Abstract
Systemic sclerosis (SSc) is a connective tissue disease characterized by progressive fibrosis of the skin and visceral organs, to date, skin fibrosis remains a clinical therapeutic challenge. Adipose-derived mesenchymal stem cells (AMSCs) have been considered extremely promising for the treatment of SSc, and the biological effects of MSCs are partly attributed to the secretion of exosomes (exos). Our aim was to determine whether exosomes derived from AMSCs have parental biological effects to AMSCs in the therapy of SSc skin fibrosis. In vitro cellular experiments, AMSCs and SSc skin fibroblasts were cocultured in direct contact and transwell indirect contact at a ratio of 1:5 and 1:10, respectively, then exosomes were extracted from the cell culture supernatant of AMSCs and identified, and the exosomes were cocultured with fibroblasts to investigate the effects of AMSCs and exosomes on fibroblast collagen synthesis. Repeated subcutaneous injections of bleomycin (BLM) to construct a model of SSc skin fibrosis in vivo experiments, then AMSCs and exosomes were injected subcutaneously to investigate their effects on skin fibrosis in the BLM mice. The results revealed that exosomes had similar biological functions to AMSCs, by inhibiting the TGF-β1/Smad3 axis, which alleviated collagen synthesis in skin fibroblasts from SSc patients and skin fibrosis in BLM models. In conclusion, AMSCs-derived exosomes may be “rising star candidates” for the treatment of SSc skin fibrosis.
Similar content being viewed by others
Introduction
Systemic sclerosis (SSc) is a multisystem involvement autoimmune connective tissue disease characterized by skin and organ fibrosis, with a 3-year survival rate of 47–56%. Uncontrolled fibrosis of the skin and internal organs in patients with SSc can lead to serious and sometimes life-threatening complications, such as interstitial lung disease (ILD), cardiac involvement, pulmonary hypertension, gastrointestinal complications, and scleroderma renal crisis1. The fibrotic process of SSc has been recognized for more than 40 years, the use of biologic agents targeting anti-inflammatory and anti-fibrotic mechanisms has been proposed, however, limited specific disease-modifying treatments are available to effectively control of fibrosis in patients with SSc2. So far, the organ-based approach, focusing on treatment aimed at stabilizing lung function in SSc-ILD has yielded more success, the generic tyrosine kinase inhibitor nintedanib slows the decline in lung function in SSc patients, but has not been effective in treating skin symptoms3,4. Skin involvement has the highest average incidence of clinical manifestations of SSc (> 90%)5, and the severity and distribution of skin fibrosis is associated with vital organ involvement, fingertip ulceration, quality of life, and SSc-related mortality. Although several open and randomized open studies have reported on the beneficial effect of cyclophosphamide (CYC) on SSc skin, and two small randomized controlled trials (RCTs) have shown a modest effect of methotrexate on skin fibrosis in early diffuse cutaneous SSc6, Unfortunately, most RCTs targeting skin fibrosis have failed to meet their primary endpoints, such as CD80/CD86 blockers abatacept and tocilizumab7. Therefore, the development of novel treatments for skin fibrosis is still a major unmet clinical need to slow disease progression and reduce long-term complications.
In recent years, cell therapy, including hematopoietic stem cells8 and mesenchymal stem cells (MSCs)9, has received widespread attention. MSCs are an attractive approach due to their low immunogenicity and immunosuppressive function, and their additional anti-fibrotic, angiogenic, and anti-inflammatory properties make them a promising treatment for SSc patients10,11. In 1976, Friedenstein first identified MSCs in bone marrow, MSCs can be derived from various tissues, such as bone marrow, adipose tissue, placenta, umbilical cord and dental pulp12. Among them, Adipose-derived mesenchymal stem cells (AMSCs) are more readily available and more immunomodulatory compared to other sources of MSCs, and maintain the phenotype and pluripotency of MSCs at higher culture passages, it has been preferred in several SSc clinical trials and promotes disease remission13. However, the risks of MSCs therapy, such as immune rejection and tumorigenicity, have partially limited its clinical application.
MSCs mainly act via soluble paracrine factors, and extracellular vesicles (EVs) are thought to mediate, at least in part, the paracrine activity of MSCs14. MSC-derived extracellular EVs (MSC-EVs) display the main functions of the parental cells and, therefore, have attacted considerable attention as a possible therapeutic strategy for SSc15. EVs can be broadly classified into three subtypes based on their size: exosomes (30-150 nm), microvesicles (100–1000 nm), and apoptotic bodies (50–4000 nm). Exosomes (exos) are nano-sized membrane vesicles that are initiative secreted by almost all cells and exist in all the body fluids16, they contain components (protein, DNA, and RNA) of the cells that secrete them. They are taken up by distant cells, where they can affect cell function and behavior. Intercellular communication through exosomes appears to be involved in the pathogenesis of several disease, including cancer, neurodegeneration, and inflammatory diseases17. Exosomes, as a “cell-free” substance, may circumvent the adverse effects of MSCs while having great potential for disease prediction and targeted therapy in SSc.
