Abstract
Suppression of Tumorigenicity 2 (ST2) is a member of the interleukin-1 receptor/ Toll-like receptor superfamily, and its specific ligand is Interleukin-33 (IL-33). IL-33/ ST2 signaling has been implicated in numerous inflammatory and allergic diseases, as well as in promoting malignant behavior of tumor cells and angiogenesis. However, the precise role of ST2 in gastric cancer angiogenesis remains incompletely elucidated. We observed a significant correlation between high expression of ST2 in gastric cancer tissues and poor prognosis, along with various clinicopathological features. In vitro experiments demonstrated that the IL-33/ ST2 axis activates the PI3K/AKT/NF-κB signaling pathway through TRAF6, thereby promoting VEGFA-mediated tumor angiogenesis; meanwhile sST2 acts as a decoy receptor to regulate the IL-33/ST2L axis. Consistent findings were also observed in subcutaneous xenograft tumor models in nude mice. Furthermore, we investigated the molecular mechanism by which IL-33 promotes ST2L expression in GC cells via upregulation of transcription factors YY1 and GATA2 through intracellular signaling pathways.
Similar content being viewed by others
Introduction
Gastric cancer (GC) ranks among the most prevalent malignancies worldwide, resulting in over 10,000 deaths annually1. Despite advancements in diagnosis and perioperative treatment over recent decades, most cases are diagnosed at an advanced stage with an overall poor prognosis2,3,4. Therefore, more in-depth studies are warranted to delineate genetic contributions to gastric cancer development and identify novel therapeutic targets.
As an important cytokine in the tumor microenvironment of gastric cancer, interleukin can interact with its receptor to transmit signals between cells and affect tumor growth. Multiple studies have shown that activation of the IL-33/ST2L axis contributes to the progression of lung carcinoma, breast carcinoma, colorectal cancer, and gastric cancer by remodeling TME5,6,7,8,9,10. IL1RL1 encodes four isoforms of ST2 via alternative splicing: ST2L, sST2, ST2V, and ST2LV11,12. ST2L is a membrane-embedded receptor while sST2 is a soluble form of ST2 which can act as a decoy receptor of IL-33, thereby blocking IL-33/ST2L signaling13.
IL-33 binds to the heterodimer ST2L/IL-1RAcP complex on the target cell membrane and activates various intracellular kinases and factors, including MyD88, TRAF6, IRAK1, and IRAK4, leasing downstream gene transcription induction through NF-κB, p-38, JNK and ERK pathways14,15,16,17. As a key cytokine regulating the immune system, most studies have focused on the effect of IL-33 on ST2L-expressing mast cells, macrophages, regulatory T cells, and other immune cells in the tumor microenvironment8,18,19,20. Targeting IL-33/ST2L axis in tumor immunotherapy or as an adjunct to immune checkpoint blockade therapy.
Despite immune cells, endothelial cells, fibroblasts, and tumor cells can also express ST2L. Huang et al. found that IL‑33/ST2L promotes the malignant progression of GC cells via MAPK pathway10. Furthermore, ST2 expression is elevated in poorly differentiated GC and metastases and the IL-33/ST2 axis enhances the proliferation of GC stem cells and organoids21. These studies suggest that the ST2 protein may be a valuable biomarker for gastric cancer progression and pathogenesis. Given the important role of the IL-33/ST2 axis in tissue vascular repair and angiogenesis, it has also been found that IL-33 can promote the expression and angiogenesis of VEGF in the breast cancer site of wild-type mice, but has no promoting effect on ST2-/- mice22,23. ST2L plays an important role in remodeling tumor microenvironment, but whether it can induce gastric cancer angiogenesis and its mechanism remains to be further explored.
In our study, we demonstrated that the IL-33/ST2L axis activates the PI3K/Akt/NF-κB pathway through the adaptor protein TRAF6, promoting VEGFA-dependent tumor angiogenesis, while sST2 acts as a decoy receptor to block this effect. Importantly, our evidence suggests that IL-33 can activate Akt signaling, enhance the expression of transcription factors YY1 and GATA2, and up-regulate the transcription of ST2L, further amplifying IL-33/ST2L-mediated signaling.
Results
ST2 is highly expressed in gastric cancer tissues, which is associated with poor prognosis
As shown in Fig. 1A, the immunohistochemical staining of gastric cancer tissue and normal gastric epithelial tissue from the HPA database revealed a significantly higher expression of ST2L in gastric tumor samples compared to normal samples. Analysis of TCGA data demonstrated a significant correlation between ST2 expression levels and gastric cancer progression, with an increasing trend observed as the cancer stage advanced (Fig. 1B). Furthermore, Kaplan-Meier analysis indicated a significant association between high IL1RL1 mRNA expression levels and shorter overall survival in patients with gastric cancer (Fig. 1C). Based on the data in the TCGA database, we found that IL1RL1 expression was significantly correlated with Lymph Node Stage, Cancer Metastasis Stage Code, and TNM stage (Table 1). Thus, we speculated that the high expression of IL1RL1 in patients with gastric cancer may be associated with poor prognosis. Given the crucial role of angiogenesis in tumor development, we further investigated the relationship between IL1RL1 expression and angiogenic markers PECAM1 and CD34. The result revealed a positive correlation between IL1RL1 expression and these angiogenic markers (Fig. 1D). Our results suggest that elevated ST2 expression in gastric cancer tissues may promote angiogenesis thereby facilitating disease progression.
ST2 is highly expressed in gastric cancer tissues, which is associated with poor prognosis. (A) Immunohistochemical staining of ST2 in human normal gastric epithelial tissue and gastric cancer tissue from HPA database. (B) Relationship between IL1RL1 mRNA expression in gastric cancer patients and cancer stage (C) Kaplan-Meier analysis of the relationship between overall survival (OS) and IL1RL1 expression in patients with gastric cancer based on the data of TCGA database. (D) Correlation analysis of co-expression of IL1RL1 and angiogenic markers PECAM1and CD34 in gastric cancer tissues based on TCGA database.
