Abstract
The relationship between albumin-corrected anion gap (ACAG) and severe disorder of consciousness (SDOC), in-hospital mortality, and long-term mortality in patients with ischemic stroke (IS) remains unclear. This study investigates the association of ACAG with SDOC and other outcomes in IS using data from the MIMIC-IV database. A total of 2,379 IS patients were included, with a demographic breakdown showing 51% were male and an SDOC incidence of 16.4%. Analysis through Cox proportional hazards models indicated that ACAG is significantly associated with the risks of both SDOC and mortality. Additionally, restricted cubic spline(RCS) analysis suggested a nearly linear relationship between increasing ACAG levels and the incidence of SDOC. Kaplan-Meier curves demonstrated significant differences in the incidence rates of SDOC, in-hospital mortality, and long-term mortality across varying ACAG levels. The findings suggest that ACAG serves as an independent predictor for SDOC, in-hospital mortality, and long-term mortality in IS patients. Nonetheless, further prospective studies are needed to confirm these causal relationships.
Similar content being viewed by others
Introduction
Stroke is the second leading cause of death and the third leading cause of disability, with annual direct and indirect costs exceeding $891 billion worldwide1. Ischemic stroke (IS) accounts for 87% of all stroke cases2. Severe disorder of consciousness (SDOC) is a common clinical presentation in IS patients, resulting in higher in-hospital mortality and poorer discharge outcomes3. Few studies have investigated the incidence of SDOC in IS. Current research reported that the triglyceride glucose index and glycemic variability are predictors for cerebrovascular disease with SDOC3,4 and measures associated with blood glucose metabolism. Therefore, further exploration of risk factors is needed for predicting SDOC in stroke patients. Accurate prediction of IS patient outcomes, especially the occurrence of SDOC, remains a key challenge in clinical practice.
Serum anion gap (AG) is a clinical measure of acid-base homeostasis that can be easily and quickly obtained. Increased AG usually indicates metabolic acidosis, which is often associated with poor prognosis in various acute and chronic conditions5,6,7. Albumin-corrected AG (ACAG) is a modified measure that accounts for the effect of serum albumin level on AG and provides a more accurately reflection of the metabolic status of patients8,9,10. ACAG has been strongly associated with short- and long-term mortality across various diseases8,10,11, and is a valuable clinical biochemical marker that offers crucial information for the prognostic assessment of these conditions12,13.
The relationship between ACAG level and the incidence of SDOC and outcomes of IS patients is currently unknown. Therefore, this study aims to examine the association of ACAG with SDOC and outcomes of IS patients using the MIMIC-IV database in order to assist clinicians in the early detection and treatment of high-risk patients.
Methods
Study population
The data used in this study originated from The Medical Information Mart for Intensive Care-IV (MIMIC-IV) 2.2 database developed by the Laboratory for Computational Physiology of Massachusetts Institute of Technology. MIMIC-IV is a publicly available clinical database sourced from the detailed records of 431,231 hospital admissions at the Beth Israel Deaconess Medical Center between 2008 and 2019. Patient identifiers have been removed to protect their privacy, and therefore, patient consent and ethical approval were not required. After completing relevant training, author Ying Chen (ID: 62685292) was given access to the database.
Study population inclusion criteria: Patients diagnosed with IS according to ICD-9 or ICD-10. Exclusion criteria were: (a) age ≥ 18 years; (b) lack of AG and albumin test data; (c) presence of SDOC [Glasgow Coma Scale (GCS) ≤ 8] before admission; for patients with multiple hospitalizations, only data from the first hospitalization were used.
Patient characteristics
Relevant medical information was extracted from the MIMIC-IV database using Structured Query Language (SQL). These information included demographics (age, sex race, body mass index (BMI), marital status), laboratory data [albumin (ALB), alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN), creatine kinase (CK), creatine kinase isoenzyme (CKMB), chloride ion, serum creatinine (SCR), blood glucose (GLU), hemoglobin (HB), glycated hemoglobin A1c (HBA1C), bicarbonate (HCO3), potassium ion (Potassium), lymphocytes, sodium ion (Sodium), neutrophils (Neutrophil), platelets (Platelet), total bilirubin (TBIL), total cholesterol (TC), triglycerides (TG), white blood cell count (WBC)], prior comorbidities [hypertension (HBP), diabetes mellitus (DM), hyperlipidemia (HL), anemia, cancer, atrial fibrillation (AF), coronary artery disease (CAD), chronic kidney disease (CKD), respiratory failure (RF), heart failure (HF), alcohol use, and tobacco use identified according to ICD-9 or ICD-10], disease severity [Sequential Organ Failure Assessment (SOFA), Oxford Acute Severity of Illness Score (OASIS), Simplified Acute Physiology Score II (SAPSII), Acute Physiology Score III(APS III) ], and medication use (use of antiplatelet and anticoagulant agents prior to onset of DOC.)
