Abstract
Adlay millet seeds are well known for excellent health benefits. However, using fungal fermentation to improve their nutritional and functional constituents and the underlying mechanisms has not been thoroughly investigated. Herein, we used Rhizopus oryzae as starter and applied metabolomics combining with quantitative verification to understand the changes of the nutritional and functional profiles of adlay millet seeds. Results showed that a total of 718 metabolites from 18 compound classes were identified. The fermentation with R. oryzae varied 203 differential metabolites, of which 184 became more abundant and 19 got less abundant, and many components such as amino acids, nucleotides, vitamins, flavonoids, terpenoids, and phenols significantly increased after the fermentation process. Interestingly, we found that R. oryzae synthesized high levels of two important beneficial compounds, S-adenosylmethionine (SAMe) and β-Nicotinamide mononucleotide (β-NMN), with their contents increased from 0.56 to 370.26 μg/g and 0.55 to 8.32 μg/g, respectively. The Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis of enriched metabolites revealed the amino acid metabolic pathways were important for conversion of the primary and secondary metabolites. Specifically, aspartate can up-regulate the biosynthesis of SAMe and β-NMN. These findings improved our understanding into the effects of R. oryzae fermentation on enhancing the nutritional and functional values of cereal foods.
Similar content being viewed by others
Introduction
Adlay millet (Coix lachryma-jobi L.) is being widely cultivated in East and Southeast Asia for centuries, including in countries like China, India, Japan, Korea, and Malaysia1. Adlay millet seeds are primarily consumed as a food source and utilized in medicines for their beneficial physiological and pharmacological effects on human health2. Adlay millet seed was once an important staple and very nutritious cereal food source in some parts of Asia, containing about 70% starch, 12.2–16.7% protein, 5.1–9.4% lipid, and 1.3–3% dietary fiber3. The processing methods of adlay millet seed include oil extraction, starch isolation, and extraction of proteins4. For food application, the adlay millet seed can be used as ingredients in broths, soups, porridge, pastries, tea, and beverages. It can also be used as raw material for fermented wine and beer3,4. Since the Ming dynasty (14h-17th centuries CE) in China, adlay millet seed has been used in traditional Chinese medicine for various purposes, including inducing diuresis, stimulating lung and spleen function, treating heat-related ailments, reducing inflammation, and treatment of cardiovascular diseases and hypertension5. Modern studies found that adlay millet seed extract has functions such as antioxidant, anti-inflammatory, anti-obesity, anti-cancer, antidiabetes, immunomodulatory activity, lipid regulation, skin protection, and gastroprotection2,4. These health-promoting effects are thought to be related to the bioactive substances naturally present in adlay millet seed, such as fatty acids, esters, polysaccharides, sterols, phenols, flavonoids, alkaloids, triterpenoids, tocopherols, lactams, lignans, etc.2,3. However, the presence of fibers in adlay millet seed binds many active ingredients, making it challenging for the human body to digest and absorb, resulting in low bioavailability6.
Microbial fermentation is an effective and economical approach to improve the nutritional value, flavor, texture, shelf-life, digestibility, bioavailability, and functional components of cereal grains7,8. The abundant enzymes (cellulases, amylases, proteases, and phytases) produced by microbial metabolic activities modify the chemical composition of cereal grains7. It was reported that many food-grade microorganisms have been widely used to contribute to desirable modifications of nutritional quality traits of adlay millet seed. Fermentation of dehulled adlay by Monascus sp. produced higher total phenol content than unfermented adlay, and greatly enhanced its antioxidant capacity9. Defatted adlay fermented with yeast showed increased content of gama-aminobutyric acid (GABA), free amino acids, and total phenolic10. The Bacillus subtilis fermented adlay millet seeds showed high yield of tetramethylpyrazine, and contained higher free amino acids, fatty acids, GABA, triterpenes, phenolics, flavonoids, and coixenolide11. Lactobacillus plantarum was also used to ferment adlay millet seeds, and the nutritional compounds, including free amino acid, free fatty acid, soluble dietary fiber as well as organic acids of fermented adlay were significantly increased12. Adlay millet seeds fermented by Monascus purpureus showed abundant total phenols and flavonoids13. In addition, fermentation could also improve the qualities of beverages made from adlay seed. For example, the Lactiplantibacillus plantarum strain L42g was an effective starter to produce functional adlay beverages with GABA and antimicrobials14. The co-fermentation of adlay seed-chrysanthemum beverage with lactic acid bacteria led to enhanced phenolics bioaccessibility, and high antioxidant activity15. The Trametes versicolor fermented Rosa roxburghii tratt and adlay seed liquid increased the content of vitamin C, GABA, and total live bacteria in yogurt16.
The members of fungus Rhizopus (R. microsporus, R. oligosporus, and R. oryzae), has been used for thousands of years as major components of starter cultures for mixed-culture, solid state rice fermentation of Chinese sweet rice wine (‘daqu’)17. The complex mixture of microbial species produces numerous enzymes, alcohol, amino acids, and many other flavor and bioactive compounds, which improve the nutritional value, sensory properties, and functional qualities of rice18. Recent studies have shown that R. oryzae fermentation of rice bran increased the total contents of phenolics, flavonoids, carotenoids, anthocyanins, and enhanced the antioxidant capacity19. Defatted adlay bran fermented with R. oryzae showed increased results of ferric reducing antioxidant power (FRAP) and 2,2′-Azinobis-(3-ethylbenzthiazoline-6-sulphonate) (ABTS+) radical scavenging capacity20. The R. oryzae is generally recognized as safe (GRAS) by the Food and Drug Administration (FDA)21. In previous studies, R. oryzae was one of the typical strains used for production of lactic acid, fumaric acid, lipase, amylase, protease biosynthesis, and dicarboxylic acids21. However, there are few reports about the use of R. oryzae in fermenting adlay millet seed and its influence on the nutritional properties.
Metabolomics has been widely used as an analytical approach in different fields of food science. Hundreds of metabolites involved in cereal species and fermentation processes can be comprehensively analyzed22. It enables a comprehensive exploration of key functional molecules and their possible effects on biological systems23. The widely targeted metabolomics was applied to analyze metabolic profiling of six different coarse cereals, millet, coix (adlay millet seed), buckwheat, quinoa, oat, and grain sorghum, of which 768 metabolites were indentified, and glutamate and thiamine were selected as unique bioactive metabolites of coix24. Non-targeted lipidomics combining multivariate chemometrics and algorithms successfully screened 72 lipids out of 1211 lipids as potential markers to trace the geographical origin of coix seeds from four major Chinese areas25. Metabolomics has been also applied in many cereals to analyze the diversity and variability of metabolites, including the cereal profiling of carbohydrates, amino acids, vitamins, lipids25, flavonoids, terpenoids, phenolic24,26, as well as their antioxidant activities and implications for human gut health26. The metabolic characteristics of fermented cereals were also studied by metabolomics. The primary metabolites mainly identified in fermented cereals were mono- and poly-saccharides, amino acid, peptides, fatty acids, nucleosides, steroids, and organic acids22. The phenols are the most significant secondary metabolites studied in fermented cereals22,27, other metabolites, for instance, flavonoids, isoflavones, alkaloids, tannins, alcohols, phytic acid28, lignans27, and flavor substances29 have also attracted wide attention. The untargeted metabolomics of adlay millet seeds fermented by Monascus purpureus showed a total of 270 differential metabolites, of which amino acids, peptides, and analogues enriched the most metabolites13. However, to the best of our knowledge, there has been no report of a comprehensive, i.e., metabolomic analysis of the detailed nutritional and functional compositional changes resulting from R. oryzae fermentation of adlay millet seed.