ST2L expression is associated with proliferation, migration, and VEGFA-dependent angiogenesis in vitro
ST2 belongs to the interleukin-1 receptor family and mainly presents two isoforms in vivo: transmembrane ST2 (ST2L) and soluble ST2 (sST2). To further explore the role of ST2 in gastric cancer development, we first attempted to detect sST2 secreted by tumor cells by ELISA. However, the amount of sST2 in the cell culture supernatant was too small to reach the detection limit. Therefore, we hypothesized that among the two subtypes of ST2 in gastric cancer cells, the membrane protein ST2L plays a more important role in the progression of gastric cancer. To further investigate the impact of ST2L expression on the biological behavior of tumor cells, gastric cancer cells (BGC-823 and HGC-27) were transfected with scramble/ST2L KD siRNA/pcDNA/ST2L OE plasmid (Fig. 2A and B). The MTT assay revealed that upregulation of ST2L enhanced the proliferation of gastric cancer cells, whereas downregulation of ST2L led to an inhibition in tumor cell viability (Fig. S1A and B). Both wound healing and transwell assays demonstrated that ST2L overexpression significantly increased the migratory capacity of gastric cancer cells, whereas knockdown of ST2L had an inhibitory effect on cell migration (Fig. 2C, D, and S1C). As angiogenesis is a crucial requisite for tumor metastasis, and our findings indicate a significant positive correlation between the expression of ST2L and angiogenesis markers in gastric cancer patients, we aim to investigate the potential impact of ST2L expressed by gastric cancer cells on tumor angiogenesis. Transwell assay was used to examine the effect of different conditioned mediums (CMs) on the migration ability of HUVECs. The results showed that CM prepared by ST2L overexpressed GC cells significantly promoted HUVEC migration, while CM prepared by ST2LKD GC cells inhibited HUVEC migration (Fig. 2E and S1D). Tube formation assays demonstrated that compared with the respective control group, HUVECs cocultured with ST2L OE cell CM had a significant increase in vascular junctions, while the number of junctions in HUVECs cocultured with ST2L KD cell CM decreased (Fig. 2F and S1E). Since VEGFA is a vital factor in tumor angiogenesis, we wondered whether ST2L expression could regulate VEGFA expression and thus affect tumor angiogenesis. Here, we noted that overexpression of ST2L significantly facilitated VEGFA protein levels in both BGC-823 and HGC-27 cells, while ST2L knockdown inhibited VEGFA expression in GC cells (Fig. 2G, H). To further quantify ST2L-mediated VEGFA secretion in gastric cancer cells, we performed ELISA assay, which showed that overexpression of ST2L was able to promote VEGFA secretion, whereas knockdown of ST2L inhibited VEGFA concentration in the cell supernatant (Fig. 2I). In summary, these findings suggest that ST2L expressed by gastric cancer cells can regulate angiogenesis through VEGFA.
ST2L expression is associated with proliferation, migration and VEGFA-dependent angiogenesis in vitro. (A, B) The effects of transfection of ST2L overexpressing plasmid or siRNA in BGC-823 cells was measured by Western blot and qRT-PCR. Wound healing assay (C) and Transwell analysis (D) was used to detect the migration ability of BGC-823 cells. The permeabilized cells were dissolved in methanol, and the optical density (OD) value at 570 nm was detected to quantify the number of cells. (E) Transwell assay showed the migration ability of HUVECs after co-culture with the corresponding CMs. (F) Formation of vascular was measured by HUVECs tube formation assay. The number of tube junctions were measured by imagej. (G, H) Western blot analysis of VEGFA protein levels in GC cells. (I) ELISA assay was conducted to measure the VEGFA secretion. *P < 0.05, **P < 0.01, ***P < 0.001. Original blots/gels are presented in Supplementary Figure. The samples were derived from the same experiment and the gels/blots were processed in parallel.
ST2L induces gastric cancer angiogenesis in vitro by binding to its ligand IL-33, and this effect can be blocked by sST2
As the sole ligand of ST2L in vivo, IL-33 shows an important effect on inflammatory response and angiogenesis. The STRING database demonstrated the interaction between IL-33 and ST2 (IL1RL1) (Fig. 3A). Consistent with this, analysis of the TCGA database revealed that IL-33 expression was positively correlated with IL1RL1 expression in gastric cancer patients (Fig. 3B). Additionally, survival analysis indicated that gastric cancer patients with higher expression of IL-33 had significantly worse overall survival (Fig. 3C).
ST2L induces gastric cancer angiogenesis in vitro by binding to its ligand IL-33, and this effect can be blocked by sST2. (A) The protein-protein interactions of IL1RL1-associated proteins. (B) Correlation analysis of IL1RL1 and IL-33 expression in STAD patients based on the data from TCGA database. (C) Kaplan-Meier analysis of OS related to the IL-33 expression in STAD patients according to the data from TCGA database. The effect of the corresponding CM on the migration (D, F, H) and the vascular formation (E, G, I) of HUVECs. (J, K) Western blot analysis of VEGFA protein expression level. (L, M) The concentration of VEGFA in the cell supernatant of each group was detected by ELISA. *P < 0.05, **P < 0.01, ***P < 0.001. Original blots/gels are presented in Supplementary Figure. The samples were derived from the same experiment and the gels/blots were processed in parallel.
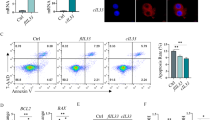
To further demonstrate the interaction between IL-33 and ST2L on the surface of gastric cancer cells to mediate gastric cancer angiogenesis, we treated gastric cancer cells with 30ng/ml human recombinant IL-33 and then the CM was collected. The migration and tube formation ability of HUVECs were significantly increased after treatment with the above conditioned medium, which were reversed by the addition of VEGFA antibody. (Fig. 3D, E and Fig. S2A, B). However, when we knocked down the expression of ST2L 48 h before adding IL-33, it completely reversed the aforementioned effects (Fig. 3F, G and Fig. S2C, D). Similarly, knockdown of ST2L reversed the IL-33-mediated increase in VEGFA expression in GC cells as well as in the supernatant (Fig. 3J, L and S2G). This suggests that ST2L receptors on the membrane of gastric cancer cells are able to interact with IL-33 to promote VEGFA-mediated angiogenesis.
Furthermore, soluble ST2 (sST2), acting as an extracellular decoy receptor, can bind to IL-33 and competitively block its interaction with ST2L. We then examined whether the application of sST2 could inhibit IL-33/ST2L axis activation-induced angiogenesis in GC cells. Gastric cancer cells were pretreated with 200ng/ml sST2 for 2 h before adding 10ng/ml IL-33 to six-well plates for culturing another 24 h. The addition of sST2 also partially reversed the IL-33-induced HUVECs migration and increased the number of junctions (Fig. 3H, I and S2E, F). sST2 also reversed IL-33-mediated VEGFA protein expression and secretion (Fig. 3K, M and S2H).
Taken together, the results suggest that ST2L on the GC cell membrane interacts with IL-33 to induce VEGFA-mediated angiogenesis, while sST2 acts as a decoy receptor to block this effect.
IL-33/ST2L axis regulates the TRAF6-mediated PI3K/Akt/ NF-κB signaling pathway to promote VEGFA-dependent vascular growth in gastric cancer cells
Next, we aimed to investigate which intracellular signaling pathway is activated by the IL-33/ST2L axis to mediate VEGFA-related angiogenesis. Figure 3A displayed a protein-protein interaction between ST2 and TRAF6. Choi et al. found that IL-33 stimulates endothelial NO production through the ST2/TRAF6-Akt-eNOS signaling pathway, thereby promoting angiogenesis24. Moreover, the IL-33/ST2 axis can activate the PI3K/AKT pathway through TRAF6 to promote inflammation-induced lymphangiogenesis25. Numerous studies have shown that the PI3K/Akt signaling pathway is involved in VEGF-dependent tumor angiogenesis. Therefore, we hypothesized that the IL-33/ST2L axis may activate the PI3K/AKT pathway through the adaptor molecule TRAF6 to mediate VEGFA-dependent tumor angiogenesis.
