Abstract
Tamarix tetragyna is a plant grows in Mediterranean area and some Arab countries. It possesses numerous medicinal values. Purpose of our study is to explore biological activity of tamarix tetragyna extracts of both leaves and stem with investigating their phytochemical composition. The investigated extracts’ phyto-constituent composition was determined using gas chromatographic-mass spectrometric method. In addition, in vitro cytoxicity activity versus cancer cell lines such MCF-7, HepG-2, HCT-116, and A-549 was examined by MTT assay method, together with exploring its apoptosis effect by flow cytometry and western blot analysis techniques. Moreover, some phytochemical compounds were identified, and in-silico evaluated against anticancer molecular targets. Plant extracts showed good cytotoxic activity against both A-549 and HCT-116 cancer cell lines. With an IC50 value of 23.90 µg/ml that led to apoptosis and G2/M-phase arrest in A-549 cells, cytotoxicity data demonstrate leaves’ extract effectiveness against these cells. Upon GC-MS analysis, it revealed presence of some bioactive components such as Stigmast-5-en-3-ol and 2-methoxy-4-vinyl phenol, which are known for their cytotoxic activity. Our findings suggest that methanolic extracts of Tamarix tetragyna parts may have potential therapeutic uses as anticancer against A-549 cells, which opens up further avenues for investigation into its industrial applications.
Similar content being viewed by others
Introduction
Use of plants as a source of medicine has been inherited and is an important component of the health care system. Numerous medicinal plants have been utilized for ages in traditional medicine to cure a variety of diseases1. A wide variety of chemicals in medicinal plants can be used to treat both chronic and infectious disorders2. Several plant extracts have been shown to have a high therapeutic activity. These extracts were made utilizing a variety of organic solvents, including ethanol, methanol, and acetone3.
More than 60 species of halophyte plants belonging to the genus Tamarix of the Tamaricaceae family, often known as “Tamarisk” and “salt cedar,” are cultivated all over the world. These plants have salt-coated needle-like leaves that are produced by salt glands and are covered in salt4. Although tamarisk species are best recognized for flourishing in hot, arid areas, they can also be found there5. Tamarix species are grown in dry regions to aid in stabilizing sand dunes6, but their development in moist climates is prohibited because they act as invasive plants that hinder the development of other species7. Tamarix tetragyna is a perennial shrub with height of about 2.76 m. Its flowering period is between March and October every year8.
The strong antioxidant system found in Tamarix species is a result of particular phenolic chemicals, terpenoids (carotenoids and essential oils), and vitamins. These elements are required for plants to regularly grow, develop, and guard against damage and infection9. Along with their many therapeutic advantages, these compounds have the ability to function as antioxidants and antibacterial agents, as well as hepato-protective and chemo-preventive properties, anti-inflammatory, anti-allergic, antithrombotic, cardio-protective, and vasodilator effects10.
T. tetragyna possess a wide range of biological properties, including antioxidant, antibacterial, and α-glucosidase inhibiting actions, as well as anticancer and hepato-protective effects, according to several pharmacological studies. These biological activities were attributed to presence of flavonoids, terpenes, tannins, quinones, and phytoestrogens11,12,13. T. tetragyna extract was found to be bacteriostatic against B. subtilis, Clostridium sporogenes, S. aureus, Neisseria meningitidis, and M. catarrhalis as well as bactericidal against Corynebacterium diphtheria14,15. Furthermore, a leaf extract from T. nilotica demonstrated strong antibacterial activity against a range of human pathogenic microorganisms16. Various cancer cell lines have been used in studies on Tamarix species. By altering cell cycle proteins such cyclin B1 and p38, T. gallica hydromethanolic extract decreased cell growth and proliferation in human colorectal Caco-2 cells, and the potency was virtually the same in shoot, leaf, and flower extracts17. Distinct extracts from T. senegalensis (syn: T. nilotica), showed anticancer activities in human liver (Huh-7) and lung (A-549) carcinoma cells, with ethyl acetate displaying the strongest activity18.
The antibacterial properties of T. gallica, or French tamarix, have also been researched. Excellent effectiveness against phyto-pathogenic organisms of the genus Fusarium was demonstrated by its bark aqueous ammonia extract19. T. aphylla has a number of biological activities relevant to traditional medicine, including antidiabetic, antibacterial, anti-inflammatory, wound-healing, antifungal, and anticholinesterase properties20.
