Abstract
Despite the association between spinal cord injury (SCI) and various neurological diseases, the risk of Parkinson’s disease (PD) in SCI is not elucidated yet. Especially, the role of SCI severity and injury level in the risk of PD development is not investigated. Based on the nation-wide cohort data the Korean National Health Insurance Service between 2010 and 2018, we investigated the incidence of PD in 7,182 patients with SCI compared with 24,844 age- and sex-matched controls. Adjusted hazard ratio and 95% confidence interval (CI) were calculated using Cox proportional hazards regression. We compared the risk of PD based on the degree of disability (without disability, mild, severe) and SCI level (cervical, thoracic, and lumbar). During the mean follow-up duration of 4.31 years, patients with SCI had a higher risk of PD compared with matched controls. The PD risk was greater among patients with SCI with disability than in those without disability, especially those with mild disability. Additionally, cervical-level injury was associated with the highest risk in patients with SCI without disability, while thoracic-level injury was associated with the highest risk in those with disability. Our study found patients with SCI have increased risk of PD, particularly those with disability and thoracic-level injuries.
Similar content being viewed by others
Introduction
Parkinson’s disease (PD) is the most common neurodegenerative movement disorder, characterized by akinetic-rigidity, resting tremor, gait disturbance, and postural instability, caused by dopaminergic neuron loss in the substantia nigra pars compacta1. Aggregation of α-synuclein (α-syn) and formation of Lewy bodies (LB) are key pathogenic mechanisms of PD2. In Korea, the incidence and prevalence of PD are 22.4–27.8 cases per 100,000 person-years and 0.11–0.14%, respectively3.
It is well known that traumatic brain injury (TBI) increases the risk of PD4,5, and the severity of TBI is also related to the higher risk of PD4. Even though TBI and spinal cord injury (SCI) can be combined and share clinical features6,7, the association between spinal cord injury (SCI) and PD is less understood, unlike TBI. Spinal cord injury (SCI) is a devasting disease of the central nervous system that can lead to neuronal damage, inflammation, and neurodegeneration, and it is associated with the development of a wide range of neurological conditions including stroke, dementia, multiple sclerosis, and restless leg syndrome8,9. A population-based study conducted by Yeh et al. found that patients with SCI had higher risk of developing PD than matched controls after adjusting for age, sex, comorbidities, and socioeconomic status10. Although the exact mechanism linking SCI and PD is not understood, the previous study suggested inflammation and related accumulation of alpha-synuclein (α-syn) as potential contributors to PD development in SCI10. Additionally, aberrant α-syn enhances the pathologic changes after SCI11, but the links of SCI severity and injury level with risk of PD development have not been investigated.
In this population-based study, we investigated the association between PD and SCI according to severity of disability and injured spinal cord level using a nationally representative database.
Methods
Data source and study setting
This study is based on data from the Korean National Health Insurance Service (KNHIS), the single national health insurer with uniform insurance policies that cover about 97% of the South Korean population12. The remaining 3% of Medicaid beneficiaries are covered by the Medical Aid Program (MAP), although those data also are managed by the KNHIS13. Therefore, the KNHIS database includes demographic and medical claims information on all Korean people14, and all the data of South Korean population were included in our study. Claims data is presented as diagnosis codes according to the 10th revision of the International Classification of Diseases (ICD-10). KNHIS also administers the national health check-up program, which is a bi-annual, free evaluation for all people 40 years and over and all employees regardless of age13. The national health check-up program includes a wide range of health assessment items, including questionnaires on health behaviours (regular exercise, smoking status, and alcohol consumption), anthropometric measurements (height, weight, and body-mass index (BMI), and blood pressure], and laboratory tests (blood sugar, cholesterol, etc.).
Study subjects
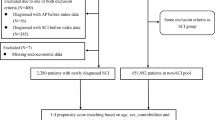
Eligible patients with SCI were defined as those who had one or more admission claims with the appropriate ICD-10 code (cervical-level SCI, S14; thoracic-level SCI, S24; or lumbar-level SCI, S34). Between January 1, 2010, and December 31, 2018, a total of 18,239 patients with SCI were identified and matched with 54,717 controls without SCI in a 1:3 ratio based on age, sex, and index year. We included patients who underwent the national health examination program within two years of the index date to obtain baseline characteristics for adjustment. We excluded patients who (1) were younger than 40 years (696 in SCI and 2,689 in controls); (2) had a history of TBI (136 in SCI and 43 in controls); (3) had PD (54 in SCI and 76 in controls); and (4) had missing data for matched variables of age, sex, and index year (347 in SCI and 1,012 in controls). Finally, 7,182 patients with SCI and 24,844 controls were included in the analyses. The flowchart for selection of eligible participants is illustrated in Fig. 1.