Western blot showed that IL-33 could increase the protein level of TRAF6 in GC cells and activate the PI3K/Akt signaling pathway, leading to elevated expression levels of p-PI3K and p-Akt, while knockdown of ST2L or addition of sST2 significantly inhibited TRAF6 expression and phosphorylation levels of PI3K and Akt (Fig. 4A, B and Fig. S3A, B). Akt activation is a key regulator of Iκ-B degradation and NF-κB activation in NF-κB-dependent gene transcription. In addition, studies have found that NF-κB also promotes tumor angiogenesis by upregulating VEGFA, ultimately leading to tumor hematologic metastasis and growth26. Therefore, we further examined the phosphorylation levels of IκBα and NF-κB. As expected, p-IκBα and p-NF-κB protein levels increased significantly after incubation with IL-33, and this effect could be blocked by ST2L knockdown or sST2 (Fig. 4A, B and Fig. S3A, B). These results suggest that IL-33 activates the PI3K/AKT pathway through ST2L/TRAF6, which further induces phosphorylation of IκBα to promote the activation of NF-κB.
IL-33/ST2L axis regulates the TRAF6-mediated PI3K/Akt/NF-κB signaling pathway to promote VEGFA-dependent vascular growth in gastric cancer cells. (A, B and C) Western blot analysis of the protein levels of the corresponding protein in each group of cells. TRAF6 KD cells were treated with IL-33, and CM were collected and added to HUVECs for determination of (D) migration ability and (E) tube formation. (F, G) The protein expression and secretion of VEGFA in TRAF6 KD cells treated with IL-33 was detected by western blot assay and ELISA. ST2L OE cells were treated with Akt inhibitor (Triciribine) or p-IκBα inhibitor (BAY 11-7082), CM were collected and added to HUVECs, and (H) migration ability and (I) tube formation was conducted. (J, K) The protein level and secretion of VEGFA was measured by western blotting and ELISA. *P < 0.05, **P < 0.01, ***P < 0.001. Original blots/gels are presented in Supplementary Figure. The samples were derived from the same experiment and the gels/blots were processed in parallel.
In order to clarify the key role of TRAF6 in connecting the IL-33/ST2L axis with the downstream pathway, we successfully constructed siRNA to knock down TRAF6 expression in gastric cancer cells (Fig. S3C, D). Western blot analysis showed that TRAF6 knockdown significantly inhibited IL-33-mediated PI3K/Akt phosphorylation, indicating that TRAF6 plays a critical role in IL-33/ ST2L-mediated PI3K/Akt activation (Fig. 4C). Transwell and tube formation assay showed that compared with the control group (IL-33 + scramble), CM prepared after 48 h IL-33 treatment of TRAF6 KD cells could partially reverse the increase of HUVECs’ migration and angiogenesis capacity (Fig. 4D, E and Fig. S3E, F). Similarly, IL-33-induced upregulation of VEGFA expression and secretion in GC cells was reversed by TRAF6 knockdown. (Fig. 4F, G and S3G).
To further demonstrate the above signaling pathways in regulating gastric cancer angiogenesis, we conducted transwell and HUVECs tube formation assays. Compared with the group added with CM from ST2L OE cells, the migration rate (Fig. 4H and S3H) and the tube formation (Fig. 4I and S3I) of HUVECs decreased noticeably adding the CM from ST2L OE cells treated with 10 mg/ml Triciribine (p-Akt inhibitor) or 10 mg/ml BAY 11-7082 (p-IκBα inhibitor). Similarly, the up-regulation of VEGFA protein induced by ST2LOE can also be reversed by Triciribine or BAY 11-7082 (Fig. 4J, K and S3J).
Taken together, we can conclude that the IL-33/ST2L axis regulates the activation of IκBα and NF-κB through the TRAF6/PI3K/Akt signaling pathway to promote VEGFA-dependent angiogenesis in gastric cancer, while sST2 can competitively bind to IL-33 and reverse the above effects.
Overexpression of ST2L promotes GC progression in vivo, while sST2 or blocking akt pathway can slow GC progression in vivo
It has been demonstrated that the IL-33/ST2L axis promotes VEGFA-dependent angiogenesis in GC cells by regulating TRAF6/PI3K/Akt/NF-κB signaling pathway. Next, to further elucidate its role in tumor development in vivo, we established a xenograft tumor model, and the experimental design is shown in Fig. 5A. When tumor volume reached 100 mm3, Triciribine(15 mg/kg) was injected intraperitoneally twice a week, or sST2(0.1 mg/kg) was injected intratumorally twice a week. Tumor volumes were recorded every five days and tumor growth curves were plotted. After 21 days of modeling, the mice were euthanized, and the solid tumors were dissected. The results showed that subcutaneous inoculation of ST2L OE tumor cells resulted in faster tumor growth and larger and heavier solid tumors compared with the control group, which were partially reversed by Triciribine or sST2 (Fig. 5B-D). Intratumoral injection of sST2 slowed tumor growth and reduced tumor burden compared with the control group (Fig. 5B-D). Immunohistochemical staining of tumor tissue also showed that overexpression of ST2L significantly promoted CD34 expression, and administration of Triciribine or sST2 reduced vascular density (Fig. 5E, F).
Overexpression of ST2L promotes GC progression in vivo, while sST2 can slow GC progression in vivo. (A) Experimental plan of subcutaneous xenograft tumor model in nude mice. (B) Growth curves of subcutaneous tumors. Image (C) and weight (D) of xenograft tumors. (E) Immunohistochemical staining of tumor tissue. (F) Quantification of immunohistochemical data. *P < 0.05, **P < 0.01, ***P < 0.001.
