Abstract
To investigate the effect of low-doses esketamine on spinal anesthesia-induced hypotension in women with preoperative anxiety undergoing elective cesarean section, the randomized controlled trial enrolled 120 women aged 18–35 years who preoperative State-Trait Anxiety Inventory State scores > 40, conducted from September 2022 to August 2023 in Xuzhou Central Hospital, China. Women in the esketamine group received a single intravenous injection of 0.2 mg/kg esketamine after sensory block level achieved. The incidence of hypotension in the esketamine group was significantly lower than the control group at T2 (10% [6 of 60]; P < 0.001), T3 (5.0% [3 of 60]; P = 0.007) and T4(5.0% [3 of 60]; P = 0.004). Despite being higher in the esketamine group, the overall rates of hypertension (11.7% [7 of 60]; P = 0.186), tachycardia (23.3% [14 of 60]; P = 0.246), and bradycardia (0.0% [0 of 60]; P = 0.079) were no significantly difference between the two groups. STAI-S scores was significantly lower in the esketamine group (mean [SD] 37.52[7.14]) than in the control group (mean [SD] 41.03[9.66], P = 0.39) in postoperative day 1. Spinal anesthesia combined with intravenous low-doses esketamine infusion can significantly reduce the incidence of hypotension in women with preoperative anxiety undergoing elective cesarean section.
Similar content being viewed by others
Introduction
For elective cesarean deliveries, spinal anesthetic is the recommended option due to its quicker onset of action, decreased risk of complications, and greater benefits1. While hypotension is the most frequent adverse consequence of spinal anesthesia2,3, it may result in negative side effects such vomiting, nausea, dyspnea4,5, even more reduced uteroplacental blood flow, which could culminate in umbilical arterial hemorrhagic acidosis, and a lower fetal Apgar score6. Thus, hypotension significantly affects the prognosis of both the mother and the fetus. According to recent research, women's preoperative anxiety significantly influences the development of hypotension following spinal anesthesia, and up to 70% of these women may require vasoactive medications during cesarean sections2,7.
The dextro-isomer of ketamine, esketamine, is known to sustain circulatory excitability through sympathomimetic action8. Furthermore, esketamine has been observed in recent research to be beneficial for those with anxiety and depression9. However, it is currently unknown how esketamine affects hemodynamics following spinal anesthesia in parturient with preoperative anxiety. So, we designed a randomized controlled trial to investigate the effect of low-doses esketamine on spinal anesthesia-induced hypotension in women with preoperative anxiety undergoing elective cesarean section, along with to assess the safety and efficacy of esketamine for both the mothers and the fetus.
Method
Study design and patient enrollment
This was a single-center, double-blind, randomized, placebo-controlled study, registered in the Chinese Clinical Trial Registry (ChiCTR2300078343, 05/12/2023). An informed consent form was signed by each of the 120 eligible participants who had elective cesarean sections performed at Xuzhou Central Hospital from September 2022 to August 2023. The study was performed in accordance with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Inclusion criteria included (1) women aged 18–35 years; (2) full-term singleton pregnancy with regular prenatal checkups; (3) ASA classification I–II and BMI of 19–29 kg/m2; (4) planned elective cesarean section under spinal anesthesia;(5) preoperative State-Trait Anxiety Inventory- State (STAI-S) scores ≥ 40. Maternal with the following conditions were not eligible for the trial: (1) ASA classification III or higher; (2) a history of contraindication to the use of esketamine or nonsteroidal anti-inflammatory drugs (NSAIDs) (severe risk of elevated blood pressure and intracranial pressure, poorly controlled or untreated hypertension, severe arrhythmia, untreated or inadequately treated hyperthyroidism, and gastrointestinal bleeding); (3) history of psychiatric disorders or preoperative use of relevant anxiolytic or sedative medications; (4)history of chronic pain, or central nervous system disorders; (5) severe obstetric complications, such as preeclampsia and eclampsia, placenta previa, or placenta previa; (6) stillbirths or neonatal malformations; and (7) history of gastroesophageal reflux. Subjects with change of surgical procedure, change of anesthesia, unanticipated serious complications, or postoperative ICU admission during the trial would be excluded.
Ethics approval
The study was approved by the Clinical Research Ethics Committee of Xuzhou Central Hospital (XZXY-LK-20220526-044).