Fibrosis is characterized by the presence of α-smooth muscle actin-positive (a-SMA), apoptosis-resistant myofibroblasts. These contractile cells secrete not only matrix molecules but also TGF-β and other profibrotic mediators that further promote extracellular matrix accumulation and remodeling (COL1, COL3). Of the multitude of soluble mediators involved in SSc, TGF-β is generally considered to be the master regulator of fibrosis18. Nevertheless, the regulation of skin fibroblasts in SSc patients by AMSCs-derived exosomes has rarely been probed in SSc-associated fibrosis, and AMSCs-derived exosomes have not been adequately investigated in BLM-induced SSc fibrosis mouse models. Taken together, our study explores whether exosomes inheriting the biological functions of parental AMSCs exert antifibrotic effects by classic TGF-β1/Smad3 axis in SSc skin by comparing the effects of AMSCs and exosomes on collagen synthesis in SSc skin fibroblasts and skin fibrosis in BLM mice.
Methods
Patient samples
Skin tissue samples (n = 5) from diffuse SSc patients were obtained from Huashan Hospital and Zhongshan Hospital affiliated with Fudan University, AMSCs obtained from adipose tissue derived from liposuction surgery in healthy adults (18 to 45 years old) were purchased from Shanghai Saiye Company. All subjects signed an informed consent form and were approved by the ethics committee. All experiments were performed in accordance with relevant guidelines and regulations.
Isolation of fibroblasts
Fibroblasts were isolated from skin tissues by enzymatic digestion, and the skin tissues were rinsed three times with sterile PBS, and the subcutaneous tissues were scraped and washed repeatedly. The tissues were cut into 1 –2 mm pieces and placed in a new Petri dish with Dispase II enzyme added overnight. The next day, the tissues were washed with sterile PBS again, the epidermis and dermis were separated from the basal layer, the dermis was removed and trypsin was added, the tissues were digested at 37 °C for 2–3 h, the cell suspension was filtered through a cell strainer, and washed repeatedly with complete medium consisting of Dulbecco’s Modified Eagle’s Medium (DMEM), 10% fetal bovine serum (FBS), and 1% penicillin-streptomycin centrifuged, and the supernatant was discarded to finally obtain fibroblasts.
Cell culture
Fibroblasts were cultured in DMEM supplemented with 10% fetal bovine serum, 1% penicillin, and streptomycin (Thermo Fisher Scientific, USA) in a 5% CO2 incubator at 37 °C to 80% confluence and passaged every 3 days. AMSCs were purchased from Shanghai Saiye company and their quality was tested, they were cultured in DMEM/F12 complete medium. Fibroblasts and AMSCs were used between passages 2 to 5.
AMSCs-fibroblasts direct contact coculture
Fibroblasts were inoculated in 6-well cell culture plates at 1 × 105 cells/well, AMSCs were inoculated on the surface of fibroblasts at 2 × 104 cells/well (AMSCs : fibroblasts = 1:5) and 1 × 104 cells/well (AMSCs : fibroblasts = 1:10), and the control group contained no AMSCs. The cell cocultures were incubated with AMSCs in complete DMEM/F12 medium.
AMSCs-fibroblasts indirect contact coculture
Fibroblasts were inoculated at 1 × 105/well in the lower chamber using 12-well transwell culture plates, and AMSCs were inoculated at 2 × 104/well (AMSCs : fibroblasts = 1:5) and 1 × 104/well (AMSCs : fibroblasts = 1:10) in the upper chamber, respectively. and the control group did not contain AMSCs. The cell cocultures were incubated with AMSCs in DMEM/F12 medium.
Exosomes extraction and characterization
Collect AMSCs cell culture supernatant, centrifuge at 3,000 g for 10 min at 4 °C, remove cellular debris in the supernatant, add exosomes extraction reagent, vortex and shake, leave at 4 °C for 2 h, then centrifuge at 10,000 g for 60 min at 4 °C. Aspirate the supernatant, blow the precipitate well with PBS, and centrifuge at 12,000 g for 2 min at 4 °C, and the supernatant will be the extracted exosomes. The concentration of exosomes was measured using a BCA protein assay kit (Beyotime, Shanghai, China). Transmission electron microscopy (TEM), nanoparticle tracking analysis (NTA), and Western blot (WB) were used to analyze the shape and surface markers of exosomes.