The antioxidant activity of T. aphylla leaf extract has been investigated, and it was discovered that, when compared to regular ascorbic acid, its ethyl acetate extract is a potent antioxidant21. Several T. articulata parts were extracted and investigated. It was discovered to have encouraging antibacterial action, antioxidant qualities, and an anti-proliferative effect on colorectal and breast cancer cells22.
Although tamarix species are plants that are abundant in phytochemical substances, they are plagued by a number of problems, such as lack of mechanistic techniques and ambiguity in the currently available data23,24,25. In the present work, our aim is to evaluate the biological activities of T. tetragyna methanolic extracts of leaves and stem, such as anticancer capabilities against variable cancer cell lines together with exploration of its apoptotic effect. Also, this work describes phytochemicals identified in methanolic extract of T. tetragyna through using GC-MS analysis technique. Further in-silico docking studies against anticancer molecular targets were performed to explore ability of bioactive phytochemicals towards inhibition of cancer cell growth.
Materials and methods
All methods were carried out in accordance with relevant institutional guidelines and regulations.
Chemicals and solvents
The highest quality and analytical grade chemicals and solvents were all purchased from Sigma-Aldrich (St. Louis, MO, USA).
Collection and extraction of plant material
In the Saudi Arabian province of Hail, T. tetragyna was collected and identified by Dr. Naila Hassan Alkefai, University of Hafr Al-Batin. A voucher specimen (No. UOHCOP025) has been deposited at the College of Pharmacy, University of Hail. The leaves and stem were separated, and each was ground and converted into a powder. The powdered leaves and stem were air dried and then macerated with 70% methanol several times for further extraction. Then the vacuum was used to concentrate the methanolic extracts and residues were obtained for leaf extract (TML) and stem extract (TMS).
GC-MS (gas chromatography-mass spectrometry) analysis
GC-MS tools were applied on plant methanolic leaves’ extract (TML). Thermo MS-DSQ II; 5.0 Thermo GC-TRACE ultra ver. equipment is from Thermo Scientific Co. DB-5 Non-polar Capillary column; 30Mts with ID of 0.25 mm thickness were chosen as the best chromatographic conditions to be used. A flow rate of 1.0 ml/min was used. Temperature was programmed to rise at a rate of 6 °C/min while using a 1 L injection volume over a range of 70 to 260 °C. Using a capillary column TG-5MS with dimensions of 30 m x 0.25 mm x 0.25 m film thickness and a Trace GC-TSQ mass spectrometer (Thermo Scientific, Austin, TX, USA). The temperature in the column oven was initially set at 50 °C and then increased by 5 °C/min to 250 °C and held for 2 min. Afterward, the temperature was raised to 300 °C at a rate of 30 °C per minute while being held for 2 min. The temperature of the MS transfer line was 260 °C, whereas the injection unit was maintained at 270 °C. A constant flow rate of 1 ml/min of helium was employed as a carrier gas throughout the examination.
Biological activity
Cancer cell lines
American Type Culture Collection (ATCC, Rockville, MD) provided MCF-7 cells (human breast cancer cell line), HepG-2 cells (human hepatocellular carcinoma), HCT-116 cells (colorectal carcinoma), and A-549 cells (lung carcinoma). On RPMI-1640 medium supplemented with 10% inactivated fetal calf serum and 50 mg/ml gentamycin, the cells were cultured. The cells were sub-cultured two to three times per week and kept at 37 °C in a humid environment with 5% CO2.
Cell viability assay
The tumor cell lines were dispersed on Corning® 96-well tissue culture plates at a concentration of 5 × 104 cells/well. The plates underwent a 24-hour incubation. Three replicates of the extracts were then combined to create a total of twelve concentrations for each ingredient. For each 96-well plate used as a control, medium or 0.5% DMSO were used. After 24 h of incubation, the MTT test was used to measure the number of viable cells26.
Apoptosis analysis (annexin V-FITC assay) of A549 cells
Leaves’ extract was applied to cultured A549 (lung cancer cell lines) at IC50 concentration (23.9 µg/ml). A549 cells were extracted after 72-hour treatment period. They were rinsed twice in PBS for 20 min and with binding buffer. In addition, 100 mL of kit binding buffer containing suspended cells received 1 mL of FITC-Annexin V. It was incubated at 4 °C for 40 min. Cells were washed and re-suspended in 150 mL of binding buffer in which 1 mL of DAPI (1 µg/mL in PBS) was included.
Cell cycle analysis using flow cytometry
In order to ascertain the effect of the tested substance on the cell cycle distribution of the A-549 cell line, cell cycle analysis was carried out by staining the DNA with a fluorescent dye and measuring its intensity. Since the dye stains DNA stoichiometrically, it is possible to identify aneuploid populations and differentiate cells in G0/G1, S phase, and G2/M. Different sample types can be stained using different procedures, but the overall analysis stays the same27.