Randomization and blinding
The SPSS software version 26.0 (IBM Corp.) was used to create random assignments at a 1:1 ratio. Sequentially numbered envelopes held allocations. Before the anesthesiologist was put under anesthesia, non-trial collaborators unwrapped the envelopes in the order that they were recruited, prepared esketamine or an identical saline placebo, and gave the study medication to the attending anesthesiologist. The investigator in charge of data collection and follow-up was blind to the group assignment.
Research protocol
No preanesthetic drugs were given to any of the subjects. Following admittance to the operation room, an intravenous access was regularly opened; heart rate and oxygen saturation were continuously tracked; and every five minutes, noninvasive blood pressure (NBP) was measured. Every patient received regular perioperative rehydration, and oxygen was delivered through a mask at a flow rate of 5L/min. Spinal anesthesia was performed with parturient in the left lateral decubitus position. 2% lidocaine was administered for local anesthesia. A 25G needle was used for subarachnoid puncture in the L3-4 intervertebral space. Once the cerebrospinal fluid was detected, 15 mg of 0.5% ropivacaine was slowly injected. After the procedure, the patient was immediately moved to the supine position with 15° leftward tilt. The upper sensory block was targeted between the T4 and T6 levels, patients who did not meet blocking requirements would be excluded. Specifically, patients in the esketamine group received a single intravenous injection of 0.2 mg/kg esketamine after block level achieved; while the control group received an injection of the same volume of saline.
Adverse hemodynamic events were continuously monitored. Hypotension was defined as systolic blood pressure less than 90 mmHg or more than 20% decrease from baseline; hypertension was defined as systolic blood pressure greater than 160 mmHg or more than 20% increase from baseline; tachycardia was defined as heart rate greater than 100 beats per minute or more than 20% increase from baseline; bradycardia was defined as heart rate less than 60 beats per minute or more than 20% decrease from baseline. An initial infusion of 4 μg/ml norepinephrine will be given if hypotension present10, other events were managed routinely.
In this study, diclofenac sodium 100 mg combined with acetaminophen 1000 mg in a single rectal dose was taken for multimodal analgesia instead of intravenous opioids for postoperative analgesia, as recommended by the guidelines11,12.
Outcomes
The incidence of intraoperative maternal hypotension was the main outcome indicator in this trial. At the same time, the patient’s blood pressure would be recorded in detail at the following six time points: T0: before spinal anesthesia (basal blood pressure); T1: immediately after spinal anesthesia; T2: 2 min after esketamine or saline administration; T3: beginning of surgery; T4: delivery of the fetus; T5: End of surgery.
Secondary outcome indices included (1) adverse hemodynamic event, including hypertension, tachycardia, bradycardia and various arrhythmias during surgery; (2) STAI-S scores in postoperative day 1 and day 7; (3) the incidence of neuropsychiatric symptoms during surgery and in postoperative day 1, including somnolence, hallucination, dizziness and other related symptoms; (4) the modified Observer’s Assessment of Alertness/Sedation(OAA/S) scores at T2, T4, T5; (5) neonatal umbilical vein blood pH, and neonatal Apgar scores at 1 and 5 min at the time of delivery; (6) intraoperative and postoperative day 1 nausea and vomiting; (7) the postoperative day 1 visual pain score (VAS).
Data collection
Demographic information, maternal gestational week, gestational diabetes mellitus, gestational hypertension, baseline blood pressure, and baseline heart rate (heart rate before anesthesia) were all included in the baseline data. And intraoperative information, including duration of the procedure, hemodynamic data (blood pressure, heart rate), use of vasoactive medications, and neonatal outcomes.
Statistical methods and sample size calculation
The rate of intraoperative hypotension in women undergoing elective cesarean sections under spinal anesthesia is 60–70%, according to prior studies6,13,14. With α set at 0.05 and power at 90%, assuming the incidence of hypotension in the control group is 60%, and 50% reduction in the incidence of maternal hypotension in the esketamine group compared to the control group, this required 53 patients in each group to be statistically significant. Given the approximately 10% drop rate, we projected to include 60 patients in each group, for a total of 120 patients. Version 15.0 of the PASS software was used to estimate the sample size (NCSS).
For continuous variables, the Kolmogorov–Smirnov test was used to assess the distribution. Normally distributed variables were expressed as means and comparisons between groups were made using the two-tailed independent samples t-test. Non-normally distributed variables were expressed as medians and comparisons between groups were made using the Mann–Whitney U test. Categorical variables were analyzed using the χ2 test and expressed as percentages.