Uptake of exosomes by fibroblasts
Purified exosomes were labeled with a PKH67 green fluorescence kit (Sigma-Aldrich). The 100 µL of exosomes were resuspended with 200 µL of DiluentC, and 0.8 µL of PKH67 ethanol dye solution was mixed with 200 µL of DiluentC, incubated for 4 min at 4 °C in the dark, and 500 µL of FBS for 1 min at room temperature to stop staining. PKH67-labeled Exos was incubated with fibroblasts at 37 °C for 24 h. Fibroblasts were fixed with 4% paraformaldehyde, washed with PBS, and stained with DAPI (D9542, Sigma). Finally, the uptake of Exos by the fibroblasts was observed with a fluorescence microscope (ECLIPSE E80i, Nikon, Tokyo, Japan).
Preparation of scleroderma mouse model
24 female C57BL/6 mice were purchased and randomly and equally divided into four groups: control, BLM model group, AMSCs treatment group, and exosomes treatment group. After 1 week of laboratory acclimatization, the backs of the mice were shaved the day before disease induction. The control group was continuously injected subcutaneously with an equal volume of saline every day, and the other three groups were continuously injected subcutaneously with BLM (2 U/kg) every day. After 4 weeks, AMSCs (1107/kg) and exosomes (2.5 mg/kg) were injected subcutaneously in a single injection in the shaved area in the AMSCs-treated and exosomes-treated groups, respectively, and saline was injected subcutaneously in the BLM-treated group, and the mice were euthanized and their skin tissues were collected 1 week later.
Histological assessment
Skin specimens were fixed in 4% paraformaldehyde for 4 h, paraffin-embedded, and sectioned at 4 μm. Hematoxylin and eosin (H&E) and Masson’s stain were used to assess dermal thickness and collagen content, respectively. Images were observed and captured at 100× magnification using K-Viewer software and quantified using Image J software (National Institutes of Health, Bethesda, MD, USA). Five randomly selected fields of view were observed, and dermal thickness was defined as the distance between the epidermal-dermal junction and the dermal-subcutaneous fat junction. Collagen content was defined as the area of collagen deposition as a percentage of the total area.
Quantitative real-time polymerase chain reaction (qPCR)
We extracted total RNA with Trizol (Thermo Fisher Scientific, USA) and measured the concentration of RNA with a Nanodrop instrument. PrimeScript RT Master Mix (Takara, Biomedical Technology, Beijing) was was used for cDNA reverse transcription of 1 µg of total RNA. 2x SYBR Green qPCR Mastermix (Selleck, USA) and specific primers α-SMA, COL1A1, COL3A1, TGF-β1 and GAPDH were used for RT-qPCR. Analyses were performed using Bio-Rad or QS6 Applied Biosystems software and quantified using the 2−ΔΔCt method with gene expression ratios normalized to GAPDH. Primer sequences are provided in Supplementary Table S1.
Western blot
Total proteins from skin tissues or fibroblasts were lysed on ice for 15 min using RIPA buffer containing protease and phosphatase inhibitors (Beyotime Biotechnology, Shanghai, China), then centrifuged at 13,000 × g for 15 min at 4 °C and the supernatant of cell lysates was collected. The Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, USA) was used to quantify the protein concentration. Loading buffer was used to denature the protein samples, and the gels were transferred to PVDF membranes after electrophoresis and blocking the nonspecific sites. Primary antibodies (Abways, China) a-SMA, COL1A1, COL3A1, TGF-β1, pSmad, Smad, GAPDH, Alix, CD63 were diluted 1:1000, and the protein bands were incubated with the primary antibody at 4 °C overnight, then the secondary antibody (Beyotime Bio-technology, China) was diluted 1:2000, and incubated for 1 h at room temperature. ECL kit (Millipore, USA) was used for blotting, and Tanon software was used to observe and quantify the bands.
Statistical analysis
All statistical analyses were performed with GraphPad Prism 9 software (GraphPad Software, San Diego, CA, USA). Independent two-group t-tests were used to analyze two sets of data with normal distribution, and those among multiple groups were analyzed by one-way ANOVA, with differences considered statistically significant at P < 0.05. Normality test and homogeneity test of variance were performed on the data, with data presented as mean ± standard deviation.
Results
Manifestations in SSc patient skin and isolated fibroblasts
We collected skin tissues from the forearms of five patients with diffuse SSc, and fibroblasts were isolated using enzymatic digestion and cultured in vitro, a simplified schematic is shown in Fig. 1A, and the protocol is described in Methods. SSc patients with waxy shiny skin with skin pigmentation and hyperpigmentation, hand skin sclerosis with joint contracture, fingers half bent into claw-like shape, skin ulcers on the knuckles, skin on the back of the neck feels like leather when touched, surface is smooth and dry, and vellus hair disappears (Fig. 1B). The growth of fibroblasts was observed under the microscope, and Fig. 1C shows that fibroblasts grow against the wall with a long spindle-shaped morphology, protruding cytoplasm, and a radial growth pattern, which is consistent with the characteristics of fibroblasts.