Flow Diagram of Study Population Enrolment. Between January 1, 2010, and December 31, 2018, a total of 18,239 patients with spinal cord injury (SCI) were identified and matched with 54,717 controls without SCI in a 1:3 ratio based on age, sex, and index year. Of these, 696 patients with SCI and 2,689 controls were younger than 40 years and were excluded, along with 136 patients with SCI and 43 controls with a history of traumatic brain injury, 54 patients with SCI and 76 controls with Parkinson’s disease, and 347 patients with SCI and 1,012 controls with missing data for matched variables. Finally, the study included 7,182 patients with SCI and 24,844 controls in the analysis. SCI = spinal cord injury; TBI = traumatic brain injury; PD = Parkinson’s disease.
This research was reviewed and approved by the Institutional Review Board of Samsung Medical Centre (IRB No. SMC- SMC 2020-12-068), and informed consent was waived because deidentified retrospective data was used. All methods were performed in accordance with the relevant guidelines and regulations.
Spinal cord injury severity
To assess differences in outcomes based on SCI severity, patients were divided into two groups: with disability and without. The presence and severity of disability in patients with SCI were determined by disability grade recorded in the National Disability Registry (NDR) of the Ministry of Health and Welfare (MOHW). In South Korea, people with disabilities are registered with the NDR to receive government benefits. SCI is classified as a physical disability within the NDR and is assessed for severity only by neurologists, neurosurgeons, or physiatrists. The disability grade is determined based on a state of impairment that has persisted for at least six months since the injury.
Disability is classified into six grades based on degree of weakness and number of affected limbs and can be used as a severity indicator. Disability severity was further divided into mild (grade 4–6) and severe (grade 1–3)15. For example, patients who lost all function in both arms or both legs are categorized into grade 1. Those with severe impairments in the function of both arms or who have completely lost function in one arm, or one leg are considered to have a grade 3 physical disability. In contrast, if patients have moderate disability in the function of both legs, they would be classified into grade 4. As in the above examples, disability grade can serve as an indicator of the severity of neurological symptoms related to SCI and is used as a measure of SCI severity in our analysis.
Study outcomes and follow-up
We investigated the incidence of newly diagnosed PD between patients with SCI and matched controls during the follow-up period. Patients with newly diagnosed PD were identified using the ICD-10 code for PD (G20) and the PD registration code (V124). The Korean government implemented a registration program in 2006 for rare intractable diseases to reduce co-payments by up to 10% (the usual co-payment is 30%). A physician certificate is required to register for this program, and the definition is considered reliable and has been used in many previous studies16,17. The V124 code is assigned to patients with PD diagnosed by a neurologist or neurosurgeon and is not assigned to secondary parkinsonism conditions such as vascular parkinsonism, drug-induced parkinsonism, traumatic brain injury, post-infectious parkinsonism, or hypoxic brain damage.
Covariates
Comorbidities were identified based on claims and prescription records prior to the index date. Diabetes mellitus was defined as having one or more claims for ICD-10 codes E10–14 and one or more claims for a prescription of an antidiabetic medication. Hypertension was defined as having one or more claims for ICD-10 codes I10–11 or one or more claims for a prescription of an antihypertensive medication. Dyslipidaemia was defined as having one or more claims for ICD-10 code E78 and one or more claims for a prescription of a lipid-lowering medication. We classified smoking status into none-, ex-, and current-smoker categories. Similarly, alcohol consumption was categorized as none, mild, and heavy based on a daily consumption threshold of 30 g.