AG and ACAG levels were calculated by: AG (mmol/L) = (sodium + potassium) - (chloride + bicarbonate)14, and ACAG (mmol/L) = [4.4-{albumin (g/dL)}] *2.5 + AG15.
Handling of missing data: For variables with less than 20% missing data, missing values were imputed using the multiple imputation method based on a random forest model. Variables with > 20% missing data were classified based on the reference range or median of the data provided in the database and included as dummy variables in the analysis.
Outcome measures
The primary outcome measure was the incidence of SDOC, defined by GCS score ≤ 8, during hospitalization. Secondary outcome measures were in-hospital and long-term mortality.
Statistical analysis
Continuous variables with a normal distribution are expressed as mean (standard deviation (SD)) and compared using the t-test. Continuous variables without normal distribution are expressed as median (interquartile range (IQR)) and compared using the Mann-Whitney U test. Categorical variables are presented as frequency and percentage (%) and compared between groups using the Pearson chi-square test or Fisher’s exact test. The Cox proportional hazards models were used to assess the association between ACAG level and the risks of primary and secondary outcomes, while correcting for multiple confounding factors (Model 1: unadjusted; Model 2: adjusted for age, sex, BMI and race; Model 3: To prevent multiple collinearity, variables with a variance inflation factor ≥ 5 were excluded, and variables from Model 2 and marital status, ALT, AST, CK, CKMB, Chloride, SCR, GLU, HB, HBA1C, Neutrophil, Platelet, TC, WBC, AF, Alcohol use, Anemia, Cancer, CHD, CKD, DM, HF, HBP, HL, RF, Tobacco use, Anticoagulant drugs, Antiplatelets drugs, APSIII, and SAPS II were included). Subsequently, the potential nonlinear relationship between ACAG level and outcomes was explored using restricted cubic spline (RCS). Subgroup analyses were also performed to explore the relationship between ACAG and outcomes in different patient populations. All data were analyzed using R4.4.1, and a P < 0.05 was considered statistically significant.
Results
Baseline characteristics
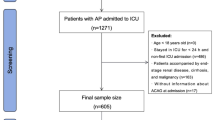
A total of 2379 patients with IS were included in this study, and the patient selection process is shown in Fig. 1. The median age was 71 years, and there were 51% males and 64% white people. The median length of stay (LOS) was 6 days, and SOC occurred in 389 patients (16.4%). ALT, SCR, GLU, neutrophils, and ACAG levels were higher in the SDOC group than in the non-SDOC group. In addition, the SDOC group had significantly higher number of patients with AF, anemia, RF, and neutrophil > 8.6k/µL than the non-SDOC group (P < 0.001). Patients with GCS > 8 had a notably higher rate of antiplatelet and anticoagulant medication use than those with GCS ≤ 8(P < 0.001), (Table 1). Stratification of patients according to ACAG levels revealed that the incidence of SDOC (GCS ≤ 8; P < 0.001), in-hospital mortality (P < 0.001), and long-term mortality (P < 0.001) were gradually increased as ACAG levels. The prevalence of anemia, CHD, HF, and RF, as well as GLU, leukocytes, and various disease severity scores were significantly higher in the top ACAG levels than in the other groups (all P < 0.001) (Table 2).
Cox proportional hazard analysis
When ACAG was handled as a continuous variable, ACAG was significantly positively correlated with SDOC (model1 HR = 1.055; 95% CI: 1.035–1.077; model2 HR = 1.049; 95% CI: 1.027–1.071; model3 HR = 1.032; 95% CI: 1.012–1.053) and long-term mortality (model1 HR = 1.082; 95% CI: 1.070–1.096; model2 HR = 1.094; 95% CI: 1.080–1.109; model3 HR = 1.065; 95% CI; 1.044–1.087). These associations remained significant after inclusion of confounders (Table 3).