AMSCs inhibit collagen synthesis in SSc skin fibroblasts by direct co-culture
AMSCs were cocultured with SSc patient skin fibroblasts in direct contact for 48 h at the number ratios of the blank group, 1:5 and 1:10, respectively, and a schematic diagram of the direct contact co-culture is shown in Fig. 2A. Detection of α-SMA, COL3A1, and TGF-β1 mRNA levels showed that the expression of α-SMA, COL3A1, and TGF-β1 mRNA was reduced in the coculture group compared with the control group (Fig. 2B–D). In addition, the results of detecting α-SMA, COL3A1, and TGF-β1 proteins in the cells of each group showed that the 1:5 and 1:10 co-culture groups inhibited the protein synthesis of α-SMA, COL3A1, and TGF-β1(Fig. 2E–H). The 1: 5 co-culture group had a significant inhibitory effect on the mRNA and protein levels of pro-fibrotic phenotype, suggesting that direct contact co-culture of AMSCs may inhibit collagen synthesis in SSc skin fibroblasts by suppressing the expression of pro-fibrotic markers, the efficacy is AMSCs quantitative gradient dependent.
Effects of direct contact of AMSCs on SSc fibroblasts. (A) Schematic diagram of direct contact co-culture; (B–D) Real-time PCR was performed to detect α-SMA, COL3A1, and TGF-β1 mRNA in direct co-culture of AMSCs and SSc skin fibroblasts for 48 h. (E–H) Western blot analysis of the α-SMA, COL3A1,TGF-β1 proteins in direct co-culture of AMSCs and SSc skin fibroblasts for 48 h. Data are presented as the mean ± SD, *p < 0.05, **p < 0.01.
AMSCs inhibit collagen synthesis in SSc skin fibroblasts by indirect coculture
Since AMSCs act not only through cell-cell direct contact but also paracrine secretion, to further clarify the role of paracrine effect of AMSCs, we used transwell cell culture plates to separate the two types of cells, with AMSCs spread in the upper chamber and skin fibroblasts in the lower chamber, and a schematic diagram is shown in Fig. 3A. Indirect contact co-culture was performed for 48 h, and the mRNA and protein expression of α-SMA, COL1A1, COL3A1, and TGF-β1 in skin fibroblasts were detected. The results showed that AMSCs could reduce the mRNA expression levels of α-SMA, COL1A1, COL3A1 and TGF-β1 in skin fibroblasts after indirect co-culture (Fig. 3B–E) and protein synthesis (Fig. 3F–J), suggesting that AMSCs could inhibit the collagen synthesis of skin fibroblasts through paracrine secretion, and that the efficacy is not dependent on the number of AMSCs, which may be attributed to the loss of direct cell-cell contact inhibition by paracrine secretion.
Effects of indirect contact of AMSCs on SSc fibroblasts. (A) Schematic diagram of indirect contact co-culture; (B–E) Real-time PCR was performed to detect α-SMA, COL1A1, COL3A1, TGF-β1 mRNA in indirect co-culture of AMSCs and SSc fibroblasts for 48 h. (F–J) Western blot analysis of the α-SMA, COL1A1, COL3A1,TGF-β1 proteins in indirect co-culture of AMSCs and SSc fibroblasts for 48 h. Data are presented as the mean ± SD, *p < 0.05, **p < 0.01, and ***p < 0.005.
Characterization of exosomes from AMSCs
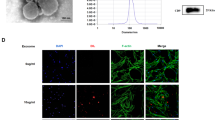
We have demonstrated that AMSCs can inhibit the pro-fibrotic phenotype of SSc skin fibroblasts not only through direct contact but also through paracrine secretion. Exosomes are considered to be important mediators of stem cell paracrine effects; therefore, we further focused on the role of AMSCs-derived exosomes on SSc skin fibroblasts. Nanoparticle tracking analyzer (NTA), transmission electron microscopy (TEM), and western blot (WB) were used to identify the exosomes. The results are shown in Fig. 4. The particle size of the samples detected by NTA was consistent with the standard, and the diameter of the exosomes was 138.9 ± 54.2 nm (Fig. 4A). Clear vesicle structures (marked by red arrows) were observed by TEM, and the vesicle size was consistent with the detection standard of exosomes (Fig. 4B). WB analysis revealed the presence of the exosome markers CD63 and Alix (Fig. 4C). The data indicated that exosomes were successfully extracted. To verify whether exosomes could be taken up by SSc skin fibroblasts, PKH67 green fluorescence was used to label exosomes, blue fluorescence is SSc skin fibroblast nuclei. Fibroblasts without exosomes intervention were used as a blank control, intervention with exosomes for 24 h, fluorescence microscopy was performed to observe the entry of exosomes into the fibroblasts, the results showed that co-localization of exosome fluorescence with fibroblast nuclear staining (Fig. 4D), suggesting that exosomes were successfully taken up by fibroblasts to perform their biological functions.