Statistical analyses
Descriptive statistics were calculated as mean and standard deviation (SD) for continuous variables and number (percentage) for categorical variables. Normality and homogeneity of variance was checked and were satisfactory. Student’s t-test for continuous variables and the Chi-square test for categorical variables were used when comparing two groups. Kaplan-Meier curves were presented as cumulative incidence probabilities of PD in SCI and controls. Cox proportional hazards analyses were performed to evaluate the association between SCI and risk of PD, and hazards ratio (HR) and 95% confidence interval (CI) were estimated. Model 1 was not adjusted; Model 2 was adjusted for age and sex; and Model 3 was additionally adjusted for residential area, income, diabetes mellitus, hyperlipidaemia, hypertension, smoking, drinking, regular exercise, and BMI18. We also evaluated PD risk among patients with SCI based on presence and severity of disability and between patients with or without injury level. All statistical analyses were conducted using SAS statistical package version 9.4 (SAS Institute Inc., Cary, NC, USA), and a p-value < 0.05 was considered statistically significant.
Results
Baseline characteristics
Clinical characteristic and patient demographics are described in Table 1. The mean age of patients with SCI was 60.5 ± 10.9, and 68.5% of patients with SCI were male. The SCI group had a larger proportion of people in the bottom 20% of the income distribution (21.6% versus 18.5%) and of those registered in rural areas compared with the control group (62.7% versus 55.4%). The prevalence of diabetes, hypertension, and dyslipidaemia was higher in SCI participants (24.0% versus 16.7%, 49.9% versus 43.5%, and 36.5% versus 33.4%, respectively). The proportion of patients who performed regular exercise was lower in the SCI group compared with the control group (20.7% versus 23.0%). The frequencies of current smoking (28.7% versus 22.9%) and heavy alcohol drinking (12.4% versus 8.5%) were higher in the SCI group. Age, sex, and BMI were similar between the SCI and control groups.
Among the 7,182 patients with SCI, 79.8% did not have a disability. Patients with SCI without disability had a higher mean age (61.9 ± 10.3) compared with those with a disability (60.1 ± 11.0), as well as lower incidence of low income (21.0% versus 23.8%), diabetes (22.5% versus 29.9%), hypertension (48.2% versus 56.4%), and dyslipidaemia (35.6% versus 40.3%). Additionally, patients with SCI without disability represented a smaller percentage of non-smokers (50.5% versus 51.5%) and those who did not consume alcohol (50.2% versus 56.3%).
Parkinson’s Disease Risk in spinal cord Injury patients compared with matched controls
The mean follow-up duration was 4.6 ± 2.6 years in the SCI group and 5.0 ± 2.5 years in the control group (p < 0.0001). Patients with SCI had a higher rate of PD diagnosis at 1.35 per 1,000 person-years, compared with 0.97 per 1,000 person-years for the control group during the follow-up period. In Model 3, risk of PD was 1.57 times higher in patients with SCI (95% CI, 1.11 − 2.22) compared with the matched controls (Table 2; Fig. 2).
Kaplan-Meier Curves of the Incidence Probability of Parkinson’s Disease in patients with SCI and a Control Group. During the mean follow-up duration of 4.31 years, patients with SCI had a higher PD risk compared with matched controls (aHR, 1.57; 95% CI, 1.11–1.22) (A). The PD risk was greater among patients with SCI with disability than in those without disability (aHR, 1.45; 95% CI, 1.10–3.48), (B) especially those with mild disability (aHR, 2.07; 95% CI, 1.05–4.08) (C). SCI = spinal cord injury.
Risk of Parkinson’s disease by disability status and severity
The PD incidence rate in the SCI with disability group was 1.93 cases per 1,000 person-years, while that in the SCI without disability group was 1.20 cases per 1,000 person-years. According to disability status, the SCI with disability group had higher PD risk than the SCI without disability group and the control group in Model 3 (Table 2). When further divided by disability severity, 557 patients with SCI had severe disability (disability grade 1–3) and 891 had mild disability (grade 4–6). Patients with SCI with mild disability had a higher adjusted HR (aHR) than controls in Model 3 (aHR, 2.07; 95% CI, 1.05–4.08), but patients with SCI without disability and those with severe disability did not show significant differences from the control group (Table 2).
Risk of Parkinson’s disease by level of spinal cord injury
In patients with SCI, the most common injury level was cervical, followed by lumbar and thoracic levels (Table 2). According to disability status and injury level, patients with SCI without disability who were injured at the cervical level showed a higher risk (aHR, 1.55; 95% CI 1.00–2.39) than controls in Model 3 (Table 2). Regarding the SCI with disability group, Model 3 revealed that patients who had thoracic-level injuries had significantly higher PD risk (aHR, 3.30; 95% CI, 1.04–10.48).