For the primary outcome, a two-sided P < 0.025 (0.05/2) was considered statistically significant. For other outcomes, a two-sided P < 0.05 was considered statistically significant unless otherwise stated after Bonferroni correction. SPSS for Windows, version 26.0 (IBM Corp.) and GraphPad Prism, version 9.0 (GraphPad Software Inc.) were used for statistics and plotting.
Results
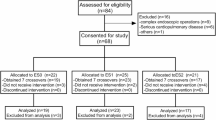
The subjects are shown in Fig. 1. Following the principle of randomized grouping, 120 patients who met the inclusion criteria were separated into two groups of 60 cases each, and entered into the analysis of results. As demonstrated in Table 1, there was no discernible difference between the two groups’ baseline information about the demographics of the mothers and the duration of the surgery.
Following spinal anesthesia (T1), the rate of hypotension in the two groups did not differ statistically significantly (38.3% [23 of 60] vs. 41.7% [25 of 60], P = 0.709). However, adhering to drug administration (T2), the esketamine group’s blood pressure significantly recovered, the incidence of hypotension was significantly lower at T2 (45.0% [27 of 60] vs. 10.0% [6 of 60], P < 0.001), T3 (21.7% [13 of 60] vs. 5.0% [3 of 60], P = 0.007) and T4(23.3% [14 of 60] vs. 5.0% [3 of 60], P = 0.004) (Fig. 2A). On the other hand, despite being higher in the esketamine group, the overall rates of hypertension (5.0% [3 of 60] vs. 11.7% [7 of 60], P = 0.186), tachycardia (15.0% [9 of 60] vs. 23.3% [14 of 60], P = 0.246), and bradycardia (5.0% [3 of 60] vs. 0.0% [0 of 60], P = 0.079) were no significantly difference between the two groups from T2 to T5 (Fig. 2B).
Comparison of adverse hemodynamic events. T0: before spinal anesthesia (basal blood pressure); T1: immediately after spinal anesthesia; T2: 2 min after esketamine or saline administration; T3: beginning of surgery; T4: delivery of the fetus; T5: End of surgery; Hypertension: Defined as systolic blood pressure greater than 160 mmHg or an increase of greater than 20% from baseline; Hypotension: Defined as systolic blood pressure less than 90 mmHg or a decrease of greater than 20% from baseline. Tachycardia: Defined as heart rate greater than 100 beats per minute or an increase of greater than 20% from baseline; Bradycardia: Defined as heart rate less than 60 beats per minute or a decrease of greater than 20% from baseline; **:P < 0.01; ***:P < 0.001.
The women in the esketamine group and control group did not exhibit any significant differences in their basal blood pressure (T0) or post-anesthesia blood pressure (T1). After the drug (T2) was administered, the esketamine group's maternal blood pressure was consistently higher than the control group's (mean [SD] 116.76[9.77] mmHg vs. 106.00[6.69] mmHg; P < 0.001). According to statistical analysis, both groups' blood pressure increased from the start of the procedure until the fetus was delivered (T3–T4). Figure 3 displays the variations in heart rate and blood pressure between the two groups.
STAI-S scores was significantly lower in the esketamine group (mean [SD] 37.52[7.14]) than in the control group (mean [SD] 41.03[9.66], P = 0.39) in postoperative day 1, but there was no significant difference in postoperative day 7. Regarding neuropsychiatric symptoms, we found that women in the esketamine group experienced dreaming (11.7% [7/60]; P = 0.019), whereas there were no comparable manifestations in the control group. Additionally, the remaining adverse neuropsychiatric symptoms were not detected during this trial.
The modified OAA/S scores of esketamine group were significantly lower than the control groups at T2 (median [IQR] 5 [5 to 5] vs. 4 [4 to 4], P < 0.001) and T4 (median [IQR] 5 [5 to 5] vs. 4 [4 to 5], P = 0.012), while in the end of surgery, there was no significant difference in the modified OAA/S scores between two groups. Furthermore, the newborns in the esketamine group had 1 min and 5 min Apgar ratings of 10 (10, 10), and their umbilical vein blood pH was 7.33 ± 0.04, which did not differ significantly from the control group. There also was no significant difference in the VAS scores or incidence of mother nausea and vomiting between two groups. (Table 2).