Identification and uptake of exosomes. (A) Determination of exosomes concentration and particle size by nanoparticle tracking analyzer (NTA); (B) Morphology of exosomes was detected by transmission electron microscopy (TEM), scale bars = 100 nm (left) and 200 nm (right); (C) Identification of exosomes surface markers by western blot (WB); (D) Fluorescence microscopy of exosomes and fibroblasts in co-culture.
Exosomes inhibit collagen synthesis in SSc skin fibroblasts
To investigate the role and mechanism of exosomes on the pro-fibrotic phenotype of SSc skin fibroblasts (SScF), fibroblasts were treated with exosomes (3 µg/mL) for 48 h, and the mRNA and protein levels of α-SMA, COL1A1, and COL3A1 were detected. The results showed that exosomes inhibited the expression of α-SMA, COL1A1 and COL3A1 mRNA (Fig. 5A–C) and protein (Fig. 5D–H) synthesized by SSc fibroblasts. TGF-β1/Smad3 is one of the most classical pathways in fibrosis, and inhibition of the TGF-β1/Smad3 pathway is of great importance in the treatment of SSc fibrosis. Western blot assessed the level of TGF-β1/Smad3 pathway, the result showed that exosomes (3 µg/mL) could significantly inhibit the classical TGF-β1/Smad3 pathway in fibrosis process(Fig. 5I–K), suggesting that exosomes can inhibit the pro-fibrotic phenotype of SSc fibroblasts by targeting the TGF-β1/Smad3 pathway.
Effect of exosomes on SSc fibroblasts. (A–C) Real-time PCR was performed to detect α-SMA, COL1A1, COL3A1 mRNA in SSc fibroblasts with exosomes(3 ug/mL) intervention for 48 h; (D–H) Western blot analysis of the α-SMA, COL1A1, COL3A1 proteins in SSc fibroblasts with exosomes (3 ug/mL) intervention for 48 h; (I–K) Western blot analysis of the TGF-β1/Smad3 pathway in SSc fibroblasts with exosomes (3 ug/mL) intervention for 48 h. Data are presented as the mean ± SD, *p < 0.05.
Mouse model of SSc fibrosis construction
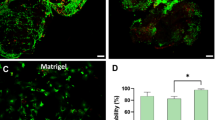
Repeated subcutaneous injections of BLM are the most widely used preclinical animal model in studies simulating fibrotic manifestations and antifibrotic therapy in SSc patients. We constructed and validated a model of BLM-induced SSc fibrosis (Fig. 6A). At the end of the four-week modeling period, none of the mice died, and there was a significant decrease in body weight in the BLM group (Fig. 6B). H&E staining of the skin showed that compared with the control, BLM mice had atrophied hair follicle glands with inflammatory cell aggregation, and the separation of the dermis from the subcutaneous fat tissue had disappeared. Masson’s staining showed that collagen fibers of BLM mice were significantly thickened, deposited in the subcutaneous dermis, and replaced the adipose tissue (Fig. 6C). The skin thickness of the two groups was measured separately, and it was found that the skin of the BLM modeling group was significantly thicker than that of the control group (Fig. 6D). The results of collagen content measurement showed that the collagen volume fraction in the skin of BLM mice was significantly increased compared with the control (Fig. 6E). WB analysis of the expression of fibrosis marker protein expression showed that the protein expression levels of α-SMA and COL3A1 were significantly higher in the skin tissues of the BLM group than the control (Fig. 6F–H). The above results indicated that the BLM-induced SSc model mice showed severe thickening of skin tissues and increased collagen expression, suggesting that the BLM-induced skin fibrosis model of SSc mice was successfully established.
Mouse model of BLM-induced fibrosis in SSc. (A) Schematic diagram of the construction of the BLM dermal fibrosis model; (B) Changes in mice body weight during modeling; (C) The respective images are the skin sections stained with H&E and Masson’s trichrome, scale bars = 200 μm; (D–E) Dermal thickness and collagen content were quantified from H&E and Masson’s staining respectively; (F–H) Western blot analysis of the α-SMA, COL3A1 proteins in skin tissue. Data are presented as the mean ± SD, *p < 0.05, **p < 0.01, and ***p < 0.005.