The aim of this study was to investigate the potential of using R. oryzae fermentation technique to enhance the nutritional and bioactive components of adlay millet seeds, and analyze the specific functional metabolites by widely targeted metabolomics in order to develop functional food. In this study, the ultra-high performance liquid chromatography-QTrap mass spectrometry (UHPLC-MS) was performed to explore global changes in the metabolites of adlay millet seeds after fermentation with R. oryzae. Differential metabolites (DMs) analysis elucidated improvements in the nutritional and functional constituents of adlay millet seeds after fermentation, which was further confirmed by the quantitative verifications of amino acids. The potential metabolic pathways related to the quality improvements of fermented adlay millet seeds were investigated by Kyoto Encyclopedia of Genes and Genomes (KEGG) annotation and pathway enrichment analysis. Further, the metabolic network of key DMs were summarized to illuminate the possible mechanisms of the biotransformation of bioactive constituents, especially the two bioactive metabolites with significant increase in abundance, i.e. S-adenosylmethionine (SAMe) and β-Nicotinamide mononucleotide (β-NMN). Our findings will provide valuable insights into the complex transformations of comprehensive nutritional factors and bioactive components during R. oryzae fermentation of adlay millet seeds, and provide a theoretical basis for the development of fermented cereal products with fortified active constituents and stronger functional activities.
Materials and methods
Chemicals and materials
Chromatographic grade formic acid and β-Nicotinamide mononucleotide standard were purchased from Sigma Aldrich (St. Louis, MO). Chromatographic grade methanol and acetonitrile were purchased from Shanghai Anpu Experimental Technology (Shanghai, China). S-adenosylmethionine and Uridine diphosphate glucose standard were purchased from Beijing spectrum analysis standard Technology Co., LTD (Beijing, China). Purified water was obtained using Merck Millipore water purifier (Ming-Che D 24UV, Merck Millipore Corp., Shanghai, China). Adlay millet seeds were originally produced in Xingren area, Guizhou Province, China, and retailed by Vanguard Co., LTD. The R. oryzae starter was obtained from Angel Yeast Co., LTD (Hubei, China). Before experiments, the R. oryzae was cultured on Potato Dextrose Agar (PDA) medium for 7–8 days, and then the mycelium was selected and observed with a microscopy (Nikon Ti-E, Nikon, Japan).
Adlay millet seeds pretreatment and fermentation
Adlay millet seeds were washed 2–3 times, and then soaked in water (1:3, w/v) overnight at room temperature. After draining the water, the soaked adlay millet seeds were crumbled for 3–5 s at 50 Hz to granules with diameter of 2–2.5 mm using a domestic blender (JYL-C020E, Joyoung, China), then the adlay millet grits were steamed in a steamer for 30 min. After cooling on a clean bench for about 10 min, the adlay millet grits were stirred with distilled water (12% w/w), then were divided into six equal parts for further experiments. These three parts of the adlay millet grits were inoculated with R. oryzae starter culture (0.6% w/w, 108–109 spores/g), then incubated for 54 h at 30 °C (ZSH-150 incubator, Shanghai Gemtop Scientific Instruments, Shanghai, China). These samples were designated as fermented adlay millet grits (FAG). The other three unfermented parts of the adlay millet grits were designated as control adlay millet grits (CAG). The FAG and CAG samples were stored at −80 °C until needed for analysis.
Metabolite extraction
The FAG and CAG samples were freeze-dried, then crushed to a powder with the mixer mill for 60 s at 50 Hz. After that, aliquots (50 mg) were precisely weighed and transferred to Eppendorf tubes of extraction solvent (700 μL, methanol/water 3:1 v/v, containing the internal standard 2-chlorophenylalanine and precooled at −40 °C). After vortex mixing for 30 s, the samples were homogenized at 35 Hz for 4 min and sonicated for 5 min in ice-water bath for 3 times. Then the samples were extracted overnight at 4 °C on a shaker and centrifuged at 12,000 rpm (reactive centrifugal force (RFC) = 13,800 (×g), R = 8.6 cm) for 15 min at 4 °C). The supernatant was filtered through a 0.22 μm microporous membrane, diluted 10 times with extraction solvent, and vortexed for 30 s and transferred to 2 mL glass vials. A mixture of 60 μL aliquots from each sample was analyzed as a QC sample and stored at −80 °C until needed for widely targeted-metabolomic analysis.
Widely targeted-metabolomic analysis
Widely targeted-metabolomics based on large-scale MS/MS has been widely used to elucidate metabolite accumulation patterns in plants and nutrition research30. In this study, the UHPLC-MS/MS was carried out using an ExionLC AD System (SCIEX, Framingham, MA, USA), fitted with a Waters Acquity UPLC HSS T3 C18 (1.8 μm, 2.1 mm × 100 mm) chromatographic column, with a 400 μL/min flow rate and 40 °C column oven temperature. The mobile phases were water/0.1% formic acid (A) and acetonitrile (B). The auto-sampler temperature was 4 °C and the injection volume 2 μL. The mobile phase linear gradient was: 0 min, 2% B; 10.0 min, 50% B; 11.0 min, 95% B; 13.0 min, 95% B; 13.1 min, 2% B; and 15.0 min, 2% B. A SCIEX QTrap 6500+ MS system was used for detection and identification of eluted compounds. Typical ion source parameters were as follows: Ion spray voltage, +5500 V/−4500 V; curtain gas: 35 psi, temperature: 400 °C; ion source gas I, 60 psi; gas II: 60 psi; declustering potential (DP), ± 100 V. Further, the standard curve method was used for the quantitative determination of SAMe, UDPG and β-NMN in CAG and FAG samples. All tests were carried out in triplicate.
Quantitative verification of amino acids
The content of amino acids in CAG and FAG samples was quantitatively analyzed by Hitachi L-8800 amino acid analyzer as described by Wu et al.31. The determination conditions were as follows: The separation column temperature was 57 °C, and the reaction column temperature was 135 °C. The sample size was 20 μL. The flow rate of buffer for elution was 0.40 mL/min and that of ninhydrin solution was 0.35 mL/min. The detection wavelengths were 440 and 570 nm, respectively. All the analyses were carried out in triplicate.