Western blot analysis
One crucial method in cell and molecular biology is western blotting. Researchers can distinguish proteins from a complicated mixture of proteins isolated from cells by utilizing a western blot. To achieve this, the method consists of three steps: size separation; transfer to a solid substrate; and target protein marking with the appropriate primary and secondary antibodies for visualization28.
In-silico studies
Docking experiments were performed utilizing a software suite to determine how phytochemical components operate as inhibitors for MDM2 (mouse double minute 2) on Co-protein linked to the p53 tumor suppressor transactivation29. The evaluation used (Nutlin-3a) as the reference standard to assess the binding scores and modes of these compounds. The compounds were prepared for docking using Chem-Office PerkinElmer Suite 2017 and a standard procedure30,31. The screened compounds and the Co-crystallized inhibitor (Nutlin-3a) were inserted into a database and saved as an MDB file.
The Protein Data Bank (PDB) provided crystallographic structure of MDM2 in association with Nutlin-3a with a resolution of 1.25 Å32 and prepared in a number of ways, including error correction, insertion of 3D hydrogens, and energy minimization33. The postures that displayed highest scores, RMSD values under 2, and advantageous interactions between screened compounds and targeted proteins were examined. A re-docking process was performed using co-crystallized ligand in created target protein’s binding pocket to confirm precision of docking program34,35,36,37.
Results and discussion
Gas chromatography–mass spectrometry (GC-MS) analysis
GC–MS chromatogram of methanolic extracts of T. tetragyna showed presence of 16 peaks that may correspond to biological substances. These peaks were further identified by comparing their mass spectral fragmentation patterns to those of established standards. Five compounds were identified, as shown in Fig. 1.
The major phytochemical substances present were 2-Methoxy-4-vinylphenol, hexadecanoic acid, ingol-12-acetate, α-tochopherol and Stigmast-5-en-3-ol, as shown in Table 1. 2-Methoxy-4-vinylphenol is an organic compound that induces cell cycle arrest, which aids in treatment of cancer38. Also, it has been tested against human pancreatic cancer cells and was found to prevent Panc-1 cell growth and metastasis39. Hexadecanoic acid is a straight chain sixteen carbon fatty acid that possess antioxidant and cholesterol lowering effects40. A plant phyto-sterol called stigmast-5-en-3-ol is widely distributed in a variety of plants. In vitro models have been used to study its anti-proliferative effects41 and its ability to decrease cholesterol has also been proven. Also, it has been reported to have anti-proliferative effects against human leukemia HL-60 and human breast cancer cells, MCF-742. Its anti-tumor effect against melanoma cells was proved together with its anti-hyper-lipidemic activity towards cholesterol, triglycerides and LDL43. Ingol-12-acetate is an ingol derivative that possess Antimicrobial, antiviral and cytotoxic activities44. Tochopherol or Vitamin E is a fat-soluble vitamin that has a strong antioxidant and anti-inflammatory effect that may enhance the immunity system45. Al-Hadid KJ has investigated antibacterial activity of TML extract and it was found that some gram –ve bacteria were sensitive to TML extract due its content of phenolic acid and flavonoids46.
On comparison with other Tamarix species that grow in Saudi Arabia, it was found that ethyl acetate extract of T. aphylla leaves contains 23 different components. These components were identified by GC-MS analysis to be; Oxacyclo-dodecan 2-one, Acetophenone, Tetrazole, Pyridine, 2,4,6-trimethyl, 1-undecanol, Digitoxin, Octadecanol, Colchicine, Oxacyclododecan 2-one, Gibberellic acid, Ricinoleic acid, and Trifluoromethyl Pentadecanol, Phenylmercuric salicylate, Vobassan1-17-oic acid, 4 dimethyl-3-oxo-methyl ester, Cholesteryl benzoate, Gamabufotalin, Beclomethasone, Gamabufotalin, and Prednisolone acetate20. This reveals unique presence of some bioactive substances in T. tetragyna such as; 2-Methoxy-4-vinylphenol, stigmast-5-en-3-ol and Ingol-12-Acetate.