AMSCs and exosomes ameliorate skin fibrosis in BLM mice
Subsequently, we used BLM models, AMSCs and exosomes were injected subcutaneously to explore the therapeutic effects of AMSCs and exosomes on skin fibrosis in BLM mice, Fig. 7A shows a schematic diagram. H&E and Masson’s staining of the skin showed that the dermis was significantly thinner, with reduced inflammatory cell aggregation and collagen fiber production in AMSCs and exosomes treated mice compared to the BLM group (Fig. 7B). Dermal layer thickness measurements showed a significant reduction in skin thickness after AMSCs and exosomes treatment (Fig. 7C). Detection of collagen content showed that the collagen volume fraction in the skin of mice in the BLM group was significantly increased compared with that in the control group, while AMSCs and exosomes were significantly decreased after treatment (Fig. 7D). WB detection of the expression of fibrosis marker proteins showed that the protein levels of α-SMA and COL3A1 in the skin tissues of the BLM group were significantly higher than those of the control group, and AMSCs and exosomes were decreased after treatment (Fig. 7E–H). Further detection of the TGF-β1/Smad3 pathway by WB revealed that the TGF-β1/Smad3 pathway was upregulated in the BLM group, and the TGF-β1/Smad3 signaling axis was inhibited by AMSCs and exosomes treatment (Fig. 7I–L), suggesting that exosomes, which have similar biological functions to parental AMSCs, could ameliorate skin fibrosis in BLM mice by inhibiting the TGF-β1/Smad3 signaling pathway.
Effects of AMSCs and exosomes on skin fibrosis in BLM mice. (A) Schematic diagram of BLM mice treated with subcutaneous injections of AMSCs and exosomes; (B) The respective images are the skin sections stained with H&E and Masson’s trichrome. Scale bars = 200 μm. (C,D) Dermal thickness and collagen content were quantified from H&E and Masson’s staining respectively. (E–H) Western blot analysis of the α-SMA, COL3A1 proteins in skin tissues. (I–L) Western blot analysis of the TGF-β1/Smad3 pathway in skin tissues. Data are presented as mean ± SD, *p < 0.05, **p < 0.01, and ***p < 0.005.
Disscussion
SSc is a severe connective tissue disease characterized by progressive organ fibrosis, which can occur in the early and late stages of SSc with different clinical progression. Persistent fibroblast activation leads to excessive extracellular matrix deposition, distortion of tissue architecture, organ dysfunction and ultimately organ failure19. Among these, the degree of dermal fibrosis is closely related to patients’ quality of life and disease prognosis, reflecting the process of internal organs20. Therefore, early control of skin fibrosis progression and targeting fibroblast fibrosis phenotype are crucial. To date, mycophenolate mofetil (MMF) is used as a first-line therapeutic agent to improve skin symptoms, but it is associated with serious side effects such as pancytopenia, myelosuppression. Other immunosuppressants agents such as methotrexate, cyclophosphamide, and glucocorticoids have not shown long-term beneficial effects on overall disease. Although the advent of biologics has opened new avenues in the treatment of SSc with significant benefits in improving lung function, these therapies are unable to meet the primary endpoint of reducing the Modified Rodnan Skin Score (MRSS)21. Therefore, current treatment options for skin fibrosis in SSc are limited and there is an urgent need for safe and effective treatments.
A recent review on MSCs for SSc reported preclinical and clinical studies of MSCs from different sources (including bone marrow, adipose tissue, and umbilical cord) for the treatment of SSc, which showed various positive clinical outcomes, such as improvement of lung function, improvement of limb circulation, and reduction of skin fibrosis and finger ulcers22. Among them, allogeneic adipose-derived MSCs are considered to be the potential gold standard MSCs for clinical application in SSc. An open cohort study found that AMSCs dramatically reduced the consequences of orofacial fibrosis in SSc. By inhibiting fibroblast proliferation and key regulators of fibrogenesis, including TGFβ-1 and CTGF, AMSCs may alleviate skin fibrosis23. Rebekka et al. demonstrated that, in a co-culture model of AMSCs and normal human dermal fibroblasts, AMSCs increased the formation of collagen types I, III, and VI in the ECM24. It appears that AMSCs could target abnormal fibroblasts and reduce pathological deposition of ECM. However, limitations such as aging and functional degeneration, local distribution and differentiation, tumorigenicity after infusion of AMSCs into the body are pose challenges to clinicians, and new therapeutic options are constantly being sought25.