Statistical analysis
Total ion chromatograms (TICs) of multiple QC sample and internal standard were used for data quality control. The reproducibility of peak retention times and peak areas between runs was very good, and the RT and peak area of the internal standard 2-chlorophenylalanine was very stable (Supplementary Fig. S1 online), indicating the data acquisition was both repeatable and stable. The data preprocessing and annotation of widely targeted-metabolomic analysis was as follows: the SCIEX Analyst Work Station Software (Version 1.6.3) was employed for multiple reaction monitoring (MRM), data acquisition, and processing32. A total of 792 peaks were extracted in this study, and 718 peaks were retained. All the data including peak area value of 718 compounds (rows) and 8 groups (columns) was analyzed by SIMCA software (V16.0.2, Sartorius Stedim Data Analytics AB, Umea, Sweden). The data were log-transformed and Center scaling (CTR) formatted, and then modeled and analyzed automatically by the software33. Unsupervised principal component analysis (PCA) with a 95% confidence interval was carried out. To visualize group separation and find significant DMs, supervised orthogonal projections to latent structures-discriminate analysis (OPLS-DA) was applied. Analytical methods including seven-fold cross validation, and permutation testing with 200 repetitions, were performed to estimate the stability, reliability, and validity of the OPLS-DA model. The screening criteria of P-value < 0.05 from Student's T-tests, and fold change ≥ 2 or fold change ≤ 0.533. Metabolic pathway enrichment analysis of DMs was performed using the KEGG and MetaboAnalyst databases.
The result of the quantitative test was provided as the mean and SD (n = 3). GraphPad Prism 8.0 software (GraphPad Software Inc.) was used to process image and analyze quantitative data. Multiple t-test was used for comparison of typical functional DMs and amino acids between FAG and CAG groups with the significant difference at the 5% level.
Research involving plants statement
This study was developed with commercial adlay millet seeds obtained from local supermarket in China, therefore nonexotic or at risk of extinction. The experimental research complied with relevant institutional, national, and international guidelines and legislation for cultivated plants.
Results and discussion
Features of R. oryzae and macroscopic appearance of adlay millet grits before and after fermentation
The R. oryzae colony exhibited a fluffy appearance on PDA medium and produced sporangia in the later stages (Fig. 1a). The sporangia was black brown and spherical with erect sporangiophores (Fig. 1b). There were obvious stolons and rhizoids without diaphragm in the mycelium (Fig. 1c). The sporangiospores were oval and the funicular of sporangia was wedge-shaped (Fig. 1d). The general characteristics of R. oryzae in this study were in line with those reported by Zheng et al.34. The adlay millet grits before fermentation was compact and well defined with each other (Fig. 1 e,h). After 24 h of fermentation, there was clear liquid seeped out with pleasant sweet scent and some bubbles, and the adlay millet grits seemed relatively intact (Fig. 1 f,i). After 54 h of fermentation, there was more glutinous liquid seeped out with clear wine aromas and more bubbles, and a membrane was formed by R. oryzae mycelium on the surface of the loosed adlay millet grits (Fig. 1g,j).
Macroscopic and microscopic features of R. oryzae and macroscopic appearance of adlay millet seed before and after fermentation. (a) Mycelium growth on PDA medium of R. oryzae. (b) Sporangia and sporangiophores. (c) Stolons and rhizoids. (d) Sporangiospores and columella. (e–g) Overview of adlay millet grits fermented for 0, 24 and 54 h, respectively. (h–j) Partial view of the bowl bottom of adlay millet grits at 0, 24 and 54 h, respectively.
Metabolomic analysis of fermented adlay millet grits samples using widely targeted-metabolomics
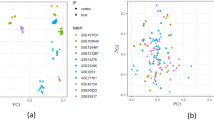
The widely targeted metabolomic analysis integrates the "universality" characteristics of non-targeted metabolomics and the "accuracy" feature of targeted metabolomics. It has several advantages, such as high throughput, ultra-sensitive, wide coverage, qualitative and quantitative accuracy35. It has not only advanced the discovery and validation of nutritional biomarkers, but also facilitated their implementation in health research36. To obtain a complete picture of metabolites in adlay millet seeds before and after R. oryzae fermentation, the widely targeted metabolomic analysis was conducted. Unsupervised principal component analysis (PCA) with a 95% confidence interval was carried out to visualize the distribution and grouping of the samples33. Differences in metabolomics data from the CAG, FAG, and QC samples were visualized by PCA (Supplementary Fig. S2a online). PC1 and PC2 explained 60.5% and 10.6% (total 71.1%) of the variation between CAG and FAG samples, respectively; all samples were within the 95% confidence interval, i.e., there were no outliers. The CAG samples were more closely grouped than the FAG samples, but the two groups were widely separated from each other and the QC samples, indicating a significant difference in metabolite profiles between CAG and FAG samples24.
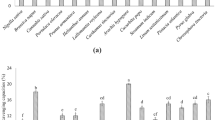
In order to obtain more reliable information about the correlation between intergroup differences in metabolites and the group of samples, the OPLS-DA was applied33. In the OPLS-DA model, the FAG samples were more closely grouped, and the groups were still well-separated (Supplementary Fig. S2b online). Permutation testing of the OPLS-DA model was performed (Supplementary Fig. S3 online); the abscissa represented the permutation retention, the point where the permutation retention equals 1 corresponds to the R2Y (goodness of fit) and Q2 (goodness of prediction) values of the original model, which were both close to 1, validating the authenticity, robustness and stability of the OPLS-DA model. Specifically, 718 metabolites were annotated in CAG and FAG samples from 18 compound classes (Fig. 2), of which the top 10 most abundant metabolites were flavonoids (113 metabolites), terpenoids (101 metabolites), alkaloids (78 metabolites), phenols (63 metabolites), amino acids and derivatives (53 metabolites), lipids and lipid-like molecules (44 metabolites), coumarins and lignans (37 metabolites), steroids and derivatives (28 metabolites), organic acids and derivatives (23 metabolites), and carbohydrates and conjugates (23 metabolites). Zhao et al. also found that flavonoids were more abundant than other detected metabolites in grains24.
Identification and classification of differential metabolites
To analyze metabolic profile differences between FAG and CAG samples at the individual compound level, a hierarchical cluster analysis of the DMs was performed based on a Euclidean distance matrix and the complete linkage method37. The abundance of most DMs was much higher in FAG than in CAG samples (Supplementary Fig. S4 online), indicating that the microbial fermentation had a great influence on the metabolic profile of adlay millet seed22. The distribution of differential metabolites was visualized in a volcano plot (Fig. 3a). A total of 203 differential metabolites were identified, of which 184 were more abundant and 19 were less abundant in FAG (Fig. 3a). The top ten DM compound classes were amino acids and derivatives (17.24%), flavonoids (11.82%), terpenoids (11.82%), alkaloids (8.37%), carbohydrates and conjugates (6.40%), lipids and lipid-like molecules (6.40%), organic acids and derivatives (5.42%), phenols (6.40%), nucleotides and derivatives (2.96%), and steroids (2.46%) (Fig. 3b). The abundances of amino acids and derivatives, nucleosides, nucleotides and derivatives, and terpenoids all increased (Fig. 3c), of which the first two classes included three metabolites exhibiting the highest fold-change, namely, SAMe, uridine diphosphate glucose (UDPG), and β-NMN (Fig. 3d). Similarly, the fermentation of adlay millet seed by Monascus purpureus resulted in the detection of 187 up-regulated metabolites, with the highest concentration of DMs found in amino acids, peptides, and analogues13. The co-fermentation of R. oryzae and Lactobacillus resulted in higher content of amino acids, nitrogen, and total phenol in oat38. In health applications, SAMe supplementation could restore hepatic glutathione (GSH) deposits and attenuate liver injury, treat arthritis, and depression, and reduce jaundice39. β-NMN can ameliorate health conditions associated with nicotinamide adenine dinucleotide (NAD+) deficiency, such as aging, cardiac, and cerebral ischemia, Alzheimer’s disease and obesity40. It seemed that the fungi imprint their unique chemical signature on the foods they inhabit by producing primary and secondary metabolites41.