Biological activity
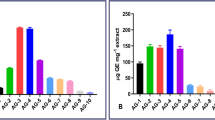
Some tamarix extracts had revealed good cytotoxic activity such as; tamarix gallica whose leaf extract showed strong anticancer effect against human colon cancer cells with IC50 of 50 µg/ml17. In addition, tamarix articulata leaf methanolic extract has produced a good anticancer effect against hepatocellular cancer cells47. The methanolic extracts of T. tetragyna stem and leaves have been investigated against HepG2, A549, HCT-116 and MCF7 cancer cell lines as shown in Figs. 2, 3 and the resulting IC50 values have been summarized in Table 2.
With an IC50 of 23.9 ± 1.31 and 30.6 ± 1.62 µg/ml, respectively, it was found that leaves’ extract has strong cytotoxic activity against both the A-549 and HCT-116 cell lines. This activity is comparable to that of standard doxorubicin (with IC50 of 1.71 ± 0.16 and 1.28 ± 0.19 µg/ml for A-549 and HCT-116 cell lines, respectively), While it shows less cytotoxic activity against HepG2 and MCF7 cancer cell lines. Stem extract showed less cytotoxic activities on all cell lines as shown in Table 2.
Moreover, as compared to MRC-5 cells, which are healthy normal cells, the extract from the leaves demonstrated good selectivity towards the A-549 and HCT-116 cancer cell lines. The A549 cells showed the highest level of selectivity, with leaf and stem extracts being nearly three times more selective, as seen in Table 3. We can infer from high selectivity that extracts from leaves and stems are less harmful to normal cells. We investigated the mechanism of action of T. tetragyna leaf extract on A549 cancer cell lines because of these encouraging results against A549 cells.
T. tetragyna methanolic extract of leaves induced G2/M phase cell-cycle arrest in A-549 cells
As leaves extract’s cytotoxic activity was shown to be highest against the A-549 cancer cell lines, we aimed to characterize bioactivity of this extract by showing its impact on the progression of cell cycle. A-549 cancer cells treated with a methanolic extract of leaves showed an increase in the percentage of cells in the G2/M phase (18.62% compared to 12.81% in untreated cells), as shown in Fig. 4. These findings indicate that percent of A-549 cancer cells that arrested in G2/M phase has been increased in presence of TML which reveals inability for cell division. Hence we can conclude that TML has induced apoptosis in A-549 cells. Thus, cell cycle analysis revealed G2/M-phase arrest and activation of apoptosis in A-549 cells.
T. tetragyna methanolic extract of leaves activated caspase-dependent and bax and p53 mediated apoptosis in A-549 cells
Analysis of the cell cycle revealed that methanolic leaf extract led to apoptosis in A-549 cells. As a result, an annexin V/propidium iodide (PI) apoptosis assay was performed using leaves’ extract on A-549 cell lines to confirm that.
As cells in late apoptosis or necrosis (both annexin and PI positive) are represented by Q2 quadrant. Hence, leaves’ extract induced late apoptosis (8.66% in Q2 compared to 0.27% only in untreated cells). As cells in early apoptosis or cell apoptosis (annexin positive and PI negative) are represented by Q4 quadrant, therefore, leaves’ extract induced early apoptosis (19.53% in Q4 compared to 0.43% only in untreated cells). In addition, TML extract caused more necrosis that is confirmed by its high percentage (4.32%) compared to that of untreated cells (1.58%) in Q1 quadrant, as shown in both Table 4 and Fig. 5.
Therefore, we may conclude that the methanolic extract of the leaves caused the death of cancer cells, primarily via inducing apoptosis with a small amount of necrosis.
Proteins called caspases are primarily in charge of apoptosis. Caspase-3 is one of caspases that can be considered as a frequently activated death protease. As shown in caspase-cascade system, activation and function of caspases are organized by different types of molecules, such as Bcl-2 family proteins, calpain, apoptosis protein inhibitor and Ca2+48. One of the proteins involved in tumor suppression and subsequent anti-proliferative activities, such as apoptosis, is p5349. After applying leaves’ methanolic extract to A549 cancer cells, western blot analysis results showed that high amounts of protein expression for cleaved caspase-3 were produced. This explains induction of apoptosis in treated A-549 cancer cells and correlates with results of Annexin V/PI assay, as mentioned in Table 5.
In silico docking
On utilizing molecular docking experiments, it was possible to test the binding relationships between the examined phytochemical elements. The results were visualized using Discovery Studio 4.0 software. DNA synthesis and cell signal transmission are two biological processes that depend on protein-protein interactions (PPIs)50. These interactions could either encourage or prevent the formation, growth, and spread of cancer. Therefore, concentrating on PPIs has great promise for the treatment of cancer. In the hunt for anticancer medications, the interaction between the proteins p53 and MDM2 (mouse double minute 2) is of great interest51.