Nakamura et al. found increased mRNA levels and exosome secretion of the exosomal markers CD63, CD9 and CD81 in skin samples and dermal fibroblasts from SSc patients compared to controls.Wermuth P.J et al. found that four pro-fibrotic miRNAs (let-7 g, miR-17, miR-23b, and miR-29a) were upregulated 5-fold and four anti-fibrotic miRNAs (let-7a, miR125b, miR140, and miR146a) were downregulated 70-fold in exosomes isolated from SSc serum compared to normal subjects. Conditioned medium from SSc dermal fibroblasts (containing exosomes) and exosomes from serum of SSc patients promote normal human dermal fibroblasts to encode collagen and other extracellular matrix components. This suggests a key role for exosomes in paracrine regulation in neighboring and distant cells, where released exosomes fuse with target cells to induce and propagate a pro-fibrotic molecular program of SSc-causing factors. In addition to this, exosomes, as a cell-free substance secreted by MSCs, have received extensive attention from researchers due to their advantages of lower munogenicity, easy extraction and purification, greater tissue permeability, and the ability to maintain the therapeutic properties of MSCs. Existing studies have focused on the role of bone marrow MSCs or umbilical cord MSCs or their exosomes26, which have achieved favorable therapeutic results in the treatment of SSc mice. Many studies have confirmed that MSC-EVs, typified by Exos, can act as antifibrotic agents through various mechanisms of action in the treatment of fibrotic diseases, whereas the efficacy of AMSCs-Exos as a highly promising substance for the treatment of BLM mouse models has not yet adequately been demonstrated. Furthermore, in vitro experiments, Rozier et al. demonstrated the efficacy of AMSCs in treating an in vitro cell model of SSc induced by TGF-β1, and further found that AMSCs-EVs had a stronger antifibrotic effect than AMSCs27. However, the direct therapeutic effect of AMSCs-derived exosomes on skin fibroblasts from diffused SSc patients has not been reported to date.
Hence, in our cellular experiments, we used skin fibroblasts from diffuse SSc patients and found that both direct and indirect co-culture of AMSCs inhibited collagen synthesis (Col1a1, Col3a1) and expression of fibrosis markers (α-SMA, TGF-β1) in SSc skin fibroblasts. Exosomes have been shown to be the key substances for AMSCs to exert biological effects, but further research is required to determine the long-term safety of exosomes, detailed mechanisms of action, and the ability to translate experimental results into clinical practice. In our study, we successfully isolated and characterized exosomes from the cell supernatants of AMSCs and found that exosomes can be taken up by fibroblasts, and also further demonstrated that exosomes can inhibit the expression of fibrosis-related molecules through the TGF-β1/Smad3 axis. In addition, we also constructed the most classical BLM-induced fibrosis mouse model of SSc, and observed that both AMSCs and exosomes could reduce the dermal thickness and collagen volume fraction, and inhibit the expression of α-SMA and COL3A1 in skin tissues of BLM mice via the TGF-β1/Smad3 axis.
In conclusion, our study suggests that exosomes can act as a cell-free substance that can circumvent the adverse effects of AMSCs while delivering their biological functions to improve SSc skin fibrosis by inhibiting the TGF-β1/Smad3 signaling pathway, and whether exosomes may act by other mechanisms, we will follow up with further in-depth studies by single-cell sequencing or proteomics. Nevertheless, we believe it is reasonable to assume that exosomes could be an alternative to AMSCs for the treatment of SSc skin fibrosis, supporting the use of exosomes as a clinical treatment for SSc skin fibrosis and and informing future clinical trials.
Data availability
No datasets were generated or analysed during the current study.
References
Volkmann, E. R., Andréasson, K. & Smith, V. Systemic sclerosis. Lancet (London England)401(10373), 304–318 (2023).
Leask, A., Naik, A. & Stratton, R. J. Back to the future: Targeting the extracellular matrix to treat systemic sclerosis. Nat. Rev. Rheumatol.19(11), 713–723 (2023).
Allanore, Y. et al. Continued treatment with nintedanib in patients with systemic sclerosis-associated interstitial lung disease: Data from SENSCIS-ON. Ann. Rheum. Dis.81(12), 1722–1729 (2022).
Kuwana, M. et al. Nintedanib in patients with systemic sclerosis-associated interstitial lung disease: Subgroup analyses by autoantibody status and modified rodnan skin thickness score. Arthritis Rheumatol. (Hoboken, NJ)74(3), 518–526 (2022).
Allanore, Y., Simms, R., Distler, O. et al. Systemic sclerosis. Nat. Rev. Dis. Primers1(15002) (2015).
Barnes, H. et al. Cyclophosphamide for connective tissue disease-associated interstitial lung disease. Cochrane Database Syst. Rev.1(1), Cd010908 (2018).
Khanna, D. et al. Tocilizumab in systemic sclerosis: A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir. Med.8(10), 963–974 (2020).
van Laar, J. M., Naraghi, K. & Tyndall, A. Haematopoietic stem cell transplantation for poor-prognosis systemic sclerosis. Rheumatol. (Oxford, England)54(12), 2126–2133 (2015).
Cras, A., Farge, D., Carmoi, T. et al. Update on mesenchymal stem cell-based therapy in lupus and scleroderma. Arthritis Res. Ther.17(301) (2015).
Peltzer, J., Aletti, M., Frescaline, N. et al. Mesenchymal stromal cells based therapy in systemic sclerosis: Rational and challenges. Front. Immunol. 9(2013) (2018).