Differential metabolites (DMs) identified in FAG samples compared with CAG samples. (a) A volcano plot of the 718 metabolites identified, showing the significantly more or less abundant DMs. (b) A pie chart of the proportions of different DM compound classes. (c) A total number and variation of different categories of DMs between FAG and CAG. (d) A matchstick diagram of the top 15 more and less abundant metabolites.
Metabolism of nutritional components
Amino acids and derivatives
Adlay millet seed has been identified as a good source of dietary protein because of its relatively high protein/carbohydrate ratio compared with other cereals1. Amino acids are fundamental for a lot of biological activities and indispensable for body functions42. In this study, the abundance of amino acids and derivatives in FAG samples notably increased (Fig. 3c, Supplementary Table S1 online), This may be due to proteases produced by R. oryzae hydrolyzing the macro molecules of protein into the more dispersible and soluble compounds of amino acids21. Similarly, the content of amino acids in yeast-fermented adlay was increased by 9.9 times after fermentation10. In particular, the abundance of SAMe increased the most markedly (11,285-fold change) in all the DMs (Table 1, Fig. 3d). SAMe is the universal methyl donor for reactions of methylation in animals, plants and microorganisms. It plays an indispensable role in most cellular metabolic processes involved in methylation reactions of transmethylation, transsulfuration, and aminopropylation43. The SAMe synthesis is highly conserved between bacteria and eukaryotes, and is important for coordinating the development and secondary metabolism in fungal44. It could explain why R. oryzae produced high levels of SAMe after fermentation. The GABA increased by 16.72-fold in FAG samples. It has been found to be effective for many physiological functions, including enhancing immunity, prevention of obesity, lowering blood pressure, etc.45. Similarly, fermentation of adlay bran by B. subtilis resulted in an almost 14-fold increase in GABA11 and yeast fermentation increased the GABA content 6-fold10. Moreover, R. oryzae fermentation of buckwheat increased the GABA content 34 fold higher than that of raw buckwheat46. Since adlay millet seed are rich in glutamate24, the GABA can easily be synthesized by R. oryzae using the high concentration of glutamate under catalysis of glutamate decarboxylase47. In general, the R. oryzae fermentation improved the trophism and abundance of important amino acids.
Carbohydrates and carbohydrate conjugates
Carbohydrate metabolism is one of the most important cellular metabolic processes and provides material and energy for growth and proliferation48. A total of 12 carbohydrate and carbohydrate conjugate DMs were identified (Fig. 3c, Supplementary Table S1 online). Stachyose decreased significantly (Fig. 3d). This may be due to the stachyose in the adlay was efficiently hydrolyzed by galactosidase produced by R. oryzae49. Similar results have been observed in other fungi, such as yeast50 and Auricularia auricular51. Kojibiose, a prebiotic was observed to increase by 100-fold in this study. Kojibiose was also detected in food such as sake and koji extracts femented with strains of Aspergillus or Rhizopus52. It was reported that Aspergillus oryzae highly expressing α-glucosidas gene could produce 0.3% kojibiose from 20% maltose substrate53.
Lipids and lipid-like molecules
Adlay millet seed is richer in lipids than most cereal grains3. 44 lipids and lipid-like molecules were annotated in FAG and CAG, including oleic acid, palmitic acid, and various unsaturated fatty acids, such as conjugated linoleic acid11, and Docosahexaenoic acid (DHA) (Supplementary Table S1 online). Among them, 14 kinds of lipids were annotated as DMs (Fig. 3b, c). This is consistent with Li's report that adlay millet seeds contain a high content of unsaturated fatty acids2. The abundance of DHA was 38 fold higher in FAG than CAG (Table 1). This may be related to the fact that lipase secreted by R. oryzae can obtain DHA by catalyzing the breakdown of oil54. On the other hand, the increased linolenic acid in the metabolites is a precursor in the biosynthesis of DHA55. DHA has several positive health effects, e.g. alleviating cardiovascular disorder, and improving inflammation56.
Nucleosides, nucleotides and derivatives
Nucleosides and nucleotides are not only components of genetic material, but also key components of cellular metabolic pathways. 12 compounds in this class were annotated in FAG and CAG, 6 of which were DMs, Uridine 5′-Diphospho-α-d-glucose (UDPG) and β-NMN were increased most (Table 1). UDPG increased more than 5000-fold in FAG compared with CAG (Fig. 3d, Table 1). As one of the most important glycosyl donors for biosynthesis of glycosides, oligosaccharides, polysaccharides, and glycoproteins, UDPG is widely found in animals, plants and microorganisms. It is closely associated with the biosynthesis of nucleotide sugars, galactose metabolism, amino sugar, and nucleotide sugar metabolism57. The increased UDPG abundance may be related to fungal response to cultivation conditions and its metabolism. It was notably the β-NMN was upregulated significantly in FAG samples (Fig. 3d, Table 1). Beta-MNM is an endogenous bioactive nucleotide and a key precursor for NAD+ biosynthesis40. It has been reported that microorganisms that can naturally synthesize β-NMN include fructophilic lactic acid bacteria58 and yeast59. Since the nicotinamide riboside kinase (NRK), a key enzyme for β-NMN biosynthesis, is present in yeast cells59, we speculate that similar NRK may also present in fungus R. oryzae cells. Nevertheless, the molecular mechanism and enzymatic properties of NRK synthesis in R. oryzae cells needs to be further studied in the future. Since β-MNM has beneficial pharmacological effects on health problems40, so fermented adlay millet seed rich in β-NMN merits further study to evaluate its potential health benefits.
Vitamins
Vitamins are important nutritional components of adlay millet seed; vitamin E and β-carotene have been previously identified in adlay millet seed2. In FAG and CAG, 10 vitamins were annotated, including vitamin E, β-carotene, ascorbic acid, nicotinic acid, vitamin A, pyridoxine, and thiamine, the last four of which were DMs (Table 1). The abundance of B vitamins, suca as nicotinic acid and pyridoxine increased 10.4 and 3.05 fold-change in FAG. Similar results were observed in fermented soybean of R. oryzae because of releasing of vitamins21. In contrast, the abundance of thiamine decreased obviously in FAG samples. Thiamine is a cofactor of several essential enzymes involved in central carbon metabolism pathways such as glycolysis, the pentose phosphate pathway and the tricarboxylic acid cycle. Under specific environmental conditions, such as those present during fermentation, R. oryzae should rely on the exogenous thiamine for maximum growth and metabolism rates, just like the strategy used by yeast to make wine60.