P53, referred as the tumour suppressor protein (TP53) influences the transcription of several downstream genes that regulate the cell cycle, initiate apoptosis, assist in DNA repair, and result in senescence52.
MDM2, is the primary p53 negative regulator. According to studies, p53 and MDM2 work together to control one another’s activity in cells through an auto-regulatory feedback loop. MDM2 limits p53’s ability to transcribe genes by transferring p53 protein to cytoplasm and subsequently designating it for destruction via E3 ubiquitin pathway. This approach enables targeted treatment of many malignancies51,52,53.
As a result, the usage of MDM2-p53 (PPI) inhibitors has been discovered as a promising strategy for treating human malignancies54,55,56,57. In this study, the primary phytochemical components of the plant leaf extract were docked against MDM2 (PDB ID: 5ZXF). In comparison to the native co-crystallized ligand (Nutlin-3a), which acts as the reference control (demonstrating a binding energy of -6.8 kcal/mol). Furthermore, Fig. 1 illustrates the binding interactions, by forming one hydrogen bond with TYR79 at distance 2.86 Å and number of hydrophobic interactions with LYS30, LEU33, PHE34, LEU36, TYR79, ILE40 and MET41. Based on Table 6 that provides detailed information on the binding scores, bonding types, and comparisons with the co-crystallized ligand (Nutlin-3a), it is worth noting that Ingol-12-Acetate and Stigmast-5-en-3-ol exhibited binding scores superior to that of the co-crystallized inhibitor of MDM2-p53 with highest energy scores − 7.8 and − 7.4 kcal/mol, respectively. Ingol-12-Acetate forming number of hydrophobic interactions with amino acids LYS30, VAL32, LEU33, PHE34, LEU36 GLY37 and GLN38. In addition, Stigmast-5-en-3-ol could interact with receptor forming hydrophobic interactions with amino acids LYS30, GLU31, LEU33, PHE34, LEU36, GLY37 and ILE40. In-silico screening of MDM2 targeted phytochemical composition of plant extract also revealed several hydrogen and hydrophobic interactions with binding energies that ranged from G −4.8 to −7.8 kcal/mol, indicating possibility of interactions with active site of MDM2, as shown in Table 6; Figs. 6, 7, 8, 9, 10, 11.
Conclusion
In this study, cytotoxic effects of T. tetragyna leaves and stem methanolic extracts were assessed. Leaves’ extract had strong cytotoxic action against both A-549 and HCT-116 cancer cells. Additionally, this extract’s apoptotic effect has been studied, which was not done with T. articulata in earlier studies. It was found that T. tetragyna extract induces a caspase-3 dependent apoptosis in A-549 cancer cell line. From apoptotic study’s results, it was observed that leaves’ extract caused G2/M phase arrest in A-549 cancer cells. Further GC-MS analysis of plant extract identified 16 phytochemical substances. Five bioactive compounds were identified and two of them were found to be significant; 2-methoxy-4-vinyl phenol and stigmast-5-en-3-ol, which are well-known for their anticancer properties. The GC-MS analysis of T. aphylla leaf extract grown in Saudi Arabia did not identify either of them, despite the fact that our study’s T. Tetrgayna has a significant anticancer impact.
Furthermore, T. articulata extracts have never been subjected to GC-MS analysis in previous studies. Further confirmation of T. tetragyna’s anticancer effect was obtained by an in-silico analysis conducted against anticancer molecular targets, an approach not previously used in studies involving T. articulata extracts. GC-MS results encourage us to make more phytochemical characterization in further coming studies. The findings of our study makes T. tetragyna an attractive candidate drug for lung cancer treatment.
Data availability
All data generated or analysed during this study are included in this published article.
References
Ezeabara, C. A. & Egenti, M. O. Phytochemical and antimicrobial investigations on various parts of Sida acuta Burm. F. J. Ayurvedic Herb. Med. 4 (2), 71–75 (2018).
Ezeabara, C. A. & Nwafulugo, S. N. Comparison of phytochemical and proximate compositions of parts of Cleome ciliata Schum. & Thonn and Cleome viscosa L. World J. Biomed. Pharm. Sci. 1 (1), 1–5 (2015).
Aurélie, R. L. et al. Antimicrobial activity of Albizia tulearensis, an endemic Fabaceae from Madagascar. World J. Biol. Pharm. Health Sci. 2 (3), 30–34 (2020).