Discussion
Consistent with earlier research findings15,16, our trial showed that esketamine administration preserves maternal hemodynamic stability during elective cesarean section. As we know, sympathetic blockade is currently thought to be the primary mechanism causing maternal hypotension brought by spinal anesthesia, and the extent of hypotension is mostly determined by the range of sympathetic blockade6,13. Through its sympathomimetic actions, esketamine stimulates the cardiovascular system in a concentration-dependent way8. This counteracts the circulatory depression caused by sympathetic blockade during spinal anesthesia, thereby lowering the likelihood of hypotension. In addition, previous research has also shown that esketamine maintains hemodynamic stability in patients under general anesthesia8,17,18,19,20,21. The compression of the inferior vena cava and volume insufficiency caused by the body position may also have an effect on the variation in the mother's blood pressure22, so that without sympathomimetic actions it finally resulted in a slight increase in the rate of hypotension in the control group at T2.
On the other hand, the esketamine group experienced greater rates of maternal hypertension and tachycardia than the control group, but the differences were not significant. The elevation in blood pressure brought on by subanesthetic doses of esketamine was transient and quickly went away after the drug was stopped, according to a meta-analysis of the hemodynamic response to these doses23. Likewise, there were no unfavorable effects from tachycardia or hypertension in our trial, and other kinds of arrhythmias was not observed. In hence, we held that it’s feasible to use 0.2 mg/kg esketamine on maternal to remain hemodynamic stability.
When evaluating maternal anxiety and depression, STAI has a strong link. And postpartum depression risk is significantly raised with a STAI-S score of > 40 in the early postpartum period24,25,26. Esketamine has been shown to be helpful for anxious and depressed patients in recent years, and in this study, we found that maternal STAI-S scores were significantly lower in the esketamine group at 1 day postoperatively than in the control group, but no significant difference was observed between the two groups at 1 week postoperatively. While, in another study of esketamine on postpartum depression, intraoperative and postoperative co-application of esketamine was shown to be effective in improving maternal depressive symptoms 1 week after surgery27. This could be because we only gave a low dose intravenous injection of esketamine following spinal anesthesia, and the anxiolytic effect faded quickly once the drug’s metabolism was finished.
About the neurological and psychological symptoms that esketamine can produce, the esketamine group showed no symptoms other than dreaming, and it's important to note that the women had cheerful, joyful dreams instead of nightmares (Table 2). In another study, the researcher reported that intravenous injection of 0.25 mg/kg of esketamine caused maternal symptoms including somnolence, nystagmus, and vertigo, but the adverse effects were transient and only appeared during the surgery15. In addition, although the modified OAA/S scores in the esketamine group were significantly lower than those in the control group at T2 and T4, there was no significant difference in the state of consciousness between the two groups at the end of the surgery.
In patients undergoing cesarean section, sympathetic blockade and hypotension during anesthesia is one of the currently recognized mechanisms of nausea and vomiting4,14,28,29. Besides, the unavoidable intraoperative manipulation of the uterus and peritoneum and intraperitoneal saline irrigation can activate the vagal nerve, which is highly emetic and significantly increases the risk of IONV and PONV29,30. Consequently, we hypothesized that esketamine might be able to antagonize the mechanisms involved in nausea and vomiting by stimulating the sympathetic nerves. However, in this trial, there was no significant difference in the incidence of nausea and vomiting between the esketamine group and the control group, despite the fact that it was slightly lower in the former. More research is required to determine whether esketamine, alone or in combination with other medications, can lower the incidence of IONV and PONV.
The two groups' postoperative VAS scores were not significantly different. This might be because the study employed a uniform method for postoperative analgesia, along with since esketamine has a mean elimination half-life of roughly 4 h, the benefits of once intravenous esketamine infusion were unable to remain for more than 24 h after surgery. The neonatal-related outcome indicators we examined as regards of neonatal safety were umbilical vein blood pH and neonatal Apgar scores at 1 and 5 min31,32. And the fetus in this research did not exhibit any adverse effects from 0.2 mg/kg of esketamine, which was within the subanesthetic dosage range33.
Limitations
Firstly, since intravenous oxytocin were not administered to all of the women in this research, we did not tally the hemodynamic changes at specific points. While maternal in the esketamine group showed comparatively consistent hemodynamic performance throughout the period. Secondly, the sample size of this study is small and is subject to comparative validation in subsequent studies. As this study only examined esketamine's effects on intraoperative maternal hemodynamics, future research could concentrate on its impact on postoperative maternal hemodynamics.