Metabolism of functional components
Flavonoids
Flavonoids form a large group of bioactive plant secondary metabolites that is abundant in fruits and seeds. It is beneficial for human health, such as anti-inflammatory, anti-aging, cardio-protective and immunomodulatory61. A total of 24 flavonoid compounds was annotated as DMs in FAG compared with CAG, namely 16 flavones, 6 flavanones, and 2 isoflavones. Of these, the compound of epiafzelechin, naringenin, neohesperidin, quercitrin, hesperidin, and apigenin were more abundant in FAG samples (Fig. 3c, Supplementary Table S1 online). All of these flavonoids compound have been identified in various parts of adlay millet seed3. Similar results were reported in other fungal fermented-adlay millet seeds. Mei et al. found that Monascus purpureus fermentation of adlay seed upregulated the afzelechin and hesperetin13.
Terpenoids
Terpenoids constitute one of the largest classes of natural compounds present in animals, plants, and microorganisms62. They have many biological activities, such as anti-bacterial, anti-parasitic, anti-inflammatory, anti-tumor, and skin protection62. In FAG samples, 24 terpenoid compounds were assigned as DMs, i.e., 3 monoterpenoids, 2 iridoids, 6 sesquiterpenes, 1 diterpene, 11 triterpenes, and 1 tetraterpenoid (Fig. 3b, Supplementary Table S1 online). All the terpenoid DMs were more abundant in FAG (Fig. 3c). This could be due to the UbiA-type sesquiterpene cyclases and class I terpenoid cyclases are ubiquitous in fungi, so the isoprenoid diphosphate substrates can be catalyzed to form monocyclic or polycyclic terpene skeletons by R. oryzae63.
Alkaloids
Alkaloids have been isolated from many medicinal herbs and represent a rich source of bioactive molecules62. A total of 17 alkaloid DMs were identified in FAG samples, of which 13 were more abundant (Supplementary Table S1 online). The abundance of rhynchophylline, 3-Hydroxy-2-methylpyridine, lupanine, trigonelline, and jervine slightly increased in FAG samples. It has been reported the alkaloid compounds, i.e. jervine, acetylcholine, and pilocarpine were also affected by R. oryzae fermentation of soybeans64. The accumulation of alkaloids is beneficial to enhance the biological activity of fermented adlay millet seed.
Phenols
Phenols are one of the most widely distributed classes of plant secondary metabolites and have been widely applied in the food and pharmaceutical industries as flavorings, antioxidants and antibacterial agents19,26. A total of 63 phenol compounds were annotated in FAG and CAG, including gallic acid, gallocatechin, p-coumaric acid, ferulic acid, catechinic11, vanillin and vanillic acid, and 4- hydroxybenzaldehyde2; of these, 13 were DMs and 12 were more abundant in FAG (Fig. 3b, c). The abundance of catechinic and ferulic acid increased about 6.0-fold and 3.4-fold, respectively (Table 1). The results was similar to previous studies observed for increased phenolics from fermented defatted rice bran by R. oryzae19, and was consistent with the increased total content of phenols after Bacillus subtilis fermentation of adlay millet seed11. The hydrolase, such as protease, cellulase and phytase, produced by R. oryzae could reduce the coating effect of fiber and other macromolecules on phenolic ingredients, which is conducive to the dissolution of phenolics into free form, increasing the apparent content and bioaccessibilities19,20,21.
KEGG annotation and related pathway enrichment of differential metabolites
The metabolism of various substances in the biological body is not carried out in isolation, but through common intermediate substances to connect and transform to each other, forming a complete network to regulate metabolism so as to perform cellular biological functions22. Based on the metabolic reaction, the possible metabolic pathways and relevant important regulatory metabolisms will illustrate the physiological processes in cells65. To aid understanding of the metabolic activities of R. oryzae fermentation on adlay millet seed in this study, the metabolic pathway enrichment analysis of 203 DMs was performed using the KEGG and MetaboAnalyst databases65. A total of 120 DMs was mapped to the KEGG data sets and they were found to be enriched in 58 metabolic pathways (Fig. 4); most DMs were related to metabolic pathways and biosynthesis of secondary metabolites. Several pathways of amino acids biosynthesis showed significant enrichment of DMs (Fig. 4). In detail, the alanine, aspartate and glutamate metabolism pathway as well as the sulfur relay system enriched abundant DMs and had high enrichment factor (Fig. 4), indicating their significant status during the fermentation of adlay millet seed by R. oryzae. In addition, the pathways of nicotinate and nicitinamide metabolism, alanine, aspartate and glutamate metabolism, arginine and proline metabolism, glycine, serine and threonine metabolism, arginine biosynthesis, cysteine and methionine metabolism, and valine, leucine and isoleucine biosynthesis also indicated their important contribution to the fermentation (Fig. 4). The results were consistent with many published findings. For instance, Mei et al. demonstrated that after M. purpureus fermentation of adlay millet seed, most of the metabolic pathways were related to amino acids metabolism, and glutamic acid, lysine, and arginine were involved in multiple metabolic pathways13. Chen et al. found that in fermented soybeans, the amino acids and derivatives in differential metabolites (such as N-Acetyl- L-methionine, L-Aspartic acid, N-α-Acetyl-L-asparagine, L-threo-3-Phenylserine, tyrosylalanine, etc.) were mostly up-regulated due to fermentation with R. Oryzae64. Other cereals, including whole grain, wheat, rye, oat, barley, foxtail millet, and brown rice, etc., which were fermented with lactic acid bacteria or yeast, indicated the nutrient profile in terms of amino acids was highly improved in fermented samples in comparison to raw materials22. Our study certified the R. oryzae fermentation had great impact on metabolic profile of amino acids in adlay millet seed.
Quantitative verification of significant DMs and amino acids
Three significant DMs with the highest increase in abundance, i.e. SAMe, UDPG, and β-NMN, and several important amino acids were further quantitative verified. As shown in Fig. 5, SAMe, UDPG, and β-NMN all exhibited obviously increase in FAG in comparison to CAG samples. The content of SAMe showed a sharp increase from 0.56 to 370.26 μg/g after R. oryzae fermentation; and the content of UDPG and β-NMN increased significantly from 0.64 to 16.14 μg/g and 0.55 to 8.32 μg/g, respectively. The content of aspartate increased from 0.35 to 0.52%. Other correlative amino acids were also obviously increased after R. oryzae fermentation, including glutamate (from 1.31 to 1.70%), proline (from 0.48 to 0.62%) and arginine (from 0.24 to 0.31%), glycine (from 0.13 to 0.19%), serine (from 0.23 to 0.32%), and threonine (0.16–0.25%). Previous studies have reported R. oryzae fermentation effectively increased the content of free amino acids in defatted adlay bran and other cereals20,31. The quantitative results were consistent with that of the metabolomics, confirming that the amino acid metabolism were important for R. oryzae fermentation of adlay millet seed to produce functional compounds.