Samadi, N., Ghaffari, S. M. & Akhani, H. Meiotic behavior, karyotype analyses and pollen viability in species of Tamarix (Tamaricaceae). Willdenowia43 (1), 195–203 (2011).
Zhang, D., Yin, L. & Pan, B. Biological and ecological characteristics of Tamarix L. and its effect on the ecological environment. Sci. China Ser. Earth Sci. 45 (1), 18–22 (2002).
Han, Z., Yin, W., Zhang, J., Niu, S. & Ren, L. Active anti-erosion protection strategy in Tamarisk (Tamarix aphylla). Sci. Rep. 3 (1), 1–7 (2013).
Whitcraft, C. R., Talley, D. M., Crooks, J. A., Boland, J. & Gaskin, J. Invasion of tamarisk (Tamarix spp.) in a southern California salt marsh. Biol. Inv. 9 (7), 875–879 (2017).
Through website; floraveg.eu/taxon/overview/tamarix.
Ksouri, R. et al. Antioxidant and antimicrobial activities of the edible medicinal halophyte Tamarix gallica L. and related polyphenolic constituents. Food Chem. Toxicol. 47, 2083–2091 (2009).
Lee, J. M. et al. Comparison of biological activities of Korean halophytes. Nat. Prod. Sci. 24, 247–252 (2018).
Liao, J. et al. Advances in researches on chemical constituents in plants of Coreopsis L. and their pharmacological activities. Drugs Clin. 27, 404–408 (2012).
Bahramsoltani, R., Kalkhorani, M., Zaidi, S. M., Farzaei, M. H. & Rahimi, R. The genus Tamarix: traditional uses, phytochemistry, and pharmacology. J. Ethnopharmacol. 246, 112245. https://doi.org/10.1016/j.jep.2019.112245 (2020).
Jdey, A. et al. Phytochemical investigation and antioxidant, antibacterial and anti-tyrosinase performances of six medicinal halophytes. S. Afr. J. Bot. 112, 508–514 (2017).
Shakeri, A., Zirak, M. R. & Sahebkar, A. Ellagic acid: a logical lead for drug development. Curr. Pharm. Design 24 (2), 106–122 (2018).
Rahman, M. A., Haque, E., Hasanuzzaman, M. & Shahid, I. Z. Anti-nociceptive, anti-inflammatory and antibacterial properties of Tamarix indica roots. Int. J. Pharmacol. 7 (4), 527–531 (2011).
Eiman, M. A. M. et al. Antibacterial properties and phytochemical screening of Tamarix nilotica leaves from Sudan. GSC Biol. Pharm. Sci. 3 (2), 6–10 (2018).
Boulaaba, M. et al. Anticancer effect of Tamarix gallica extracts on human colon cancer cells involves Erk1/2 and p38 action on G2/M cell cycle arrest. Cytotechnology 65 (6), 927–936 (2013).
Riham, O. B., Mohamed, A. E. & Rehab, S. A. Phenolic content, radical scavenging activity and cytotoxicity of Tamarix nilotica (Ehrenb.) bunge growing in Egypt. J. Pharmacogn. Phyto Ther. 5 (3), 47–52 (2013).
Sánchez-Hernández, E. et al. Phytochemical profile and activity against fusarium species of Tamarix gallica bark aqueous ammonia extract. Agronomy 13, 496 (2023).
Alshehri, S. A. et al. Pharmacological efficacy of Tamarix aphylla: a comprehensive review. Plants (Basel) 11 (1), 118 (2021).
Al-Othman, M., Alkhataf, F. & El-Aziz, A. Antioxidant and chemical constituents of ethyl acetate extract of Tamarix aphylla leaves in Saudi Arabia. Pak. J. Bot. 52 (6), 2257–2261 (2020).
Alnuqaydan, A. M. & Rah, B. Comparative assessment of biological activities of different parts of halophytic plant Tamarix articulata ( T. articulata) growing in Saudi Arabia. Saudi J. Biol. Sci. 10, 2586–2592 (2020).
Salissou, M. T. et al. Methanolic extract of Tamarix gallica attenuates hyperhomocysteinemia induced AD-like pathology and cognitive impairments in rats. Aging (Albany NY) 10 (11), 3229. https://doi.org/10.18632/aging.101627 (2018).
Fellah, O. et al. Anti-proliferative activity of ethyl acetate extracts of grown at different climatic conditions in Algeria. Acta Sci. Nat. 5 (2), 23–31 (2018).
Bahramsoltani, R., Rahimi, R. & Farzaei, M. H. Pharmacokinetic interactions of curcuminoids with conventional drugs: a review. J. Ethnopharmacol. 14 (209), 1–2 (2017).