Conclusion
Spinal anesthesia combined with intravenous low-doses esketamine infusion can significantly reduce the incidence of hypotension in women with preoperative anxiety undergoing cesarean section, and with no apparent adverse effects on the fetus.
Data availability
The datasets used and/or analyzed during this study are available from the corresponding author on reasonable request.
References
Riley, E. T. et al. Spinal versus epidural anesthesia for cesarean section: A comparison of time efficiency, costs, charges, and complications. Anesth. Analg. 80(4), 709–712 (1995).
Orbach-Zinger, S. et al. Influence of preoperative anxiety on hypotension after spinal anaesthesia in women undergoing Caesarean delivery. Br. J. Anaesth. 109(6), 943–949 (2012).
Zieleskiewicz, L. et al. Can point-of-care ultrasound predict spinal hypotension during caesarean section? A prospective observational study. Anaesthesia 73(1), 15–22 (2018).
Macones, G. A. et al. Guidelines for postoperative care in cesarean delivery: Enhanced Recovery After Surgery (ERAS) Society recommendations (part 3). Am. J. Obstet. Gynecol. 221(3), 247 (2019).
Wilson, R. D. et al. Guidelines for antenatal and preoperative care in cesarean delivery: Enhanced recovery after surgery society recommendations (part 1). Am. J. Obstet. Gynecol. 219(6), 523 (2018).
Knigin, D., Avidan, A. & Weiniger, C. F. The effect of spinal hypotension and anesthesia-to-delivery time interval on neonatal outcomes in planned cesarean delivery. Am. J. Obstet. Gynecol. 223(5), 747 (2020).
Sahin, T. et al. Association between preoperative maternal anxiety and neonatal outcomes: A prospective observational study. J. Clin. Anesthes. 33, 123–126 (2016).
Zhang, J. et al. Effect of intraoperative infusion of esketamine on quality of postoperative recovery in patients undergoing laparoscopic bariatric surgery: A randomized controlled trial. Pain Ther. 12(4), 979–992 (2023).
Smith-Apeldoorn, S. Y. et al. Maintenance ketamine treatment for depression: A systematic review of efficacy, safety, and tolerability. Lancet Psychiatry 9(11), 907–921 (2022).
Kinsella, S. M. et al. International consensus statement on the management of hypotension with vasopressors during caesarean section under spinal anaesthesia. Anaesthesia 73(1), 71–92 (2018).
Obstetrics N C F H Q M I, Chinese Society Of Perinatal Medicine C M A. Expert consensus on cesarean section (2023). Zhonghua Fu Chan Ke Za Zhi 59(1), 14–21 (2024).
Ong, C. K. S. et al. Combining paracetamol (acetaminophen) with nonsteroidal antiinflammatory drugs: A qualitative systematic review of analgesic efficacy for acute postoperative pain. Anesthes. Analges. 110(4), 1170 (2010).
Gong, R. S. et al. Effects of colloid preload on the incidence of hypotension in spinal anesthesia for cesarean section: A systematic review and meta-analysis. Chin. Med. J. 134(9), 1043–1051 (2021).
Wang, Y. Q., Zhang, X. J. & Wang, Y. Effect of intrathecal dexmedetomidine on cesarean section during spinal anesthesia: A meta-analysis of randomized trials. Drug Des. Dev. Ther. 13, 2933–2939 (2019).
Xu, L.-L. et al. Efficacy and safety of esketamine for supplemental analgesia during elective cesarean delivery. JAMA Netw. Open 6, 4 (2023).
Liang, Z. et al. Neonatal outcomes when intravenous esketamine is added to the parturients transferred from labor analgesia to emergency cesarean section: A retrospective analysis report. BMC Anesthesiol. 23(1), 168 (2023).
Zheng, L. et al. Efficacy and safety of a subanesthetic dose of esketamine combined with propofol in patients with obesity undergoing painless gastroscopy: A prospective, double-blind, randomized controlled trial. Drug Des. Dev. Ther. 17, 1347–1356 (2023).
Yang, H. et al. The median effective concentration of propofol with different doses of esketamine during gastrointestinal endoscopy in elderly patients: A randomized controlled trial. Br. J. Clin. Pharmacol. 88(3), 1279–1287 (2022).