Discussion
This study shows the risk of PD in patients with SCI using a nationwide population-based dataset. This is the first study to show such risk in patients with SCI according to severity and injured level of SCI. We showed that the risk of PD was 1.5-fold higher in SCI populations than in a matched control group. Patients with SCI with disability, particularly mild disability, had a higher risk of PD than controls. Additionally, patients with SCI with disabilities from injuries at the thoracic level showed 3.3-fold higher risk of PD than matched controls after adjusting for covariates.
Our results are consistent with those of a population-based study by Yeh et al. using the National Health Insurance Research Database of Taiwan10. They found that patients with SCI had a higher risk of PD than a control group (HR, 1.65; 95% CI, 1.16–2.33), which was similar to our estimate. Our PD incidence rate for patients with SCI (1.35 cases per 1,000) was lower than in the previous study (4.1 cases per 1,000), and the discrepancy in results may be due to study population differences. The PD incidence in the general population in Taiwan was higher (36.6 per 100,000 person-years) than that in South Korea (22.4 per 100,000 person-years). Additionally, our study used not only ICD-10 codes, but also PD registration to diagnose PD, which may have reduced the false positive rate and increased the reliability of our results.
Mechanisms of Parkinson’s disease in spinal cord injury
Yeh et al. suggest that there may be a link between SCI and PD10, but the mechanism to explain the relationship is still not elucidated. Further, the impacts of SCI severity and injury level on PD development have yet to be investigated. After SCI, acute and chronic responses are induced through a complex set of neuroprotective and reparative processes. However, if these processes are activated for an extended period, they may contribute to the development and progression of neurodegenerative disorders including PD8. Previous research focusing on PD and SCI has suggested possible pathological mechanisms, which could improve understanding of the relationship between these two conditions11,19,20,21,22,23.
Neuroinflammatory response following SCI can play a role in the development of PD23, because activation of inflammatory mediators induces oxidative stress and production of pro-inflammatory cytokines including tumour necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6. These inflammatory processes can induce persistent neuroinflammation and progressive neurodegeneration, including dopaminergic neuron damage19,23, and the neuroinflammatory process is an integral part of PD pathology21.
Additionally, excessive production of α-syn following SCI may serve as a potential trigger for PD. After SCI, α-syn expression is increased in the spinal cord, which has been linked to injury-related neuron death11,24. Aggregation of α-syn can spread throughout the central nervous system in a manner similar to that of prions, potentially contributing to progressive spread of neurodegeneration and neuroinflammation25. Given that neuronal damage caused by SCI can spread to distant parts of the injured organ and that α-syn also has a propagative property, it is possible that α-syn pathology could spread from the spinal cord to the brain. Considering that PD is a slow progressive disease with a long-lasting prodromal phase (more than 10 years)26, there is also a possibility that the opposite effect may occur. In PD, α-syn is distributed not only in the brain, but also in other body parts such as the enteric nervous system, salivary glands, heart, skin, and spinal cord20. Ferreira et al. found that the prodromal phase of PD involves incipient α-syn pathology within pools of premotor and motor neurons in the spinal cord, which then spreads into the reticular nuclei of the brainstem27. This suggests that PD may start in the spinal cord and then progress to the brainstem, potentially explaining the link between SCI and PD. Therefore, it is possible that some patients with SCI who developed PD were already in the prodromal phase, and increased α-syn in prodromal PD is associated with severe disability and worsened outcomes for SCI24. Therefore, increased α-syn pathology after SCI may contribute not only to PD progression, but also to severe disability and delayed recovery.
Physical exercise has been associated with neuroprotective effects in the nigrostriatal dopaminergic system, and patients with SCI have lower physical activity than healthy controls22. This is shown in our study by the smaller proportion of regular exercisers among patients with SCI than matched controls. It is not clear to what extent low physical activity affects PD development and progression in patients with SCI, but it is possible that reduced physical activity could accelerate the progression of PD pathology.