Rozier, P., Maria, A., Goulabchand, R. et al. Mesenchymal stem cells in systemic sclerosis: allogenic or autologous approaches for therapeutic use?. Front. Immunol. 9(2938) (2018).
Da Silva Meirelles, L., Chagastelles, P. C. & Nardi, N. B. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell Sci.119(11), 2204–2213 (2006).
Ménard, C. et al. Integrated transcriptomic, phenotypic, and functional study reveals tissue-specific immune properties of mesenchymal stromal cells. Stem Cells38(1), 146–159 (2020).
Nooshabadi, V. T. et al. The extracellular vesicles-derived from mesenchymal stromal cells: A new therapeutic option in regenerative medicine. J. Cell. Biochem.119(10), 8048–8073 (2018).
Rozier, P., Maumus, M., Maria, A. T. J. et al. Mesenchymal stromal cells-derived extracellular vesicles alleviate systemic sclerosis via miR-29a-3p. J. Autoimmun.121(102660) (2021).
Yeo, R. W. et al. Mesenchymal stem cell: An efficient mass producer of exosomes for drug delivery. Adv. Drug Deliv. Rev.65(3), 336–341 (2013).
Kalluri, R. & Lebleu, V. S. The biology, function, and biomedical applications of exosomes. Science (New York, NY) 367(6478) (2020).
Li, M. et al. Mesenchymal stem cell-derived exosomes ameliorate dermal fibrosis in a murine model of bleomycin-induced scleroderma. Stem Cells Dev.30(19), 981–990 (2021).
Hinz, B. & Lagares, D. Evasion of apoptosis by myofibroblasts: A hallmark of fibrotic diseases. Nat. Rev. Rheumatol.16(1), 11–31 (2020).
Wu, W. et al. Progressive skin fibrosis is associated with a decline in lung function and worse survival in patients with diffuse cutaneous systemic sclerosis in the European Scleroderma Trials and Research (EUSTAR) cohort. Ann. Rheum. Dis.78(5), 648–656 (2019).
Herrick, A. L., Assassi, S. & Denton, C. P. Skin involvement in early diffuse cutaneous systemic sclerosis: An unmet clinical need. Nat. Rev. Rheumatol.18(5), 276–285 (2022).
Xiao, Y., Huang, Z., Wang, Y. et al. Progress in research on mesenchymal stem cells and their extracellular vesicles for treating fibrosis in systemic sclerosis. Clin Exp Med (2023).
Almadori, A. et al. Stem cell enriched lipotransfer reverses the effects of fibrosis in systemic sclerosis. PLoS One14(7), e0218068 (2019).
Søndergaard, R. H. et al. Adipose-derived stromal cells increase the formation of collagens through paracrine and juxtacrine mechanisms in a fibroblast co-culture model utilizing macromolecular crowding. Stem Cell Res. Ther.13(1), 250 (2022).
Baer, P. C. & Geiger, H. Adipose-derived mesenchymal stromal/stem cells: Tissue localization, characterization, and heterogeneity. Stem Cells Int. 2012(812693) (2012).
Yu, Y. et al. The therapeutic effects of exosomes derived from human umbilical cord mesenchymal stem cells on scleroderma. Tissue Eng. Regen. Med.19(1), 141–150 (2022).
Rozier, P., Maumus, M. & Bony C. et al. Extracellular vesicles are more potent than adipose mesenchymal stromal cells to exert an anti-fibrotic effect in an in vitro model of systemic sclerosis. Int. J. Mol. Sci. 22(13) (2021).
Funding
The authors acknowledge the support from the National Natural Science Foundation of China (No. 82371815) and innovative research team of high-level local universities in Shanghai-Clinical and basic research on the prevention and treatment of some inflammatory diseases by integrative medicine.
Author information
Authors and Affiliations
Contributions
Y.X. and Q.X. contributed equally to this paper. All authors contributed to the conception and design of the study, acquisition of data, analysis, and interpretation. H.Z. and X.Y. revised and finalised the manuscript. The first draft of the manuscript was written by Y.X. and Q.X. All authors commented on previous versions of the manuscript. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Approval was granted by the Ethics Committee of Huashan and Zhongshan Hospitals.
Consent to participate
All authors agreed to the participate.
Consent for publication
All authors agreed to the publication of the manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xiao, Y., Xiang, Q., Wang, Y. et al. Exosomes carrying adipose mesenchymal stem cells function alleviate scleroderma skin fibrosis by inhibiting the TGF-β1/Smad3 axis. Sci Rep 15, 7162 (2025). https://doi.org/10.1038/s41598-024-72630-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-72630-6
Keywords
This article is cited by
-
Stem cell therapy in systemic sclerosis
Clinical Rheumatology (2025)