When ACAG was handled as a categorical variable, the incidence of SDOC and long-term mortality were significantly higher in the top ACAG quartile group compared to the lower quartile group, and the association remained significant after the inclusion of confounders (SDOC: model1 HR = 1.782, 95% CI: 1.339–2.372; model2 HR = 1.825; 95% CI: 1.273–2.618; model3 HR = 1.382; 95% CI: 1.129–1.692; Long-term mortality: model1 HR = 3.184, 95%CI: 2.603–3.896, model2 HR = 3.295; 95%CI: 2.676–4.058, model3 HR = 2.087, 95%CI: 1.637–2.660) (Table 3).
Kaplan–Meier survival curve analysis
Cumulative incidence curves of SDOC (GCS ≤ 8) and survival curves for in-hospital mortality and long-term mortality were plotted for each ACAG group. The results showed that the incidence of SDOC, in-hospital mortality, and long-term mortality were significantly different among the four ACAG groups (P < 0.0001) (Fig. 2).
Cumulative event and survival incidence curves (ACAG index: Q1:<15.300; Q2: 15.300–17.850; Q3: 17.850–20.175; Q4: >20.175). a: Cumulative event incidence curves for incidence of SDOC (GCS ≤ 8). b: Survival curve for the in-hospital mortality of the entire study population. c: Survival curves for the long-term mortality of the entire study population.
Non-linear association between ACAG and outcomes
The RCS was plotted to explore the potential nonlinear relationship between ACAG and outcomes. The data showed that the increase in ACAG levels was approximately linearly proportional to SDOC and in-hospital mortality (nonlinear P > 0.05) and nonlinearly proportional to long-term mortality (nonlinear P < 0.001) (Fig. 3).
RCS curves for the HR and distribution of ACAG index. (a–c) SDOC (GCS ≤ 8) cumulative incidence curves for Model 1, Model 2, and Model 3. (d–f) In-hospital mortality survival curves and histograms for Model 1, Model 2, and Model 3. (g–i) Long-term mortality survival curves and histograms for Model 1, Model 2, and Model 3. Model 1 was unadjusted. Model 2 was adjusted for gender, age, race, and BMI. Model 3 was adjusted for the variables in model 2 and further adjusted for marital status, ALT, CK, CKMB, Chloride, SCR, GLU, HB, HBA1C, Neutrophil, Platelet, TC, WBC, AF, Alcohol use, Anemia, Cancer, CHD, CKD, DM, HF, HBP, HL, RF, Tobacco use, Anticoagulant drugs, Antiplatelet drugs, APSIII, and SAPS II.
Subgroup analyses
Subgroup analysis of ACAG and SDOC indicated that age (≤ 70 years, > 70 years), DM (presence, absence), CKD (absence), and CHD (presence, absence) were significantly associated with SDOC (P < 0.05). The HR values were stable across different subgroups, with no interaction between ACAG and each subgroup (Fig. 4a). Similarly, subgroup analysis of ACAG and in-hospital mortality revealed that age (≤ 70 years, > 70 years), DM (presence, absence), CHD (presence, absence), and CKD (absence) were significantly associated with in-hospital mortality. The HR values remained stable across different subgroups, with no interaction between ACAG and each subgroup (Fig. 4b). Furthermore, subgroup analysis of ACAG and long-term mortality revealed that each subgroup was significantly associated with outcomes with stable HR values. Interaction was observed between the age and DM subgroups (P < 0.05) (Fig. 4c).
Forest plots. (a) forest plot of HRs for SDOC in different subgroups. (b) forest plot of HRs for in-hospital mortality in different subgroups. (c) forest plots of HRs for long-term mortality in different subgroups. CKD (chronic kidney disease), BMI (body mass index), DM (diabetes mellitus), CHD (coronary heart disease), CI (confidence interval), HR (hazard ratio).
Discussion
This is a retrospective cohort study based on the IS population from the MIMIC-IV database. This is the first study to demonstrate that ACAG levels are associated with increased risk of SDOC and higher in-hospital and long-term mortality in patients with cerebral infarction. Our results also revealed that ACAG is approximately linearly associated with SDOC and in-hospital mortality but not with long-term mortality.