Quantitative verification of typical functional DMs and amino acids content associated with the metabolic pathways. The left three column groups, i.e., S-adenosylmethionine (SAMe), Uridine diphosphate glucose (UDPG) and β-Nicotinamide mononucleotide (β-NMN), reveal the differences exist extremely significances between the contents of CAG and FAG samples. The right seven column groups show there are significant differences on the contents of amino acids between CAG group and FAG group. These amino acids were closely related to the synthesis of functional substances. Note: asterisk (*) on the column mean significant difference between FAG and CAG samples (*p < 0.05, ** p < 0.01, and *** p < 0.001).
Map of metabolic pathways involved in bioactive compounds biosynthesis and amino acid metabolism
The compound of SAMe and β-NMN link to a variety of health-beneficial activities39,40. The R. oryzae fermentation of adlay millet seed significantly increased the content of SAMe and β-NMN, as well as improved the metabolic profile of amino acids in this study. By analyzing the KEGG pathways involved in SAMe, β-NMN, and amino acid metabolism, i.e., the pathways of nicotinate and nicitinamide metabolism, alanine, aspartate and glutamate metabolism, cysteine and methionine metabolism, glycine, threonine and serine metabolism, as well as arginine and proline metabolism (Fig. 4), we summarized the metabolic pathway network of bioactive compounds and amino acid, as shown in Fig. 6. All DMs in the network were more abundant in the FAG samples compared with CAG samples (Supplementary Table S1 online). It’s obvious that the aspartate was involved in three pathways,, i.e., the alanine, aspartate and glutamate metabolism (Fig. 6, purple box), the cysteine and methionine metabolism (Fig. 6, blue box), and the glycine, serine and threonine metabolism (Fig. 6, green box). The aspartate was transformed from the intermediate metabolite oxaloacetate in the tricarboxylic acid cycle (TCA) cycle by the pathway of alanine, aspartate and glutamate metabolism (Fig. 6, purple box). It is considered to provide precursor for synthesis of four essential amino acids (methionine, threonine, isoleucine, and lysine)66. The first reaction leading to the biosynthesis of the four essential amino acids is catalyzed by aspartate kinase, which can be activated by alanine, serine, and valine66. In this study, the abundance of alanine in fermented adlay seed exhibited a fold change of 24.81 (Supplementary Table S1 online), which may lead to the activation of aspartate kinase to form downstream compounds, such as homoserine, an common intermediate in the threonine and methionine biosynthesis pathways67 (Fig. 6). Under the catalysis of cystathionine-γ-synthase and S-adenosylmethionine synthase, the homoserine is transformed into methionine and then into SAMe (Fig. 6, blue box)66. Thomas et al. found that yeast cells store SAMe in preferenc to methinonine, because of high concentrations of methionine would be deleterious to the cells68. This may be the reason for the accumulation of high concentrations of SAMe in R. Oryzae fermented samples. Besides, abundant aspartate could also be participated in the nicotinate and nicotinamide metabolism pathway (Fig. 6, Supplementary Fig. S5 online), leading to increased biosynthesis of nicotinic acid (Table 1). As reported by Carrillo et al., SAMe is also associated with the synthesis of nicotinamine, and a single molecule of nicotianamine requires three molecules of SAMe69. Therefore, the enhanced SAMe should increase the content of nicotinamine, which can be used as precursor for β-NMN biosynthesis through the salvage pathway38. Aspartate was also enriched in the glycine, serine and threonine metabolism pathway (Fig. 6, green box), the more abundant glycine, serine and threonine should be closely related to generation of terpenoids and steroids through methylglutarate pathway63. Other intermediate metabolite, i.e. 2-oxoglutarate in the TCA cycle, could be transformed to synthesis glutamate and GABA through the pathway of alanine, aspartate and glutamate metabolism (Fig. 6, purple box). The conversion of glutamate to GABA by microbial metabolism was also detected in yeast fermentation of defatted adlay10. Glutamate was also enriched in the pathways of arginine and proline metabolism and arginine biosynthesis (Fig. 6). The increased content of arginine and proline can be used as precursors to synthesize alkaloids. Overall, amino acid metabolism played a crucial role in the transformation of primary metabolites into secondary metabolites by R. oryzae fermentation. Specifically, the aspartate appeared to play a vital role in the metabolic network for biosynthesis of SAMe, β-NMN and other functional compounds.
Maps of metabolic pathways involved in bioactive compounds biosynthesis and amino acid metabolism. All DMs displayed in the amino acid metabolism pathways are more abundant in the FAG samples compared with CAG samples. Compounds in bold font are involved in two or more metabolic pathways at the same time. The solid arrow represents the direct conversion between substances. The dashed arrow indicates an association with the metabolic pathway of the substance indicated by the arrow. Abbreviations of amino acids are as follows: Ala: alanine; Asp: aspartate; Glu: glutamate; Cys: cysteine; Meth: methionine; Gly: glycine; Thr: threonine; Ser: serine; Arg: arginine; Pro: proline.
Conclusions
This study has established that R. oryzae fermentation can be utilized to enhance the nutritional and bioactive properties of fermented adlay products. A noticeable improvement was observed in the metabolic profile of various nutritional components, such as amino acids and their derivatives, lipids and lipid-like molecules, nucleotides and derivatives, and vitamins. Furthermore, there was an increase in the presence of several functional components like flavonoids, terpenoids, and phenols. It is worth noting that both metabolomics and quantitative measurement revealed significant boosts in the content of two important bioactive compounds: SAMe and β-NMN. Results of the KEGG annotation and pathway enrichment revealed that the pathways of alanine, aspartate and glutamate metabolism, glycine, serine and threonine metabolism, as well as cysteine and methionine metabolism were important for transformation of primary and secondary metabolisms in R. oryzae fermenting adlay millet seeds. Specifically, aspartate appeared to play a vital role for SAMe and β-NMN biosynthesis by R. oryzae. This study provides a theoretical basis for the development of functional adlay food. Further studies should combine multi-omics techniques, including genomics, transcriptomics, and metabolomics, to elucidate the functional genome bases and metabolic regulation mechanism of functional substances conversion by R. oryzae, as well as to evaluate the potential to promote human health by the fermented adlay millet seed products.
Data availability
All data generated and/or analyzed during this study are available from the corresponding author on reasonable request.
References
Corke, H., Huang, Y. & Li, J. Coix: Overview. In Encydopedia of Food Grants (eds Wrigley, C. et al.) 184–189 (Elsevier, 2016).
Li, H. et al. Research on Coix seed as a food and medicinal resource, it’s chemical components and their pharmacological activities: A review. J. Ethnoph. 319, 117309 (2024).
Zhu, F. Coix: Chemical composition and health effects. Trends Food Sci. Technol. 61, 160–175 (2017).
Igbokwe, C. J. et al. Coix seed: A review of its physicochemical composition, bioactivity, processing, application, functionality, and safety aspects. Food Rev. Int. 38(sup1), 921–939 (2022).
Li, S. Bencao Gangmu (systematic pharmacopoeia), China (1596).
Zeng, H. et al. Anti-lipid-oxidation effects and edible safety evaluation of the oil extracted by a supercritical CO2 process from coix seed fermented by Monascus purpureus. Food Sci. Hum. Wellness 12, 1119–1127 (2023).