Salam, H. S. et al. Potential apoptotic activities of hylocereus undatus peel and pulp extracts in MCF-7 and caco-2 cancer cell lines. Plants 11, 2192. https://doi.org/10.3390/plants11172192 (2022).
Darzynkiewicz, Z. Critical aspects in analysis of cellular DNA content. Curr. Protocols Cytometry 56 (7.2), 721–728 (2011).
Mahmood, T., Yang, P. C. & Western Blot Technique, theory and trouble shooting. N. Am. J. Med. Sci. 4 (9), 429–434 (2012).
Molecular operating environment (MOE). 02 Chemical computing group ULC, 910–1010 Sherbrooke St. W., Montreal, QC H3A 2R7. 2023; Canada. (2022).
Abouzied, A. S. et al. Synthesis, molecular docking study, and cytotoxicity evaluation of some novel 1,3,4-Thiadiazole as well as 1,3-Thiazole derivatives bearing a pyridine moiety. Molecules 27, 6368. https://doi.org/10.3390/molecules27196368 (2022).
Alghamdi, A. et al. Synthesis, molecular docking, and dynamic simulation targeting main protease (Mpro) of new, thiazole clubbed pyridine scaffolds as potential COVID-19 inhibitors. Curr. Issues Mol. Biol. 45, 1422–1442 (2023).
Zhu, H. et al. Targeting p53–MDM2 interaction by small-molecule inhibitors: learning from MDM2 inhibitors in clinical trials. J. Hematol. Oncol. 15, 91. (2022).
Mohammed, H. A., Abouzied, A. S., Mohammed, S. A. A. & Khan, R. A. In vivo and in silico analgesic activity of Ficus populifolia extract containing 2-O-β-D-(3′,4′,6′-Tri-acetyl)-glucopyranosyl-3-methyl pentanoic Acid. Int. J. Mol. Sci. 24, 2270. https://doi.org/10.3390/ijms24032270 (2023).
Abouzied, A. S. et al. In Silico Pharmacokinetic profiling of the identified bioactive metabolites of Pergularia tomentosa L. latex extract and in vitro cytotoxic activity via the induction of caspase-dependent apoptosis with S-Phase arrest. Pharmaceuticals 15, 1132. https://doi.org/10.3390/ph15091132 (2022).
Aroua, L. M. et al. Synthesis, molecular docking, and bioactivity study of novel hybrid benzimidazole urea derivatives: a promising α-Amylase and α-Glucosidase inhibitor candidate with antioxidant activity. Pharmaceutics 15, 457. https://doi.org/10.3390/pharmaceutics15020457 (2023).
Al-Humaidi, J. Y. et al. Synthesis, biological evaluation, and molecular docking of novel azolylhydrazonothiazoles as potential anticancer agents. ACS Omega 8, (37), 34044–34058 (2023).
McConkey, B. J., Sobolev, V. & Edelman, M. The performance of current methods in ligand–protein docking. Curr. Sci. 83, 845–856 (2002).
Jeong, J. B. & Jeong, H. J. 2-Methoxy-4-vinylphenol can induce cell cycle arrest by blocking the hyper-phosphorylation of retinoblastoma protein in benzo[a] pyrene-treated NIH3T3 cells. Biomed. Biophys. Res. Commun. 400 (4), 752–757 (2010).
Kim, D. H. et al. 2-Methoxy-4-vinylphenol attenuates migration of human pancreatic cancer cells via blockade of FAK and AKT signaling. Anticancer Res. 39 (12), 6685–6691 (2019).
Betül, G. Antioxidant, antimicrobial activities and fatty acid compositions of wild berberis spp. by different techniques combined with chemometrics (PCA and HCA). Molecules 26 (24), 7448 (2021). https://doi.org/10.3390/molecules26247448
Moon, D. O., Kim, M. O., Choi, Y. H. & Kim, G-Y. β-Sitosterol induces G2/M arrest, endoreduplication, and apoptosis through the Bcl-2 and PI3K/Akt signaling pathways. Cancer Lett. 264, 181–191 (2008).
Fernando, I. P. S. et al. Apoptotic and antiproliferative effects of stigmast-5-en-3-ol from dendronephthya gigantea on human leukemia HL-60 and human breast cancer MCF-7 cells. Toxicol. Vitro 52, 297–305 (2018).
Iyer, D. & Patil, U. K. Efficacy of stigmast-5-en-3β-ol isolated from Salvadora persica L. As antihyperlipidemic and anti-tumor agent: evidence from animal studies. Asian Pac. J. Trop. Dis. 2, S849–S855 (2012).