Therefore, The higher risk of PD in the SCI population can be attributed to several pathomechanisms including neuroinflammatory response, excessive production of α-syn following SCI, and a reduction in physical activity resulting from the SCI11,19,20,21,22,23. However, even with various mechanisms suggested in previous studies, the exact mechanism is still not clearly understood even with our results.
Differences in Parkinson’s disease risk according to disability degree in spinal cord injury
In this study, we showed that patients with SCI with disability had higher risk of PD than patients with SCI without disability, who did not show a significant difference in risk compared with matched controls. Disability status is a surrogate marker for severity of neuronal injury, motor weakness, and greater number of affected limbs28. Therefore, more severe injuries can lead to greater nervous system damage, which may explain the higher risk of PD in patients with SCI with disability.
On the other hand, there was a paradoxical decrease in PD risk when the disability group was analysed according to disability severity. To diagnose PD, physicians rely on motor symptoms, especially bradykinesia, which is defined as decrement of movement in amplitude or speed1. However, it is difficult to accurately assess bradykinesia in patients with severe disability due to muscle weakness. In addition, spasticity from the SCI can also mimic other parkinsonian symptoms like rigidity. Thus, physicians can only rely on objective tests such as dopamine transporter (DAT) imaging for early PD diagnosis, the cost and time required for which might result in underestimation of PD incidence in patients with SCI with severe disability.
Differences in Parkinson’s disease risk according to level of spinal cord injury
Among the patients with SCI without disability, SCI at the cervical level was significantly associated with the development of PD. When we assessed the correlation among the patients with SCI with disability, SCI at the thoracic level was related to the development of PD. Considering the pathological process that spreads from the spinal cord to the brain, it is expected that the more proximal part of an injury would result in a higher risk for PD. However, it was unexpected that patients with SCI with disability and cervical-level injuries did not have increased PD risk. The involved body part and the severity of clinical symptoms from SCI can be important for the diagnosis of PD due to the clinical similarity between SCI and PD as we mentioned earlier. SCI at the cervical level usually involves all the upper and lower limbs and these symptoms, such as weakness and spasticity, from SCI can mask various parkinsonian symptoms. Unlike SCI at the cervical level, SCI at the thoracic lumbar level may not involve arms and it could be easier to diagnose PD from the parkinsonian symptoms in the upper limbs. In our study, the patients with SCI with thoracic-level injuries showed higher PD risk compared with patients with lumbar-level injuries, which is consistent with our expectations. Additionally, the severity of symptoms from SCI can be also important for the diagnosis of PD. Following our hypothesis, SCI at the cervical level, the most close spinal level to the brain, was connected to the development of PD among the subjects without disability. However, the pathophysiology to explain the connection between SCI and PD is still not fully understood and further studies focusing on the level of SCI and the development of PD might be needed.
This study revealed that PD risk is higher in patients with SCI, although diagnosis may be underestimated, particularly in patients with SCI with severe disability or cervical-level injury. While there is no cure for PD, parkinsonian symptoms, especially rigidity and bradykinesia, can be effectively managed through dopamine replacement therapy29. Therefore, it is important for clinicians to proactively evaluate for PD in patients with SCI. Utilizing objective tests, such as DAT scan, and observing non-motor symptoms, such as rapid eye movement sleep behaviour disorders (RBD), can aid in PD diagnosis29.
This study has several limitations. First, we used disability grade to measure SCI severity rather than a scale specifically designed for SCI such as the American Spinal Injury Association Impairment Scale grade30. However, disability grade is often used as a surrogate marker for SCI severity because it is based on motor weakness and is assessed by specialist neurologists, neurosurgeons, or physiatrists. Despite this limitation, disability grade may still provide valuable insights into SCI severity. Second, this study does not consider the presence of prodromal PD, such as RBD. Prodromal PD refers to early PD symptoms or signs that may occur before development of full-blown PD. Further research is needed to understand the relationship between prodromal PD and risk of PD in the SCI population. Last, because this study lacks ethnic variability and included only Korean participants, generalization of the results should be limited.
In conclusion, this study found that patients with SCI have increased risk of developing PD, particularly those with disability and thoracic-level injuries. However, the risk of developing PD in patients with severe disability and cervical-level injuries may be underestimated. Given that PD is well-controlled with dopaminergic replacement therapy, it is important to consider the possibility of PD and perform a diagnostic DAT scan for patients with SCI. While it is not fully understood how these two diseases are linked, this study suggests a higher risk of PD in people with SCI based on the large population-based data analysed herein.
aIncidence rates in 1,000 person-years.