Graham, A. E. & Ledesma-Amaro, R. The microbial food revolution. Nat. Commun. 14, 2231 (2023).
Suraj, M. et al. Influence of fermentation conditions, and the blends of sorghum and carrot pulp supplementation on the nutritional and sensory quality of tef injera. Sci. Rep. 14, 12819 (2024).
Tseng, Y., Yang, J., Chang, H., Lee, Y. & Mau, J. Antioxidant properties of methanolic extracts from Monascal adlay. Food Chem. 97, 375–381 (2006).
Xu, L. et al. Impact of yeast fermentation on nutritional and biological properties of defatted adlay (Coix lachryma-jobi L.). LWT-Food Sci. Technol. 137, 110396 (2021).
Wen, A. et al. Tetramethylpyrazine from adlay (Coix lacryma-jobi) biotransformation by Bacillus subtilis and its quality characteristics. J. Food Sci. Technol. 57, 4092–4102 (2020).
Yin, H. et al. Effects of fermentation with Lactobacillus plantarum NCU137 on nutritional, sensory and stability properties of Coix (Coix lachryma-jobi L.) seed. Food Chem. 314, 126037 (2020).
Mei, Q. et al. Analysis of metabolites of coix seed fermented by Monascus purpureus. Food Biosci. 50, 102054 (2022).
Buatong, A., Meidong, R., Trongpanich, Y. & Tongpim, S. Production of plant-based fermented beverages possessing functional ingredients antioxidant, γ-aminobutyric acid and antimicrobials using a probiotic Lactiplantibacillus plantarum strain L42g as an efficient starter culture. J. Biosci. Bioeng. 134, 226–232 (2022).
Rao, H., Lin, L. & Zhao, M. Probiotic fermentation affects the chemical characteristics of coix seed-chrysanthemum beverage: Regulatory role in sensory and nutritional qualities. Food Biosci. 58, 103629 (2024).
Tian, Y. et al. The effects of Trametes versicolor fermented Rosa roxburghii tratt and coix seed quild on the nutrition, sensory characteristics and physical and chemical parameters of yogurt. Food Chem. X 28, 100969 (2023).
Nout, M. J. Rich nutrition from the poorest - cereal fermentations in Africa and Asia. Food Microbiol. 26, 685–692 (2009).
Cai, H. et al. Microbial diversity and chemical analysis of the starters used in traditional Chinese sweet rice wine. Food Microbiol. 73, 319–326 (2018).
Chen, Y. et al. Extrusion and fungal fermentation change the profile and antioxidant activity of free and bound phenolics in rice bran together with the phenolic bioaccessibility. LWT-Food Sci. Technol. 115, 108461 (2019).
Xu, L. et al. Effect of Rhizopus Oryzae fermentation on nutritional components and antioxidant activity of defatted adlay bran. J. Chin. Cereals Oils Assoc. 36, 16–22 (2021).
Londoño-Hernández, L. et al. Rhizopus oryzae – Ancient microbial resource with importance in modern food industry. Int. J. Food Microbiol. 257, 110–127 (2017).
Gupta, R. & Gaur, S. LC-MS investigated as a tool to study the metabolomic characteristics of cereal fermentation. Appl. Food Res. 4, 100365 (2024).
Yang, M. et al. Advances in understanding of health-promoting benefits of medicine and food homology using analysis of gut microbiota and metabolomics. Food Front. 1, 398–419 (2020).
Zhao, Y. et al. Metabolomics reveals nutritional diversity among six coarse cereals and antioxidant activity analysis of grain sorghum and sweet sorghum. Antioxidants 11, 1984 (2022).
Chen, P. et al. A study of the lipid profile of coix seeds from four areas based on untargeted lipidomics combined with multivariate algorithms to enable tracing of their origin. Food Res. Int. 69, 112740 (2023).
Tiozon, R. N. Jr., Sartagoda, K. J. D., Serrano, L. M. N., Fernie, A. R. & Sreenivasulu, N. Metabolomics based inferences to unravel phenolic compound diversity in cereals and its implications for human gut health. Trends Food Sci. Technol. 127, 14–25 (2022).
Gai, H. et al. Phenolic profile and antioxidant activity of Chinese rice wine fermented with different rice materials and starters. LWT-Food Sci. Technol. 111, 226–234 (2019).
Garrido-Galand, S., Asensio-Grau, A., Calvo-Lerma, J., Heredia, A. & Andrés, A. The potential of fermentation on nutritional and technological improvement of cereal and legume flours: A review. Food Res. Int. 145, 1–15 (2021).
Chen, L., Li, D. & Rong, Y. Fermentation mechanism of ginkgo rice wine using an ultra-high-performance liquid chromatography-quadrupole/time-of-flight mass spectrometry based metabolomics method. J. Food Compos. Anal. 105, 104230 (2022).
Vos, R. C. D. et al. Untargeted large-scale plant metabolomics using liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2, 778–791 (2007).
Wu, H., Liu, H. N., Ma, A. M., Zhou, J. Z. & Xia, X. D. Synergetic effects of Lactobacillus plantarum and Rhizopus oryzae on physicochemical, nutritional and antioxidant properties of whole-grain oats (Avena sativa L.) during solid-state fermentation. LWT-Food Sci. Technol. 154, 112687 (2022).
Zha, et al. SWATHtoMRM: Development of high-coverage targeted metabolomics mthod using SWATH technology for biomarker discovery. Anal. Chem. 906, 4062–4070 (2018).
Wiklund, S. et al. Visualization of GC/TOF-MS-based metabolomics data for identification of biochemically interesting compounds using OPLS class models. Anal. Chem. 80, 115–122 (2008).
Zheng, R.-Y., Chen, G.-Q., Huang, H. & Liu, X.-Y. A monograph of Rhizopus. Sydowia. 59, 273–372 (2007).
Chen, W. et al. A novel integrated method for large-scale detection, identification, and quantification of widely targeted metabolites: Application in the study of rice metabolomics. Mol. Plant 6, 1769–1780 (2013).
Kortesniemi, M. et al. Nutritional metabolomics: Recent developments and future needs. Curr. Opin. Chem. Biol. 77, 102400 (2023).
Trygg, J. & Wold, S. Orthogonal projections to latent structures (O-PLS). J. Chemometr. 16, 119–128 (2002).
Kolde, R. Pheatmap: Pretty heatmaps. R pack. Ver. 1, 726 (2019).
Guo, T., Chang, L., Xiao, Y. & Liu, Q. S-adenosyl-l-methionine for the treatment of chronic liver disease: A systematic review and meta-analysis. PLoS ONE 10, e0122124 (2015).
Reiten, O. K., Wilvang, M. A., Mitchell, S. J., Hu, Z. & Fang, E. F. Preclinical and clinical evidence of NAD+ precursors in health, disease, and ageing. Mech. Ageing Dev. 199, 111567 (2021).
Silva, M. S. & Cordeiro, C. New findings in metabolomics in food mycology. Curr. Opin. Food Sci. 57, 101175 (2024).