Chimshirova, R. et al. Antimicrobial triterpenoids and Ingol diterpenes from propolis of semi-arid region of Morocco. Molecules 27 (7), 2206. https://doi.org/10.3390/molecules27072206 (2022).
Rizvi, S. et al. The role of vitamin E in human health and some diseases. Sultan Qaboos Univ. Med. J. 14 (2), 157–165 (2014).
Al-Hadid, K. J., Al-Karablieh, N., Sharab, A. & Mutlak, I. Phytochemical analyses and antibacterial activities of erodium, euphorbia, logoecia and Tamarix species. J. Infect. Dev. Ctries. 13 (11), 1013–1020. https://doi.org/10.3855/jidc.11776 (2019).
Alnuqaydan, A. M. & Rah, B. Tamarix articulata Inhibits cell proliferation, promotes cell mechanisms and triggers G0/G1cell cycle arrest arrest in hepatocellular carcinoma cells. Food Technol. Biotechnol. 59 (2), 162–173 (2021).
Alafnan, A. et al. Beta elemene induces cytotoxic effects in FLT3 ITD-mutated acute myeloid leukemia by modulating apoptosis. Eur. Rev. Med. Pharmacol. Sci. 27, 3270–3287 (2023).
Khazaei, S. et al. Cytotoxicity and proapoptotic effects of allium atroviolaceum flower extract by modulating cell cycle arrest and caspase-dependent and p53-independent pathway in breast cancer cell lines. Evid. Based Complement. Altern. Med. 1468957https://doi.org/10.1155/2017/1468957 (2017).
Matos, B., Howl, J., Jerónimo, C. & Fardilha, M. The disruption of protein-protein interactions as a therapeutic strategy for prostate cancer. Pharmacol. Res. 161, 105145. https://doi.org/10.1016/j.phrs.2020.105145 (2020).
Joerger, A. C. & Fersht, A. R. The p53 pathway: origins, inactivation in cancer, and emerging therapeutic approaches. Annu. Rev. Biochem. 85, 375–404 (2016).
Lane, D. P. P53, Guardian of the genome. Nature 358, 15–16 (1992).
Toledo, F. & Wahl, G. M. Regulating the p53 pathway: in vitro hypotheses, in vivo veritas. Nat. Rev. Cancer 6, 909–923 (2006).
Gonzalez, A. Z. et al. Novel inhibitors of the MDM2-p53 interaction featuring hydrogen bond acceptors as carboxylic acid isosteres. J. Med. Chem. 57, 2963–2988 (2014).
Wells, J. A. & Mc-Clendon, C. L. Reaching for high-hanging fruit in drug discovery at protein-protein interfaces. Nature 450, 1001–1009 (2007).
Oliner, J. D., Kinzler, K. W., Meltzer, P. S., George, D. L. & Vogelstein, B. Amplification of a gene encoding a p53-Associated protein in human sarcomas. Nature 358, 80–83 (1992).
Chène, P. Inhibiting the p53-MDM2 interaction: an important target for cancer therapy. Nat. Revision Cancer 3, 102–109 (2003).
Funding
This work was supported by Scientific Research Deanship at University of Ha’il – Saudi Arabia, through project number (RG-23 001).
Author information
Authors and Affiliations
Contributions
Bader Huwaimel: Formal analysis, Investigation Kareem M. Younes: Conceptualization, Writing – original draft. Amr S. Abouzied: review & editing, docking study. Akram M. Elkashlan: Investigation, Methodology, Funding acquisition. Fawaz N. Alheibshy: review & editing. Ahmed Alobaida: Formal analysis, Investigation, Abdullah Turki: Methodology. Saleh A. Alquwaiay: Methodology. Naif Alqahtani: Methodology. Sulaiman A. Alsuwayagh: Methodology.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Huwaimel, B., Younes, K.M., Abouzied, A.S. et al. Phytochemical composition, in vitro cytotoxicity, and in silico docking properties of Tamarix tetragyna L.. Sci Rep 14, 25462 (2024). https://doi.org/10.1038/s41598-024-73961-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-73961-0
This article is cited by
-
In silico and in vitro antibacterial evaluation of eight Anatolian Salvia species with chemical profiling by LC-HRMS
Scientific Reports (2025)
-
Prioritization of multiple disease target compounds (MDTC) of Sorghum bicolor: a molecular docking and dynamics simulation strategy
Discover Chemistry (2025)