Liu, X., Xiao, Q. & Zhuang, S. Comparison of propofol-esketamine versus propofol for anesthesia in gastroscopy: A double-blind, randomized controlled clinical trial. Front. Med. 10, 1184709 (2023).
Huang, X. et al. Comparison of the effects of esketamine/propofol and sufentanil/propofol on the incidence of intraoperative hypoxemia during bronchoscopy: Protocol for a randomized, prospective, parallel-group trial. J. Clin. Med. 11, 15 (2022).
Fan, Q. et al. Esketamine opioid-free intravenous anesthesia versus opioid intravenous anesthesia in spontaneous ventilation video-assisted thoracic surgery: A randomized controlled trial. Front. Oncol. 13, 1145953 (2023).
Ferré, F. et al. Control of spinal anesthesia-induced hypotension in adults. Local Reg. Anesth. 13, 39–46 (2020).
Vankawala, J. et al. Meta-analysis: Hemodynamic responses to sub-anesthetic doses of ketamine in patients with psychiatric disorders. Front. Psychiatry 12, 549080 (2021).
Dennis, C.-L., Coghlan, M. & Vigod, S. Can we identify mothers at-risk for postpartum anxiety in the immediate postpartum period using the state-trait anxiety inventory?. J. Affect. Disord. 150(3), 1217–1220 (2013).
Grant, K.-A., Mcmahon, C. & Austin, M.-P. Maternal anxiety during the transition to parenthood: A prospective study. J. Affect. Disord. 108(1–2), 101–111 (2008).
Newham, J. J. et al. State-trait anxiety inventory (STAI) scores during pregnancy following intervention with complementary therapies. J. Affect. Disord. 142(1–3), 22–30 (2012).
Chen, Y. et al. Perioperative adjunctive esketamine for postpartum depression among women undergoing elective cesarean delivery: A randomized clinical trial. JAMA Netw. Open 7(3), e240953 (2024).
Shen, J. et al. The effect of low-dose esketamine on pain and post-partum depression after cesarean section: A prospective, randomized, double-blind clinical trial. Front. Psychiatry 13, 1038379 (2022).
Tan, H. S. & Habib, A. S. The optimum management of nausea and vomiting during and after cesarean delivery. Best Pract. Res. Clin. Anaesthesiol. 34(4), 735–747 (2020).
Siddiqui, M. et al. Complications of exteriorized compared with in situ uterine repair at cesarean delivery under spinal anesthesia: A randomized controlled trial. Obstetr. Gynecol. 110(3), 570–575 (2007).
Cnattingius, S., Johansson, S. & Razaz, N. Apgar score and risk of neonatal death among preterm infants. New Engl. J. Med. 383(1), 49–57 (2020).
Newborn, A. A. O. P. C. O. F. A. & Practice, A. C. O. O. A. G. C. O. O. The apgar score. Pediatrics 136(4), 819–822 (2015).
Kwok, R. F. K. et al. Preoperative ketamine improves postoperative analgesia after gynecologic laparoscopic surgery. Anesth. Analg. 98(4), 1044–1049 (2004).
Funding
This work was supported by the Jiangsu Province’s Key Discipline/Laboratory of Medicine [grant numbers JSDW202231]; the Xuzhou Medical Key Talent Training Project [grant numbers XWRCHT20210033]; and the Xuzhou Medical Young Reserve Talent Program [grant numbers XWRCHT20220017].
Author information
Authors and Affiliations
Contributions
Conceptualization: Qi Yu and Dong Yaqi. Methodology: Wang Xinghe and Sun Jia. Formal analysis and investigation: Jiang Qinyu and Li Yanyu. Writing- original draft prepartion: Dong Yaqi Writing- review and editing: Qi Yu and Dong Yaqi. Funding acquisition: Zhou Meiyan and Wang Liwei. Resources: Zheng Wenting and Hu Zhengquan. Supervision: Zhou Meiyan, Zhou Hai and Wang Liwei. Each author has sufficiently contributed to the study in terms of conception, design, data analyses, and writing of the manuscript and take responsibility for the manuscript content. All authors have reviewed and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Qi, Y., Zhou, M., Dong, Y. et al. Effect of esketamine on hypotension in women with preoperative anxiety undergoing elective cesarean section: a randomized, double-blind, controlled trial. Sci Rep 14, 17088 (2024). https://doi.org/10.1038/s41598-024-68155-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-68155-7