Data availability
Because we conducted this study based on the data from Korean National Health Insurance Service, the data supporting the findings of this study are only available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
Postuma, R. B. et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 30, 1591–1601. https://doi.org/10.1002/mds.26424 (2015).
Spillantini, M. G. et al. Alpha-synuclein in Lewy bodies. Nature. 388, 839–840. https://doi.org/10.1038/42166 (1997).
Park, J. H. et al. Trends in the incidence and prevalence of Parkinson’s disease in Korea: a nationwide, population-based study. BMC Geriatr. 19, 320. https://doi.org/10.1186/s12877-019-1332-7 (2019).
Gardner, R. C. et al. Mild TBI and risk of Parkinson disease: a chronic effects of Neurotrauma Consortium Study. Neurology. 90, e1771–e1779. https://doi.org/10.1212/WNL.0000000000005522 (2018).
Jafari, S., Etminan, M., Aminzadeh, F. & Samii, A. Head injury and risk of Parkinson disease: a systematic review and meta-analysis. Mov. Disord. 28, 1222–1229. https://doi.org/10.1002/mds.25458 (2013).
Macciocchi, S., Seel, R. T., Thompson, N., Byams, R. & Bowman, B. Spinal cord injury and co-occurring traumatic brain injury: assessment and incidence. Arch. Phys. Med. Rehabil. 89, 1350–1357. https://doi.org/10.1016/j.apmr.2007.11.055 (2008).
Valbuena Valecillos, A. D., Gater, D. R. Jr. & Alvarez, G. Concomitant Brain Injury and spinal cord Injury Management Strategies: a narrative review. J. Pers. Med. 12 https://doi.org/10.3390/jpm12071108 (2022).
Amanat, M., Vaccaro, A. R. & Salehi, M. Rahimi-Movaghar, V. Neurological conditions associated with spinal cord injury. Inf. Med. Unlocked. 16, 100245. https://doi.org/10.1016/j.imu.2019.100245 (2019).
Mahmoudi, E. et al. Traumatic spinal cord Injury and Risk of Early and Late Onset Alzheimer’s Disease and related dementia: large longitudinal study. Arch. Phys. Med. Rehabil. 102, 1147–1154. https://doi.org/10.1016/j.apmr.2020.12.019 (2021).
Yeh, T. S., Huang, Y. P., Wang, H. I. & Pan, S. L. Spinal cord injury and Parkinson’s disease: a population-based, propensity score-matched, longitudinal follow-up study. Spinal Cord. 54, 1215–1219. https://doi.org/10.1038/sc.2016.74 (2016).
Sauerbeck, A. D. et al. Alpha-synuclein increases in rodent and human spinal cord injury and promotes inflammation and tissue loss. Sci. Rep. 11, 11720. https://doi.org/10.1038/s41598-021-91116-3 (2021).
Shin, D. W., Cho, B. & Guallar, E. Korean National Health Insurance Database. JAMA Intern. Med. 176, 138. https://doi.org/10.1001/jamainternmed.2015.7110 (2016).
Shin, D. W., Cho, J., Park, J. H. & Cho, B. National General Health Screening Program in Korea: history, current status, and future direction. Precis Future Med. 6, 9–31. https://doi.org/10.23838/pfm.2021.00135 (2022).
Lee, S. W. et al. Status of hypertension screening in the Korea National General Health Screening Program: a questionnaire survey on 210 screening centers in two metropolitan areas. Clin. Hypertens. 23, 23. https://doi.org/10.1186/s40885-017-0075-z (2017).
National Health Insurance. [The Analysis of Medical Benefits in the People with Disability and Development of Stratigies for Strenthening the Medical Insurance] (Ministry of Health and Welfare, 2005).
Kim, I. Y. et al. Systemic lupus erythematosus is associated with increased risk of Parkinson’s disease. Ther. Adv. Musculoskelet. Dis. 15 (X231152653), 1759720. https://doi.org/10.1177/1759720x231152653 (2023).