Although revascularization reduces mortality and disability in IS patients16,17, IS remains an important cause of death and disability worldwide18. Therefore, early detection and early management are critical for the outcomes of high-risk patients. ACAG is a corrected form of AG that accounts for the effect of serum albumin levels on AG values8. ACAG holds potential clinical value for assessing the severity and outcomes of patients with trauma, sepsis, liver failure, and acute myocardial infarction, as it effectively reflects both hypoalbuminemia and metabolic acidosis7,10,19,20. Metabolic acidosis may exacerbate kidney injury by reducing renal blood flow and increasing the release of inflammatory mediators. Higher ACAG levels have been shown to be associated with an increased risk of acute renal failure in ICU patients21. ACAG is also an effective prognostic marker for patients with aneurysmal subarachnoid hemorrhage (aSAH). aSAH can cause acidosis through multiple mechanisms, including brain hypoxia, accumulation of acidic metabolites, and electrolyte disturbances such as hypokalemia and hypocalcemia22. ACAG has been proposed as a novel and reliable predictor for non-alcoholic fatty liver disease (NAFLD), especially in patients with central obesity. Previous study showed that ACAG may promote the development of NAFLD by contributing to lipotoxicity and glucotoxicity23. In IS patients, lactate may accumulate due to inadequate tissue perfusion and impaired aerobic metabolism, thereby increasing AG and ACAG levels24.
DOC is prevalent in acute IS (AIS) patients and strongly associated with poor clinical outcomes. Patients with DOC exhibit higher in-hospital mortality, are less likely to go home or engage in rehabilitation, and are less likely to walk independently at discharge3. In addition, DOC has been found to be useful in predicting short-term (28-day) mortality in AIS patients25. Patients with cardiovascular diseases admitted to the intensive care unit (ICU) are more likely to develop SDOC and more complex conditions, as well as experience higher mortality rates26.
Studies have shown that the triglyceride glucose index and glycemic variability can predict the occurrence of DOC in cerebrovascular diseases. However, blood glucose is greatly affected by diet, and the frequency and timing of blood glucose measurement may vary from patient to patient, which consequently affect the assessment of blood glucose4,27. There are currently very limited data on the relationship between ACAG and the occurrence and prognosis of DOC in IS. In this study, the incidence of SDOC was 16.4% in IS patients, and the in-hospital mortality and long-term mortality were 38% and up to 61% in IS patients with SDOC, respectively. We found that the SDOC group had higher ACAG values than the non-SDOC group (19.1 [16.2–22.3] vs. 17.7 [15.2–19.9]). Moreover, the top ACAG quartile group had significantly higher risks of SDOC, in-hospital mortality, and long-term mortality compared to the lower quartile group. Collectively, these findings indicate that ACAG monitoring is favorable for the early detection and management of SDOC and for reducing mortality in IS patients.
This study proposes ACAG as a potential predictor for SDOC, in-hospital mortality, and long-term death in ischemic stroke. However, several limitations should be noted. First, despite an optimized study design, this study could not identify a definite causality between the parameters, thus warranting a large-cohort prospective study. Second, the database was sourced from the United States, where the majority of the population is white, limiting the generalizability of the data to other populations. Last, due to limitations in the database, imaging data, family history, and long-term use of antihypertensives may affect the outcomes of DOC.
Conclusion
ACAG is an important predictor for SDOC and outcomes of IS patients. Although ACAG is a promising novel risk stratification tool, its effectiveness and generalizability need to be further confirmed by subsequent prospective studies.
Data availability
The data that support the findings of this study are available from the MIMIC-IV database, but restrictions apply to the availability of these data, which were used under license for the current research and so are not publicly available. Data are, however, available from the authors upon reasonable request and with permission of the holder of the database.
Abbreviations
- IS:
-
Ischemic stroke
- SDOC:
-
Severe disorder of consciousness
- AG:
-
anion gap
- ACAG:
-
Albumin-corrected AG
- MIMIC-IV:
-
Medical information mart for intensive care-IV
- GCS:
-
Glasgow coma scale
- SQL:
-
structured query language
- BMI:
-
body mass index
- ALB:
-
albumin
- ALT:
-
alanine aminotransferase
- AST:
-
aspartate aminotransferase
- BUN:
-
blood urea nitrogen
- CK:
-
creatine kinase
- CKMB:
-
creatine kinase isoenzyme
- SCR:
-
serum creatinine
- GLU:
-
glucose
- HB:
-
hemoglobin
- HBA1C:
-
hemoglobin A1c
- TBIL:
-
total bilirubin
- TC:
-
total cholesterol
- TG:
-
triglycerides
- WBC:
-
white blood cell count
- HBP:
-
hypertension
- DM:
-
diabetes mellitus
- HL:
-
hyperlipidemia
- AF:
-
atrial fibrillation
- CAD:
-
coronary artery disease
- CKD:
-
chronic kidney disease
- RF:
-
respiratory failure
- HF:
-
heart failure
- SOFA:
-
sequential organ failure assessment
- OASIS:
-
oxford acute severity of illness score
- SAPSII:
-
simplified acute physiology score II
- APS III:
-
acute physiology score III
- IQR:
-
interquartile range
- LOS:
-
length of stay
- aSAH:
-
aneurysmal subarachnoid hemorrhage
- ICU:
-
intensive care unit
References
Feigin, V. L. & Owolabi, M. O. Pragmatic solutions to reduce the global burden of stroke: a world stroke organization-lancet neurology commission. Lancet Neurol. 22, 1160–1206. https://doi.org/10.1016/s1474-4422(23)00277-6 (2023).