Transcription factors YY1 and GATA2 participate in IL-33-mediated upregulation of ST2L transcription level
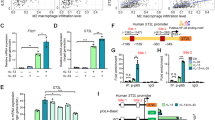
Since IL-33 exerts its biological effects mainly by interacting with ST2L and activating downstream signaling pathways, we wondered whether activation of the IL-33/ST2L axis could feedback regulate ST2L expression to further amplify the malignant progression of IL-33/ST2L-mediated angiogenesis in gastric cancer. We detected the effects of IL-33 and downstream Akt signaling on ST2L expression. The result showed that 30ng/ml IL-33 significantly upregulated the mRNA and protein levels of ST2L in BGC-823 and HGC-27 cells, and this effect was partially inhibited by Triciribine (Fig. 6A, B and S4 A). Huang et al. found a dose-dependent increase in ST2L protein levels after IL-33 treatment of AGS and MKN-45 cells, which partially supports our findings10. To further explore the mechanism of how IL-33 up-regulates the expression of ST2L in GC cells, we identified five transcription factors that may bind to the ST2L promoter site, namely YY1, GATA2, GATA3, GATA4, and TCF4 (Fig. 6C). Here, we noted that YY1 and GATA2 mRNA levels were increased upon IL-33 treatment and downregulated in the presence of Triciribine. (Fig. 6D, E). Further, we verified the YY1 and GATA2 protein levels after being treated with IL-33 and Triciribine, which also showed the same trend (Fig. 6F and S4 B). Consequently, the binding motifs of YY1 and GATA2 were predicted, and JASPAR online tool was used to identify one YY1 binding site between − 1459 bp and − 1448 bp, and three GATA2 binding sites located at -1745 ~ -1739 bp, -115 ~ -109 bp and + 94 ~ + 100 bp, respectively (Fig. 6G). CHIP assay was performed and qPCR primers were designed to amplify the ST2L promoter sequence. The result proved that the occupancy of transcription factors YY1 and GATA2 with ST2L promoter sequences was significantly increased in IL-33-treated gastric cancer cells (Fig. 6H, I and J). Therefore, we can conclude that IL-33 promotes the expression of YY1 and GATA2 through the Akt pathway, which induces increased occupancy of the promoter region of ST2L, thereby promoting the expression of ST2L. We synthesized siRNA to knock down the expression of YY1 and GATA2 respectively (Fig. 6K and S4C). Moreover, knockdown of YY1 and GATA2 in BGC-823 and HGC-27 cells significantly suppressed ST2L protein expression, while simultaneous knockdown of YY1 and GATA2 had a more significant effect on inhibiting ST2L protein expression (Fig. 6L and S4D). Thus, IL-33 can promote ST2L transcription by improving the expression of transcription factors YY1 and GATA2 through the Akt pathway.
Transcription factors YY1 and GATA2 participate in IL-33-mediated upregulation of ST2L transcription level. (A, B) The protein and mRNA levels of ST2L in each group. (C) JASPAR (http://jaspardev.genereg.net/) was used to predict the binding sites of transcription factors and ST2L promoter regions. (D, E) The mRNA levels of transcription factors were detected by qRT-PCR. (F) The protein expression of YY1 and GATA2 were measured by Western blot. (G) The binding motifs of transcription factors YY1 and GATA2 at the promoter of ST2L were analyzed using JASPAR. (H) The ChIP assay was performed using the YY1 and GATA2 antibody. (I, J) The occupancy of ST2L promoter bits was detected by ChIP-qPCR. (K) Western blot was used to verify the knockdown efficiency of YY1 and GATA2. (L) Western blotting was conducted to assess the impact of individual or simultaneous knockdown of YY1 and GATA2 on ST2L protein expression. *P < 0.05, **P < 0.01, ***P < 0.001; n.s., no significance. Original blots/gels are presented in Supplementary Figure. The samples were derived from the same experiment and the gels/blots were processed in parallel.
YY1 siRNA and GATA2 siRNA attenuated IL-33-induced ST2L production and angiogenesis
Notably, both YY1 and GATA2 have been identified to regulate IL-33-mediated ST2L transcription. Therefore, we questioned whether the simultaneous knockdown of YY1 and GATA2 has a synergistic effect. We transfected YY1 siRNA and GATA2 siRNA into BGC-823 and HGC-27 cells, respectively, or in combination, with 30ng/ml recombinant human IL-33 protein. The corresponding conditioned medium was prepared and added to HUVECs. Transwell and tube formation assays showed that knockdown of YY1 and GATA2 reduced the migration and angiogenesis ability of HUVECs, while co-knockdown of YY1 and GATA2 significantly inhibited angiogenesis (Fig. 7A, B). Similarly, western blot results also showed that YY1 GATA2 knockdown significantly inhibited IL-33-mediated increase of VEGFA protein expression and secretion, which was even lower when both YY1 and GATA2 were knocked down (Fig. 7C and D). All these results suggest that YY1 and GATA2 are able to synergistically promote IL-33-mediated increase in ST2L transcription and angiogenesis.
YY1 siRNA and GATA2 siRNA attenuated IL-33-induced ST2L production and angiogenesis. The corresponding CMs were collected and added to HUVEC cells, and the migration and angiogenesis ability was detected by transwell (A) and tube formation assay (B). (C, D) The levels of VEGFA protein and secretion of each group were detected by western blot. *P < 0.05, **P < 0.01, ***P < 0.001. Original blots/gels are presented in Supplementary Figure. The samples were derived from the same experiment and the gels/blots were processed in parallel.
Discussion
ST2 plays a crucial role in the pathogenesis of inflammatory and cardiovascular diseases. Recent research has unveiled its involvement in angiogenesis regulation and immune microenvironment remodeling. Studies have shown that ST2 is highly expressed in colorectal cancer and can be used as a potential target for immunotherapy27. In head and neck squamous cell carcinoma, anti-ST2 monoclonal antibody decreased Treg numbers, promoted anti-tumor immune response, and hindered tumor progression28. Furthermore, in 4T1 breast cancer mouse models, anti-ST2 antibodies disrupted ST2-mediated suppression of myeloid suppressor cells’ function to impede tumor growth29. Nonetheless, the expression pattern of ST2 isoforms (ST2L and sST2) in gastric cancer remains unclear. Our analysis revealed elevated ST2 levels in gastric cancer tissues compared to normal gastric epithelial tissues from the HPA database (Fig. 1A). Kwon JW et al. also found that the ST2 + subpopulation appeared spontaneously during gastric tumorigenesis, which further supports our conclusion21. Additionally, high ST2 expression was also significantly associated with poor prognosis and stage of gastric cancer patients (Fig. 1A and B). It has been found that ST2L gene deletion affects vascular remodeling by up-regulating the expression of HIF-1α and VEGF in vascular endothelial cells, which suggests that the expression of ST2L may be related to angiogenesis30. Subsequent analysis unveiled a positive correlation between IL1RL1 gene levels and angiogenic markers (Fig. 1D). These findings suggest that heightened ST2 expression may promote tumor growth and metastasis through its influence on endothelial cells within the tumor microenvironment.
Since IL1RL1 can encode several different subtypes of ST2, we hope to identify the specific subtype of ST2 that plays a pro-tumor effect in GC cells. ELISA suggested that the protein level of sST2 in the cell culture supernatant was too low to reach the detection limit. Therefore, we suspect that the gastric cancer cell membrane protein ST2L plays the most critical role among the different ST2 subtypes. Cell function experiments showed that ST2L overexpression can promote the proliferation, migration, and VEGFA-mediated angiogenesis of gastric cancer cells (Fig. 2). It has been reported that the expression of gastric cancer cells can interact with ST2L and IL-33 to activate MAPK pathway and promote EMT, thereby mediating the increase of proliferation, invasion and migration of GC cells10. It has also been reported that ST2L-mediated glioma migration and invasion are related to upregulated MMP2 and MMP9 proteins after NF-κB activation31. In this study, although we focused on the mechanism of ST2L-mediated GC angiogenesis, we also demonstrated that overexpression or knockdown of ST2L in GC cells can directly affect cell proliferation and migration, and its potential downstream mechanism remains to be further studied. ST2L activates the downstream pathway, which may promote malignant behavior of tumor cells by increasing the expression of migratory proteins or by influencing the cell cycle.