Kang, J. et al. Rheumatoid arthritis and risk of Parkinson Disease in Korea. JAMA Neurol. 80, 634–641. https://doi.org/10.1001/jamaneurol.2023.0932 (2023).
Ascherio, A. & Schwarzschild, M. A. The epidemiology of Parkinson’s disease: risk factors and prevention. Lancet Neurol. 15, 1257–1272. https://doi.org/10.1016/S1474-4422(16)30230-7 (2016).
Hellenbrand, D. J. et al. Inflammation after spinal cord injury: a review of the critical timeline of signaling cues and cellular infiltration. J. Neuroinflammation. 18, 284. https://doi.org/10.1186/s12974-021-02337-2 (2021).
Nardone, R. et al. Spinal cord involvement in Lewy body-related alpha-synucleinopathies. J. Spinal Cord Med. 43, 832–845. https://doi.org/10.1080/10790268.2018.1557863 (2020).
Tansey, M. G. et al. Inflammation and immune dysfunction in Parkinson disease. Nat. Rev. Immunol. 22, 657–673. https://doi.org/10.1038/s41577-022-00684-6 (2022).
Yang, F. et al. Physical activity and risk of Parkinson’s disease in the Swedish National March cohort. Brain. 138, 269–275. https://doi.org/10.1093/brain/awu323 (2015).
Faden, A. I., Wu, J., Stoica, B. A. & Loane, D. J. Progressive inflammation-mediated neurodegeneration after traumatic brain or spinal cord injury. Br. J. Pharmacol. 173, 681–691. https://doi.org/10.1111/bph.13179 (2016).
Amanat, M. & Vaccaro, A. R. Reducing alpha-synuclein in spinal cord injury: a new strategy of treatment. J. Neurosci. Res. 97, 729–732. https://doi.org/10.1002/jnr.24406 (2019).
Jan, A., Goncalves, N. P., Vaegter, C. B., Jensen, P. H. & Ferreira, N. The prion-like spreading of Alpha-Synuclein in Parkinson’s Disease: Update on models and hypotheses. Int. J. Mol. Sci. 22, 8338. https://doi.org/10.3390/ijms22158338 (2021).
de la Fuente-Fernandez, R. Imaging of dopamine in PD and Implications for Motor and neuropsychiatric manifestations of PD. Front. Neurol. 4, 90. https://doi.org/10.3389/fneur.2013.00090 (2013).
Ferreira, N. et al. Prodromal neuroinvasion of pathological alpha-synuclein in brainstem reticular nuclei and white matter lesions in a model of alpha-synucleinopathy. Brain Commun. 3, fcab104. https://doi.org/10.1093/braincomms/fcab104 (2021).
Marino, R. J., Ditunno, J. F. Jr., Donovan, W. H. & Maynard, F. Jr Neurologic recovery after traumatic spinal cord injury: data from the Model spinal cord Injury systems. Arch. Phys. Med. Rehabil. 80, 1391–1396. https://doi.org/10.1016/s0003-9993(99)90249-6 (1999).
Armstrong, M. J. & Okun, M. S. Diagnosis and treatment of Parkinson Disease: a review. JAMA. 323, 548–560. https://doi.org/10.1001/jama.2019.22360 (2020).
Kirshblum, S. C. et al. Reference for the 2011 revision of the International standards for neurological classification of spinal cord Injury. J. Spinal Cord Med. 34, 547–554. https://doi.org/10.1179/107902611X13186000420242 (2011).
Funding
This research was partially supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, South Korea (HI20C1073 for DWS and RS-2023-00266288 for JY).
Author information
Authors and Affiliations
Contributions
Conceptualization: KH, JY, and DWSData curation: BK, KH, and DWSFormal analysis: BK, KH, and DWSInvestigation: JHA, BK, HLC, WJ and DWSMethodology: BK, KH, and DWSWriting—original draft: JHACritical revision of the manuscript for important intellectual content: All authors.Supervision: JWC, JY, and DWS.
Corresponding authors
Ethics declarations
Conflict of interest
All the authors report no financial or non-financial conflicts of interest related to this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ahn, J.H., Kim, Bs., Han, KD. et al. Risk of Parkinson’s disease in spinal cord injury: a nationwide cohort study in South Korea. Sci Rep 14, 23016 (2024). https://doi.org/10.1038/s41598-024-74103-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-74103-2