Tsao, C. W. et al. Heart disease and stroke statistics-2023 update: a report from the American heart association. Circulation. 147, e93–e621. https://doi.org/10.1161/cir.0000000000001123 (2023).
Alkhachroum, A. et al. Association of acute alteration of consciousness in patients with acute ischemic stroke with outcomes and early withdrawal of care. Neurology. 98, e1470–e1478. https://doi.org/10.1212/wnl.0000000000200018 (2022).
Chen, T., Qian, Y. & Deng, X. Triglyceride glucose index is a significant predictor of severe disturbance of consciousness and all-cause mortality in critical cerebrovascular disease patients. Cardiovasc. Diabetol. 22https://doi.org/10.1186/s12933-023-01893-6 (2023).
Wang, X. M. et al. The serum anion gap is associated with the prognosis of coronary artery bypass grafting (CABG): analysis based on the MIMIC-IV database. Eur. Rev. Med. Pharmacol. Sci. 27, 2964–2970. https://doi.org/10.26355/eurrev_202304_31928 (2023).
Huang, Z., Wang, S. & Yang, S. Association between serum anion gap and risk of in-hospital mortality in patients with acute heart failure. Sci. Rep.https://doi.org/10.1038/s41598-024-55658-6 (2024).
Yu, W., Wen, Y., Shao, Y., Hu, T. & Wan, X. Relationship between anion gap and in-hospital mortality in intensive care patients with liver failure: a retrospective propensity score matching analysis. Am. J. Transl Res. 16, 98–108 (2024).
Wang, S., Teng, H., Han, H. & Xu, Y. The relationship between albumin corrected anion gap levels and mortality in patients with asthma in the ICU. Sci. Rep. 13https://doi.org/10.1038/s41598-023-44182-8 (2023).
Gao, P., Min, J., Zhong, L. & Shao, M. Association between albumin corrected anion gap and all-cause mortality in critically ill patients with acute kidney injury: a retrospective study based on MIMIC-IV database. Ren. Fail. 45https://doi.org/10.1080/0886022x.2023.2282708 (2023).
Sheng, H. et al. The correlation between albumin-corrected anion gap and prognosis in patients with acute myocardial infarction. ESC Heart Fail. 11, 826–836. https://doi.org/10.1002/ehf2.14639 (2024).
Zhong, L., Xie, B., Ji, X. W. & Yang, X. H. The association between albumin corrected anion gap and ICU mortality in acute kidney injury patients requiring continuous renal replacement therapy. Intern. Emerg. Med. 17, 2315–2322. https://doi.org/10.1007/s11739-022-03093-8 (2022).
Wang, R. et al. Preoperative albumin corrected anion gap is associated with in-hospital and long-term mortality in patients undergoing coronary artery bypass grafting in a retrospective cohort study. J. Thorac. Dis. 14, 4894–4903. https://doi.org/10.21037/jtd-22-1633 (2022).
Hu, T., Zhang, Z. & Jiang, Y. Albumin corrected anion gap for predicting in-hospital mortality among intensive care patients with sepsis: a retrospective propensity score matching analysis. Clin. Chim. Acta. 521, 272–277. https://doi.org/10.1016/j.cca.2021.07.021 (2021).
Chen, X. et al. Association between serum anion gap and mortality in critically ill patients with COPD in ICU: data from the MIMIC IV Database. Int. J. Chronic Obstruct Pulmonary Dis. 19, 579–587. https://doi.org/10.2147/copd.S433619 (2024).
Figge, J., Jabor, A., Kazda, A. & Fencl, V. Anion gap and hypoalbuminemia. Crit. Care Med. 26, 1807–1810. https://doi.org/10.1097/00003246-199811000-00019 (1998).