For many years, ST2 was considered an orphan receptor, until its association with IL-33 was confirmed32. There is substantial evidence that cytokines are able to influence cancer development and have either pro- or anti-tumor effects33. Through bioinformatics analysis, we found that IL-33 was significantly correlated with the expression of IL1RL1 in gastric cancer patients, and patients with high expression of IL-33 have poor prognosis. A meta-analysis also found that IL-33 expression was significantly elevated in patients with gastric cancer, liver cancer, and lung cancer, consistent with our findings34. Our study revealed that IL-33 was able to promote VEGFA-mediated angiogenesis in gastric cancer by binding to ST2L. sST2 is a soluble secretory subtype of ST2 with the same extracellular domain as ST2L, and it is able to competitively bind IL-33, thereby blocking the binding of ST2L to IL-3313,35. Our experiments demonstrated that IL-33/ST2L-mediated pro-angiogenic effects could be inhibited by the addition of sST2 (Fig. 3). Given that we observed in the first part that the overexpression or knockdown of ST2L alone can affect the expression and secretion of VEGFA in gastric cancer (GC) cells, thereby promoting the migration of endothelial cells and angiogenesis, we suspect that IL-33 derived from GC cells may influence tumor angiogenesis through an autocrine pathway. Furthermore, Choi et al. discovered that ST2L expressed by endothelial cells can also be activated by the IL-33-ST2L/TRAF6-Akt-eNOS signaling pathway, thus impacting the functionality of endothelial cells24. Although our experimental design cannot entirely rule out the impact of IL-33 from GC cells on HUVECs through a paracrine mechanism, considering the complexity of the cytokine network in the tumor microenvironment, we believe that, despite the potential influence of paracrine IL-33 from surrounding cells on HUVECs, the interaction of ST2L expressed by tumor cells with IL-33, activating the TRAF6-mediated PI3K/Akt/NF-κB pathway demonstrated in this study, indeed contributes to angiogenesis in HUVECs in a VEGFA-dependent manner. The question of whether IL-33 from tumor cells can promote tumor angiogenesis through a paracrine form is an issue that warrants our attention in subsequent research.
ST2 activation has been reported to activate a variety of intracellular kinases and factors resulting in influencing the expression of downstream genes17,35. Several studies have shown that the IL-33/ST2L axis activates the downstream signaling pathway through TRAF6, thus affecting the expression of downstream proteins15,25,36. In our experiments, we illustrated that the IL-33/ST2L axis increases the protein level of TRAF6. As an important intracellular regulator with unique receptor binding specificity, TRAF6 can mediate signal transduction reactions such as toll-like receptor and interleukin-1 receptor (IL-1R), and ultimately activate PI3K/Akt, NF-κB, and MAPK pathways37,38,39,40. We found that the IL-33/ST2L-mediated up-regulation of TRAF6 protein levels further activates PI3K/Akt phosphorylation, which mediates the phosphorylation of IκBα and ultimately activates NF-κB, leading to an increase in VEGFA protein level (Fig. 4). We also demonstrated that restoring the level of sST2 in the tumor area by intratumoral injection could inhibit tumor growth, and intraperitoneal injection of Akt inhibitor could reduce tumor vascular density and alleviate tumor burden (Fig. 5).
The above experiments proved that the IL-33/ST2L axis can activate the Akt signaling pathway to further mediate the phosphorylation of NF-κB and promote VEGFA protein expression. We then wondered whether the IL-33/ST2L axis could also activate intracellular signaling pathways that ultimately regulate ST2L transcription. IL-13 upregulates YY1 expression by activating the AKT pathway, leading to fibroblast activation, and PI3K/AKT/YY1 is involved in IL-13-induced lung cancer cell behavior41,42. The transcription factor YY1 regulates ST2 expression to prevent doxorubicin-mediated cardiotoxicity43. GATA2 can regulate the expression of ST2 in hepatocellular carcinoma cells and affect the cellular immune function induced by HBV infection44. It has also been suggested that GATA2 controls human ST2 gene transcription in mast cells45. Our study revealed that transcription factors YY1 and GATA2 can be regulated by IL-33/ST2L/Akt, and promote ST2L transcription by binding to the promoter region of ST2L (Figs. 6 and 7).
By blocking the intracellular Akt signaling pathway, the activation of IL-33/ST2L in gastric cancer cells can be blocked from the intracellular pathway, thus inhibiting the expression of VEGFA. In addition, inhibiting the expression of ST2L protein in tumor cells or increasing the level of sST2 protein in tumor sites can reduce the binding of IL-33 and ST2L, leading to the reduction of tumor load and delaying tumor progression (Fig. 8). Therefore, the development of new specific inhibitors targeting tumor cell-derived ST2L or increasing local sST2 levels in tumor areas may be a novel strategy for the treatment of gastric cancer.
Materials and methods
Public data collection
To assess ST2 expression at the protein level, IHC images of ST2 protein expression in normal gastric tissue and gastric carcinoma tissue were downloaded from the Human Protein Atlas (HPA) database (http://www.proteinatlas.org).
GEPIA2 (http://gepia2.cancer-pku.cn/) was used for the expression analysis of IL1RL1 in different pathological stages of stomach cancer (STAD). The stomach cancer patient data used to analyze the overall survival rate (OS) were provided from The Cancer Genome Atlas (TCGA, https://tcga-data.nci.nih.gov/docs/publications/tcga/). Kaplan-Meier method with log-rank test was used to compare OS between different groups.
The expression association between IL1LRL and angiogenesis markers (PCAM1 and CD34) in gastric cancer samples was identified via the TIMER2 database (timer.cistrome.org), using Spearman’s correlation coefficient analysis.
Clinical data of gastric cancer patients from TCGA database were downloaded using cbioportal database (https://www.cbioportal.org/), and the correlation between IL1RL1 mRNA expression and clinicopathological features was verified by chi-square test.
Cell culture
Human gastric cancer cells (BGC-823 and HGC-27) were cultured in RPMI-1640 media with 10% fetal bovine serum (Gibco) and 100 U/ml penicillin/streptomycin (Invitrogen Corporation). Human umbilical vein endothelial cells (HUVECs) were cultured in Endothelial Cell Medium (Sciencell, San Diego, USA). All cells were obtained from Shanghai Institute of Biological Sciences, Chinese Academy of Sciences (Shanghai, China) and were cultured with 5% carbon dioxide at 37℃.
Vector construction
ST2L overexpression plasmid was synthesized by DNA Bioscience Co.Ltd, Shanghai, China. The human siRNA sequence was designed by Shanghai GenePharma Co., Ltd., Shanghai, China. siRNA used for transfection are listed in Table S1.
Lipo8000™ transfection
Cells were resuspended at a density of 1 × 106 cells/well and seeded in 6-well plates. After 24 h the transfection complex was prepared according to manufacturer’s instructions using Lipo8000™ transfection reagent (Beyotime Biotechnology, Shanghai, China). The total intracellular RNA was extracted after 24 h, and the total intracellular protein was extracted after 72 h to verify the transfection efficiency.
