1387 (2021).
Pieters, L. La ‘Sangre de Drago’ Una Droga Tradicional de Sudamérica. (Quito, 1998).
Garavito, G. et al. Antimalarial activity of some Colombian medicinal plants. J. Ethnopharmacol. 107, 460–462 (2006).
Selina Wamucii. Croton leptostachyus—Uses, Benefits & Care. Croton leptostachyus—Uses, Benefits & Care https://www.selinawamucii.com/plants/euphorbiaceae/croton-leptostachyus/.
Bérubé, C., Borgia, A., Simon, G., Grenier, D. & Voyer, N. Total synthesis of Crotogossamide using an on-resin concomitant cyclization/cleavage reaction. Phytochem. Lett. 26, 101–105 (2018).
Selina Wamucii. Euphorbiaceae Family Croton Genus. Euphorbiaceae Family Croton Genus https://www.selinawamucii.com/plants/euphorbiaceae/croton/.
Perez, R., Condit, R. & Smithsonian Tropical Research Institute. Croton smithianus Croizat. Smithsonian Tropical Research Institute https://panamabiota.org/stri/taxa/index.php?taxon=63667&clid=71.
CLSI - Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard, Tenth Edition M07-A10. (Wayne, 2015).
Abate, G., Mshana, R. & Miörner, H. Evaluation of a colorimetric assay based on 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) for rapid detection of rifampicin resistance in Mycobacterium tuberculosis. 2, 1011–1016 (1998).
Balouiri, M., Sadiki, M. & Ibnsouda, S. K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 6, 71–79 (2016).
Harborne, J. B. Methods of plant analysis. A guide to modern techniques of plant analysis. In Phytochemical Methods 1–32 (Chapman and Hall, London, 1973).
Biswas, S., Koul, M. & Bhatnagar, A. K. Effect of salt, drought and metal stress on essential oil yield and quality in plants. Nat. Prod. Commun. 6, 1559–1564 (2011).
Souza, G. S. de, Bonilla, O. H., Lucena, E. M. P. de & Barbosa, Y. P. Chemical composition and yield of essential oil from three Croton species. Ciência Rural 47, 1 (2017).
Azevedo, M. et al. Antioxidant and antimicrobial activities of 7-hydroxy-calamenene-rich essential oils from croton cajucara benth. Molecules 18, 1128–1137 (2013).
Oliveira, G. D. de, Rocha, W. R. V. da, Rodrigues, J. F. B. & Alves, H. da S. Synergistic and antibiofilm effects of the essential oil from croton conduplicatus (Euphorbiaceae) against methicillin-resistant Staphylococcus aureus. Pharmaceuticals 16, 55 (2022).
da Costa, A. C. V. et al. Chemical composition and antibacterial activity of essential oil of a Croton rhamnifolioides leaves Pax & Hoffm. Semin. Cienc Agrar. 34, 2853 (2013).
de Vasconcelos, E. C. et al. Modeling the effect of Croton blanchetianus Baill essential oil on pathogenic and spoilage bacteria. Arch. Microbiol. 204, 618 (2022).
Carvalho, R. J. P. et al. Antimicrobial activity the essential oil from croton pluriglandulosus carn. Leaves against Microorganisms of Clinical Interest. J. Fungi 9, 756 (2023).
Almeida, J. et al. Chemical composition of essential oils from croton conduplicatus (Euphorbiaceae) in two different seasons. J. Essent. Oil Bear. Plants 17, 1137–1145 (2014).
Morais, S. et al. Essential oils from croton species: Chemical composition, in vitro and in silico antileishmanial evaluation, antioxidant and cytotoxicity activities. J. Braz. Chem. Soc. https://doi.org/10.21577/0103-5053.20190155 (2019).
Ayele, D. T., Akele, M. L. & Melese, A. T. Analysis of total phenolic contents, flavonoids, antioxidant and antibacterial activities of Croton macrostachyus root extracts. BMC Chem. 16, 30 (2022).
Araújo, F. M. et al. Antibacterial activity and chemical composition of the essential oil of Croton heliotropiifolius Kunth from Amargosa, Bahia Brazil. Ind Crops Prod. 105, 203–206 (2017).
Acknowledgements
Our sincere thanks to Dr. Álvaro Cogollo Pacheco for his invaluable guidance and expertise during this study. His contributions greatly enriched our research.
Funding
This article was funded by Universidad Cooperativa de Colombia (INV1374), Institución Universitaria Colegio Mayor de Antioquia and Universidad de Antioquia.
Author information
Authors and Affiliations
Contributions
JAS, EG, and ACOG conceived the study and secured funding. ICS, EG, FA, and ACOG planned and conducted the field trips and laboratory procedures. ICS, JAS, EG, and ACOG analyzed the results. JAS, EG, and ACOG drafted the manuscript. All authors critically reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sánchez, I.C., Segura Caro, J.A., Galeano, E. et al. Essential oils from Colombian Croton spp. exhibit antibacterial activity against methicillin-resistant Staphylococcus aureus (MRSA) and ampicillin- and streptomycin-resistant Escherichia coli. Sci Rep 14, 30643 (2024). https://doi.org/10.1038/s41598-024-65961-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-65961-x