Powers, W. J. et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 50, e344–e418. https://doi.org/10.1161/str.0000000000000211 (2019).
Majoie, C. B. et al. Value of intravenous thrombolysis in endovascular treatment for large-vessel anterior circulation stroke: individual participant data meta-analysis of six randomised trials. Lancet (London England). 402, 965–974. https://doi.org/10.1016/s0140-6736(23)01142-x (2023).
Fan, J. et al. Global burden, risk factor analysis, and prediction study of ischemic stroke, 1990–2030. Neurology. 101, e137–e. https://doi.org/10.1212/wnl.0000000000207387 (2023).
Yin, F. et al. Association between albumin-corrected anion gap and in-hospital mortality of intensive care patients with trauma: a retrospective study based on MIMIC-III and IV databases. PloS One. 19, e0300012. https://doi.org/10.1371/journal.pone.0300012 (2024).
Guo, H. & Wang, J. Association between albumin-corrected anion gap and in-hospital mortality and sepsis-associated acute kidney injury. Med. Sci. Monit. 30, e943012. https://doi.org/10.12659/msm.943012 (2024).
Zhao, X. et al. Association between albumin-corrected anion gap level and the risk of acute kidney injury in intensive care unit. Int. Urol. Nephrol. 56, 1117–1127. https://doi.org/10.1007/s11255-023-03755-2 (2024).
Wang, R., Rong, J., Xu, J. & He, M. A prognostic model incorporating the albumin-corrected anion gap in patients with aneurysmal subarachnoid hemorrhage. Front. Neurol. 15https://doi.org/10.3389/fneur.2024.1361888 (2024).
Lu, Y., Zhan, Y. Z., Li, W. & Liao, S. L. Relationship between albumin-corrected anion gap and non-alcoholic fatty liver disease varied in different waist circumference groups: a cross-sectional study. Eur. J. Med. Res. 29https://doi.org/10.1186/s40001-024-01811-w (2024).
Yao, H., Tian, J. & Cheng, S. Association of anion gap and albumin corrected anion gap with acute kidney injury in patients with acute ischemic stroke. Adv. Clin. Exp. Med.https://doi.org/10.17219/acem/186814 (2024).
Fang, L. et al. Development and validation of a nomogram for predicting 28-day mortality in patients with ischemic stroke. PLoS One. 19, e0302227. https://doi.org/10.1371/journal.pone.0302227 (2024).
Chang, C. W. J., Provencio, J. J. & Shah, S. Neurological critical care: the evolution of cerebrovascular critical care. Crit. Care Med. 49, 881–900. https://doi.org/10.1097/ccm.0000000000004933 (2021).
Cai, W. et al. Association of glycemic variability with death and severe consciousness disturbance among critically ill patients with cerebrovascular disease: analysis of the MIMIC-IV database. Cardiovasc. Diabetol. 22https://doi.org/10.1186/s12933-023-02048-3 (2023).
Acknowledgements
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Writing - original draft preparation: Ying Chen; Writing - review and editing: Lan Chu; Conceptualization: Ying Chen; Methodology: Ying Chen; Formal analysis and investigation: MingYao, You; Resources: Lan Chu; Supervision: MingYao, You, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The data used in this study originated from The Medical Information Mart for Intensive Care-IV (MIMIC-IV) 2.2 database developed by the Laboratory for Computational Physiology of Massachusetts Institute of Technology. Patient identifiers have been removed to protect their privacy, and therefore, patient consent and ethical approval were not required.All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, Y., You, MY. & Chu, L. Association of albumin-corrected anion gap with severe consciousness disorders and outcomes in ischemic stroke: a retrospective MIMIC analysis. Sci Rep 14, 26006 (2024). https://doi.org/10.1038/s41598-024-76324-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-76324-x
Keywords
This article is cited by
-
Albumin-corrected anion gap and risk of mortality among US adults
Archives of Public Health (2025)
-
Prognostic value of albumin-corrected anion gap in severe chronic kidney disease with sepsis: association with mortality and clinical outcomes
European Journal of Medical Research (2025)
-
Albumin-corrected anion gap predicts acute kidney injury in critically ill patients with acute pancreatitis: a retrospective cohort study
BMC Nephrology (2025)
-
Serum albumin corrected anion gap levels are associated with poor prognosis in patients with acute ischemic stroke
Scientific Reports (2025)