Western blot analysis
Protein extraction and Western blot analysis were conducted as previously described46. The cells were lysed using cold RIPA lysate, and centrifuged at 12,000 rpm for 25 min to remove cell debris. The cellular protein was diluted by saline and the concentration of each group was determined using the Bradford method. 30 µg protein was loaded and separated on SDS-PAGE and was electro-transferred to a polyvinylidene difluoride (PVDF) membrane. The membranes were blocked with 5% bovine serum albumin (BSA) for 90 min, and then incubated overnight at 4℃ with primary antibodies. The antibodies used in Western blot are listed in Table S2. Protein bands were photographed using a Tanon5500 ECL chemiluminescence system.
ELISA
Used lipo8000™ to transfect the plasmid, and collected the cell culture fluid after 48 h, then centrifuged at 12,000 rpm for 5 min. The supernatant was collected and used the Human VEGF-A (Vascular Endothelial Cell Growth Factor A) ELISA Kit (E-EL-H0111, Elabscience Co.) to measure the VEGF-A concentration.
Quantitative real-time polymerase chain reaction (qRT-PCR)
RNA extraction and qRT-PCR assay were conducted as previously described47. TRIzol regent (Invitrogen, USA) was used to extract total RNA from the cell lines according to the manufacturer’s instructions. The cDNA was synthesized with 1st Strand cDNA Synthesis Kit (Yeason, China). QRT-PCR analysis was performed using qPCR SYBR Green Master Mix (Yeason, China). The total RNA levels were normalized with GAPDH. The results were calculated using the 2−△△CT method and were expressed as the mean ± SD. The primers used in qRT-PCR are listed in Table S3.
Cell viability assay
Cells transfected with ST2L overexpressing plasmid or siRNA were added to the 96-well plate at a concentration of 5 × 103 cells/well. After culturing for 0, 24, 48, and 72 h, respectively, added 20 µl MTT (0.5% MTT, Sigma Chemical, St Louis, MO, USA) to each well. After incubating at 37℃ for 4 h the culture medium was aspirated, and then added 100 µl DMSO to each well. Using Microplate reader (Tecan Safire 2; Tecan Group Ltd., Männedorf, Switzerland) to measure the absorbance of the well at 492 nm.
Preparation of conditioned medium (CM)
Transfected the corresponding plasmid into gastric cancer cells. After the cells reached 80% density, they were cultured in serum-free (RPMI) 1640 medium for another 24 h. The supernatant was collected after centrifugation at 2000 ×g for 20 min at 4℃. The CM was stored at -80℃.
Transwell migration assay
Cell migration was analyzed by transwell chamber (Corning, NY, USA). HUVECs were resuspended in the corresponding conditioned medium at a density of 1 × 105 cells /ml. 200 µl of cell suspension was added to the upper chamber, and 500 µl of medium containing 10% serum was added to the lower chamber. The cells in the lower chamber were fixed with 4% paraformaldehyde for 20 min after continued culture for 24 h, stained with 0.5% crystal violet (Solarbio, Beijing, China) for 5 minutes, and then the cells in the upper chamber were wiped with a cotton swab. Cells were photographed with an inverted microscope (Nikon). Finally, transmembrane cells were lysed with methanol and optical density (OD) values at 570 nm were measured using a microplate reader (Biotek, Winooski, VT, USA).
Wound-healing assay
Cells were seeded in 6-well plates, and when cell density reached 70%, the corresponding plasmids or siRNA were transfected using lipo8000™ reagent. After 24 h, the cell culture layer was scraped with a sterile pipette tip (200 µl). Floating cells were washed off with PBS solution and serum-free medium was added. Cells were placed in an incubator, and wounds were photographed at 0 and 24 h. Three different areas were randomly selected to measure the scratch distance and the average value was taken.
Endothelial cell tube formation assay
The Endothelial cell tube formation assay was performed as previously described. In short, the matrigel (#356234, corning, NY, USA) was melted overnight at 4℃. 50 µl matrigel was added to each well of the 96-well plate, then was placed in the 37℃ incubator for 60 min. Resuspend HUVECs in CM at a density of 5 × 105 cells/ml, and added 100 µl of cell suspension to the wells. After being placed at 37℃ for 4–6 h, observed the formation of the tube with an inverted microscope. Angiogenesis analyzer, a plug-in of ImageJ software was used to count the number of tube junctions in each well.
Chromatin immunoprecipitation (ChIP) assay
The ChIP assay was performed according to the protocol of the ChIP assay kit (Beyotime Biotechnology, China). To put it simply, cultured cells were first immobilized with formaldehyde and the chromatin was precipitated by corresponding transcription factor antibodies listed in Table S2. Finally, the promoter region of ST2L was amplified by PCR and analyzed by agarose gel electrophoresis or qRT-PCR45.
The primer sequences:
F-ST2L- promoter: 5' - GTGATCATCGGGTTCAGCTTATC - 3';
R-ST2L- promoter: 5' - GCTTTACTAAATACAACAGCCAGCCT - 3';
Xenograft tumor model in nude mice
BGC-823 cells expressing pcDNA or ST2L OE plasmid were injected subcutaneously into the inner wing of 4-week-old male BALB/C nude mice (JSJ laboratories, Shanghai, China) at a density of 5 × 106/ml. When the tumor volume reached 100 mm3, the drug was injected: Triciribine (15 mg/kg, i.p., twice a week) or sST2 (0.1 mg/kg, i.m., twice a week). Animals were euthanized by CO2 asphyxiation after 3 weeks, and the tumors were isolated and weighted. Animal experiments were conducted in mice using protocols approved by the IACUC of East China University of Science and Technology. All experiments performed on animals were performed in accordance with institutional guidelines. In addition, we followed the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines.
Immunohistochemistry (IHC)
The IHC assay was carried out as previously described48. CD34 antibody (Cat. No. CY5196, Abways) was used to measure tumor tissue angiogenesis.
Statistical analysis
All results were expressed as mean ± standard deviation (SD). All experiments were performed at least three times. GraphPad Prism 10.2 software was used for statistical analysis. Student’s t test (two-tailed) was used to analyze the significance of the differences between groups. One-way ANOVA was applied with Bonferroni correction to compare data from multiple groups.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- HUVECs:
-
Human Umbilical Vein Endothelial Cells
- KD:
-
knockdown
- OE:
-
overexpression
- STAD:
-
Stomach adenocarcinoma
- NC:
-
negative control
- OD:
-
optical density
- OS:
-
overall survival
- ST2:
-
suppression of tumorigenicity 2
- sST2:
-
soluble suppression of tumorigenicity-2
- ChIP:
-
chromatin immunoprecipitation
- TCGA:
-
The cancer genome atlas
References
Sung, H. et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249. https://doi.org/10.3322/caac.21660 (2021).