Liang, X. N. et al. Quantitative analysis of amino acids in human and bovine colostrum milk samples through iTRAQ labeling. J. Sci. Food. Agric. 98, 5157–5163 (2018).
Hu, Y. et al. Penicillium oxalicum S-adenosylmethionine synthetase is essential for the viability of fungal cells and the expression of genes encoding cellulolytic enzymes. Fungal Biol. 125, 1–11 (2021).
Gerke, J., Bayram, Ö. & Braus, G. H. Fungal S-adenosylmethionine synthetase and the control of development and secondary metabolism in Aspergillus nidulans. Fungal Genet. Biol. 49, 443–454 (2012).
Diana, M., Quílez, J. & Rafecas, M. Gamma-aminobutyric acid as a bioactive compound in foods: A review. J. Funct. Foods 10, 407–442 (2014).
Park, N. et al. The effect of fermented buckwheat on producing l-carnitine and c-aminobutyric acid (GABA) enriched designer eggs. J. Sci. Food Agric. 97, 2891–2897 (2017).
Zhang, J. et al. Glutamate application maintains quality and antioxidant capacity of fresh-cut carrots by modulating GABA shunt, phenylpropanoid and ROS metabolism. Food Chem. 443, 138545 (2024).
Bhagavan, N. V. Carbohydrate metabolism, I. glycolysis and TCA cycle. In Medical Biochemistry 4th edn (ed. Bhagavan, N. V.) 225–246 (Harcourt/Academic Press, 2002).
Gajdhane, S. B., Bhagwat, P. K. & Dandge, P. B. Statistical media optimization for enhanced production of α-galactosidase by a novel Rhizopus oryzae strain SUK. Biocatal. Agric. Biotechnol. 8, 301–309 (2016).
Tudor, K. W., Jones, M. A., Hughes, S. R., Holt, J. P. & Wiegand, B. R. Effect of fermentation with Saccharomyces cerevisiae strain PJ69-4 on the phytic acid, raffinose, and stachyose contents of soybean meal. Prof. Anim. Sci. 29, 529–534 (2013).
Cai, G., Yi, X., Wu, Z., Zhou, H. & Yang, H. Synchronous reducing anti-nutritional factors and enhancing biological activity of soybean by the fermentation of edible fungus Auricularia auricular. Food Microbiol. 120, 104486 (2024).
Sato, A. & Aso, K. Kojibiose (2-O-α-d-glucopyranosyl-d-glucose): Isolation and structure: Isolation from Hydrol. Nature 180, 984–985 (1957).
Nagayoshi, E., Ozeki, K., Hata, M., Minetoki, T. & Takii, Y. Transglycosilation activity of Aspergillus oryzae-derived α-glucosidas. J. Biol. Macromol. 15, 13–27 (2015).
Ashjari, M., Mohammadi, M. & Badri, R. Selective concentration of eicosapentaenoic acid and docosahexaenoic acid from fish oil with immobilized/stabilized preparations of Rhizopus oryzae lipase. J. Mol. Catal. B: Enzym. 122, 147–155 (2015).
De Souza, N. E., Matsushita, M. & Visentainer, J. V. Fatty acids: Structure, classification, nutrition and health. Dyn. Mus. Arch. Interdiscip. 2, 102–107 (2012).
Ostermann, A. I. & Schebb, N. H. Effects of omega-3 fatty acid supplementation on the pattern of oxylipins: A short review about the modulation of hydroxy-, dihydroxy-, and epoxy-fatty acids. Food Funct. 8, 2355–2367 (2017).
Bedford, C. T., Hickman, A. D. & Logan, C. J. Structure-activity studies of glucose transfer: Determination of the spontaneous rates of hydrolysis of uridine 5’-diphospho-α-d-glucose (UDPG) and uridine 5’-diphospho-α-d-glucuronic acid (UDPGA). Bioorg. Med. Chem. 11, 2339–2345 (2003).
Sugiyama, K. et al. Nicotinamide mononucleotide production by fructophilic lactic acid bacteria. Sci. Rep. 11, 7662 (2021).
Bieganowski, P. & Brenner, C. Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a preiss-handler independent route to NAD in fungi and humans. Cell 117, 495–502 (2004).
Labuschagne, P. W. J. & Divol, B. Thiamine: A key nutrient for yeasts during wine alcoholic fermentation. Appl. Microbiol. Biotechnol. 105, 953–973 (2021).
Dias, M. C., Pinto, D. C. G. A. & Silva, A. M. S. Plant flavonoids: Chemical characteristics and biological activity. Molecules 26, 5377 (2021).
Omari, N. et al. Natural bioactive compounds targeting epigenetic pathways in cancer: A review on alkaloids, terpenoids, quinones and isothiocyanates. Nutrients 13, 3714 (2021).
Luo, P. et al. Biosynthesis of fungal terpenoids. Nat. Prod. Rep. 41, 48–783 (2024).
Chen, J. et al. Solid-state fermentation with Rhizopus oryzae HC-1 improves the metabolites profiling, antioxidant activity and gut microbiota modulation effect of soybeans. LWT-Food Sci. Technol. 187, 115253 (2023).
Kanehisa, M., Sato, Y., Kawashima, M., Furumichi, M. & Tanabe, M. KEGG as a reference resource for gene and protein annotation. Nucl. Acids Res. 44, D457–D462 (2016).
Jander, G. & Joshi, V. Recent progress in deciphering the biosynthesis of aspartate-derived amino acids in plants. Mol. Plant 3, 54–65 (2010).
Gophna, U., Bapteste, E., Doolittle, W. F., Biran, D. & Ron, E. Z. Evolutionary plasticity of methionine biosynthesis. Gene. 355, 48–57 (2005).
Thomas, D. & Surdin-Kerjan, Y. The synthesis of the two S-adenosyl-methionine synthetases is differently regulated in Saccharomyces cerevisiae. Mol. Gen. Genet. 226, 224–232 (1991).
Carrillo, J. T. & Borthakur, D. Characterization of a plant S-adenosylmethionine synthetase from Acacia koa. Plant Physiol. Biochem. 210, 108618 (2024).
Funding
This work is supported by the Guangxi Science and Technology Base and Talents Special Project (GuikeAD21220059); the Foundation for the Scientific Research Base and Distinguished Talents in Guangxi (GuikeAC22080006); the Guangxi First-class Disciplines (Agricultural Resources and Environment); and the National Natural Science Foundation of China (32101367). We also thank the supports from Guangxi Key Laboratory of Biology for Mango.
Author information
Authors and Affiliations
Contributions
C.L.: Conceptualization, Methodology, Writing—Original Draft Preparation. J.W.: Visualization. M.S.: Data Curation, Methodology. X.H.: Writing-Review and Editing. Z.W.: Resources. Q.L.: Investigation. T.L.: Writing-Review and Editing, Supervision. Z.Z.: Supervision, Project Administration and Funding Acquisition.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, C., Wei, J., Shi, M. et al. Metabolomic analysis reveals the positive effects of Rhizopus oryzae fermentation on the nutritional and functional constituents of adlay millet seeds. Sci Rep 14, 17435 (2024). https://doi.org/10.1038/s41598-024-68478-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-68478-5