Sisic, L. et al. Postoperative follow-up programs improve survival in curatively resected gastric and junctional cancer patients: a propensity score matched analysis. Gastric Cancer. 21, 552–568. https://doi.org/10.1007/s10120-017-0751-4 (2018).
Smyth, E. C., Nilsson, M., Grabsch, H. I., van Grieken, N. C. & Lordick, F. Gastric cancer. Lancet. 396, 635–648. https://doi.org/10.1016/s0140-6736(20)31288-5 (2020).
Song, Z., Wu, Y., Yang, J., Yang, D. & Fang, X. Progress in the treatment of advanced gastric cancer. Tumour Biol. 39, 1010428317714626. https://doi.org/10.1177/1010428317714626 (2017).
Kim, J. Y. et al. Interleukin-33/ST2 axis promotes epithelial cell transformation and breast tumorigenesis via upregulation of COT activity. Oncogene. 34, 4928–4938. https://doi.org/10.1038/onc.2014.418 (2015).
Luo, P. et al. The IL-33/ST2 pathway suppresses murine colon cancer growth and metastasis by upregulating CD40 L signaling. Biomed. Pharmacother. 127, 110232. https://doi.org/10.1016/j.biopha.2020.110232 (2020).
Li, Y. et al. IL-33 facilitates proliferation of colorectal cancer dependent on COX2/PGE(2). J. Exp. Clin. Cancer Res. 37, 196. https://doi.org/10.1186/s13046-018-0839-7 (2018).
Zhao, Y. et al. Increased expression of ST2 on regulatory T cells is associated with cancer associated fibroblast-derived IL-33 in laryngeal cancer. Pathol. Res. Pract. 237, 154023. https://doi.org/10.1016/j.prp.2022.154023 (2022).
Wang, C. et al. IL-33 signaling fuels outgrowth and metastasis of human lung cancer. Biochem. Biophys. Res. Commun. 479, 461–468. https://doi.org/10.1016/j.bbrc.2016.09.081 (2016).
Huang, N. et al. IL–33/ST2 promotes the malignant progression of gastric cancer via the MAPK pathway. Mol. Med. Rep. 23 https://doi.org/10.3892/mmr.2021.12000 (2021).
Yanagisawa, K., Takagi, T., Tsukamoto, T., Tetsuka, T. & Tominaga, S. Presence of a novel primary response gene ST2L, encoding a product highly similar to the interleukin 1 receptor type 1. FEBS Lett. 318, 83–87. https://doi.org/10.1016/0014-5793(93)81333-u (1993).
Larsen, K. M., Minaya, M. K., Vaish, V. & Peña, M. M. O. The role of IL-33/ST2 pathway in Tumorigenesis. Int. J. Mol. Sci. 19 https://doi.org/10.3390/ijms19092676 (2018).
Homsak, E. & Gruson, D. Soluble ST2: a complex and diverse role in several diseases. Clin. Chim. Acta. 507, 75–87. https://doi.org/10.1016/j.cca.2020.04.011 (2020).
Chang, C. P., Hu, M. H., Hsiao, Y. P. & Wang, Y. C. ST2 Signaling in the Tumor Microenvironment. Adv. Exp. Med. Biol. 1240, 83–93. https://doi.org/10.1007/978-3-030-38315-2_7 (2020).
Funakoshi-Tago, M. et al. TRAF6 is a critical signal transducer in IL-33 signaling pathway. Cell. Signal. 20, 1679–1686. https://doi.org/10.1016/j.cellsig.2008.05.013 (2008).
Pinto, S. M. et al. A network map of IL-33 signaling pathway. J. Cell. Commun. Signal. 12, 615–624. https://doi.org/10.1007/s12079-018-0464-4 (2018).
Cayrol, C. & Girard, J. P. Interleukin-33 (IL-33): a critical review of its biology and the mechanisms involved in its release as a potent extracellular cytokine. Cytokine. 156, 155891. https://doi.org/10.1016/j.cyto.2022.155891 (2022).
Eissmann, M. F. et al. IL-33-mediated mast cell activation promotes gastric cancer through macrophage mobilization. Nat. Commun. 10, 2735. https://doi.org/10.1038/s41467-019-10676-1 (2019).
Dixit, A. et al. Targeting TNF-α-producing macrophages activates antitumor immunity in pancreatic cancer via IL-33 signaling. JCI Insight. 7 https://doi.org/10.1172/jci.insight.153242 (2022).
Sun, X. et al. Inflammatory cell-derived CXCL3 promotes pancreatic cancer metastasis through a novel myofibroblast-hijacked cancer escape mechanism. Gut. 71, 129–147. https://doi.org/10.1136/gutjnl-2020-322744 (2022).
Kwon, J. W. et al. A synergistic partnership between IL-33/ST2 and wnt pathway through Bcl-xL drives gastric cancer stemness and metastasis. Oncogene. 42, 501–515. https://doi.org/10.1038/s41388-022-02575-5 (2023).
Milosavljevic, M. Z. et al. Deletion of IL-33R attenuates VEGF expression and enhances necrosis in mammary carcinoma. Oncotarget. 7, 18106–18115. https://doi.org/10.18632/oncotarget.7635 (2016).
Jovanovic, I. P. et al. Interleukin-33/ST2 axis promotes breast cancer growth and metastases by facilitating intratumoral accumulation of immunosuppressive and innate lymphoid cells. Int. J. Cancer. 134, 1669–1682. https://doi.org/10.1002/ijc.28481 (2014).
Choi, Y. S. et al. Interleukin-33 induces angiogenesis and vascular permeability through ST2/TRAF6-mediated endothelial nitric oxide production. Blood. 114, 3117–3126. https://doi.org/10.1182/blood-2009-02-203372 (2009).
Han, L. et al. Interleukin-33 promotes inflammation-induced lymphangiogenesis via ST2/TRAF6-mediated Akt/eNOS/NO signalling pathway. Sci. Rep. 7, 10602. https://doi.org/10.1038/s41598-017-10894-x (2017).
Wang, R. et al. B7-H3 promotes colorectal cancer angiogenesis through activating the NF-κB pathway to induce VEGFA expression. Cell. Death Dis. 11, 55. https://doi.org/10.1038/s41419-020-2252-3 (2020).
Van der Jeught, K. et al. ST2 as checkpoint target for colorectal cancer immunotherapy. JCI Insight. 5 https://doi.org/10.1172/jci.insight.136073 (2020).
Wen, Y. H. et al. Stromal interleukin-33 promotes regulatory T cell-mediated immunosuppression in head and neck squamous cell carcinoma and correlates with poor prognosis. Cancer Immunol. Immunother. 68, 221–232. https://doi.org/10.1007/s00262-018-2265-2 (2019).
Xiao, P. et al. Interleukin 33 in tumor microenvironment is crucial for the accumulation and function of myeloid-derived suppressor cells. Oncoimmunology. 5, e1063772. https://doi.org/10.1080/2162402x.2015.1063772 (2016).
Liu, J. et al. IL-33 initiates vascular remodelling in hypoxic pulmonary hypertension by up-regulating HIF-1α and VEGF expression in vascular endothelial cells. EBioMedicine. 33, 196–210. https://doi.org/10.1016/j.ebiom.2018.06.003 (2018).
Zhang, J. F. et al. IL–33 enhances glioma cell migration and invasion by upregulation of MMP2 and MMP9 via the ST2-NF-κB pathway. Oncol. Rep. 38, 2033–2042. https://doi.org/10.3892/or.2017.5926 (2017).
Baekkevold, E. S. et al. Molecular characterization of NF-HEV, a nuclear factor preferentially expressed in human high endothelial venules. Am. J. Pathol. 163, 69–79. https://doi.org/10.1016/s0002-9440(10)63631-0 (2003).
Landskron, G., De la Fuente, M., Thuwajit, P., Thuwajit, C. & Hermoso, M. A. Chronic inflammation and cytokines in the tumor microenvironment. J. Immunol. Res. 2014 (149185). https://doi.org/10.1155/2014/149185 (2014).
Chen, X. J. et al. Correlations between serum IL33 and tumor development: a meta-analysis. Asian Pac. J. Cancer Prev. 15, 3503–3505. https://doi.org/10.7314/apjcp.2014.15.8.3503 (2014).
Coyle, A. J. et al. Crucial role of the interleukin 1 receptor family member T1/ST2 in T helper cell type 2-mediated lung mucosal immune responses. J. Exp. Med. 190, 895–902. https://doi.org/10.1084/jem.190.7.895 (1999).
Nishizaki, T. IL-33 suppresses GSK-3β activation through an ST2-independent MyD88/TRAF6/RIP/PI3K/Akt pathway. Heliyon. 4, e00971. https://doi.org/10.1016/j.heliyon.2018.e00971 (2018).
Cheng, C. Y. et al. CORM-2 prevents human gingival fibroblasts from lipoteichoic acid-induced VCAM-1 and ICAM-1 expression by inhibiting TLR2/MyD88/TRAF6/PI3K/Akt/ROS/NF-κB signaling pathway. Biochem. Pharmacol. 201, 115099. https://doi.org/10.1016/j.bcp.2022.115099 (2022).
Li, Y. et al. LncRNA NORAD mediates the proliferation and apoptosis of diffuse Large-B-Cell lymphoma via regulation of miR-345-3p/TRAF6 Axis. Arch. Med. Res. 53, 271–279. https://doi.org/10.1016/j.arcmed.2022.01.004 (2022).
Guo, B. et al. Salidroside attenuates HALI via IL-17A-mediated ferroptosis of alveolar epithelial cells by regulating Act1-TRAF6-p38 MAPK pathway. Cell. Commun. Signal. 20, 183. https://doi.org/10.1186/s12964-022-00994-1 (2022).
Zou, Z. L., Sun, M. H., Yin, W. F., Yang, L. & Kong, L. Y. Avicularin suppresses cartilage extracellular matrix degradation and inflammation via TRAF6/MAPK activation. Phytomedicine. 91, 153657. https://doi.org/10.1016/j.phymed.2021.153657 (2021).
Guo, J. et al. IL-13 induces YY1 through the AKT pathway in lung fibroblasts. PLoS One. 10, e0119039. https://doi.org/10.1371/journal.pone.0119039 (2015).
Zhang, Y. et al. miR–29a suppresses IL–13–induced cell invasion by inhibiting YY1 in the AKT pathway in lung adenocarcinoma A549 cells. Oncol. Rep. 39, 2613–2623. https://doi.org/10.3892/or.2018.6352 (2018).
Lax, A. et al. Silencing of microRNA-106b-5p prevents doxorubicin-mediated cardiotoxicity through modulation of the PR55α/YY1/sST2 signaling axis. Mol. Ther. Nucleic Acids. 32, 704–720. https://doi.org/10.1016/j.omtn.2023.04.031 (2023).
Chen, S., Wu, L., Peng, L., Wang, X. & Tang, N. Hepatitis B virus X protein (HBx) promotes ST2 expression by GATA2 in liver cells. Mol. Immunol. 123, 32–39. https://doi.org/10.1016/j.molimm.2020.04.024 (2020).
Baba, Y. et al. GATA2 is a critical transactivator for the human IL1RL1/ST2 promoter in mast cells/basophils: opposing roles for GATA2 and GATA1 in human IL1RL1/ST2 gene expression. J. Biol. Chem. 287, 32689–32696. https://doi.org/10.1074/jbc.M112.374876 (2012).
Tao, Q. et al. Hypoxia promotes the expression of Von Willebrand factor in breast cancer cells by up-regulating the transcription factor YY1 and down-regulating the hsa-miR-424. Eur. J. Pharmacol. 934, 175308. https://doi.org/10.1016/j.ejphar.2022.175308 (2022).
Yin, Q. et al. ADAM28 from both endothelium and gastric cancer cleaves Von Willebrand factor to eliminate Von Willebrand factor-induced apoptosis of gastric cancer cells. Eur. J. Pharmacol. 898, 173994. https://doi.org/10.1016/j.ejphar.2021.173994 (2021).
Tao, Q. et al. Breast cancer cells-derived Von Willebrand Factor promotes VEGF-A-related angiogenesis through PI3K/Akt-miR-205-5p signaling pathway. Toxicol. Appl. Pharmacol. 440, 115927. https://doi.org/10.1016/j.taap.2022.115927 (2022).
Funding
This work was sponsored by Natural Science Foundation of Shanghai [grant number 21ZR1416900], Shanghai 2023 Science and Technology Innovation Action Plan Medical Innovation Research Special Project (23Y11905400) and Shanghai Frontiers Science Center of Optogenetic Techniques for Cell Metabolism (Shanghai Municipal Education Commission).
Author information
Authors and Affiliations
Contributions
Yanqing Zhu and Xin Liang conceived the study. Yanqing Zhu carried out the experiments and analyzed the data. Yuxin Lu, Yifei Zhu, Xiaolu Ren, and Qinyi Deng: methodology, data acquisition. Yanqing Zhu and Xin Liang drafted the manuscript. Yanqing Zhu, Xin Liang, and Muqing Yang edited and revised the manuscript. All authors have read and agreed to this version of the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Animal studies
Animal experiments conducted in mice were using protocols approved by the IACUC of East China University of Science and Technology.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhu, Y., Lu, Y., Zhu, Y. et al. ST2L promotes VEGFA-mediated angiogenesis in gastric cancer by activating TRAF6/PI3K/Akt/NF-κB pathway via IL-33. Sci Rep 14, 26393 (2024). https://doi.org/10.1038/s41598-024-76763-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-76763-6
Keywords
This article is cited by
-
Effects of PELP1 on proliferation, metastasis and angiogenesis of epithelial ovarian cancer
Medical Oncology (2025)
-
Monotropein represses the proliferation and angiogenesis via the AKT/NF-κB pathway in lung cancer
Naunyn-Schmiedeberg's Archives of Pharmacology (2025)