Abstract
Vitamin D (vitD) deficiency is frequently observed in patients with pulmonary arterial hypertension (PAH) and, in these patients, low levels of vitD correlate with worse prognosis. The aim of this study was to examine the expression and the antiproliferative role of vitD receptor (VDR) and its signalling pathway in the human pulmonary vasculature. VDR presence and expression was analyzed in lungs, pulmonary artery smooth muscle cells (PASMC) and endothelial cells (PAEC) from controls and PAH-patients. VDR expression and VDR-target genes were examined in PASMC treated with calcitriol. The antiproliferative effect of 48 h-calcitriol was studied in PASMC by MTT and BrdU assays. VDR is expressed in PASMC. It is downregulated in lungs and in PASMC, but not in PAEC, from PAH-patients compared to non-hypertensive controls. Calcitriol strongly upregulated VDR expression in PASMC and the VDR target genes KCNK3 (encoding TASK1), BIRC5 (encoding survivin) and BMP4. Calcitriol produced an antiproliferative effect which was diminished by silencing or by pharmacological inhibition of survivin or BMPR2, but not of TASK1. In conclusion, the expression of VDR is low in PAH-patients and can be rescued by calcitriol. VDR exerts an antiproliferative effect in PASMC by modulating survivin and the BMP signalling pathway.
Similar content being viewed by others
Pulmonary arterial hypertension (PAH) is a multifactorial chronic disorder characterized by pulmonary vascular remodeling and pulmonary artery (PA) vasoconstriction1. A key hallmark is the vascular remodeling due to an excessive and uncontrolled proliferation and resistance to apoptosis of PA smooth muscle cells (PASMC) and endothelial cells (PAEC), which leads to intimal thickening and PA obliteration2,3. As a consequence, the right ventricle (RV) tends to adapt to afterload with compensatory RV hypertrophy, which may follow with RV failure and premature death.
In recent years, we4 and others5 have demonstrated that vitamin D (vitD) deficiency is much more frequent in PAH-patients than in the general population or even compared to patients with other cardiovascular diseases. In PAH-patients or animal models, lower levels of vitD are associated with worse functional class, reduced 6-min-walking-distance, increased mean pulmonary artery pressure (mPAP), increased pulmonary vascular resistance, higher levels of BNP/pro-BNP, decreased cardiac output, reduced therapeutic response to phosphodiesterase 5 inhibitors and/or reduced survival4,5,6,7.
The active form of vitD, 1,25(OH)2vitD (i.e., calcitriol) activates the vitD receptor (VDR), a transcriptional factor that regulates the expression of specific target genes8. VDR is expressed in many tissues and cell types, including endothelial and smooth muscle cells. It exerts a wide variety of actions unrelated to its well-known effects on calcium and phosphorus homeostasis, i.e., the so-called non-calcemic actions. These effects have potential relevance in key processes within the cardiovascular and respiratory systems, such as cell proliferation, differentiation and migration, control of vascular tone, immunomodulation and regulation of metabolism among others9.
The role of VDR in pulmonary vasculature and PAH has not been explored. The aim of the present study is to characterize the presence, expression profile and effects mediated by VDR in the pulmonary vasculature, its antiproliferative role in PAH and the potential mechanism.
Results
Lung VDR gene expression is downregulated in PAH
We searched the expression of VDR in a public transcriptome dataset comparing 27 different organs. VDR expression in the lungs was lower than in gastrointestinal organs, the kidneys and the skin but higher than in most other organs (Fig. 1A). In another public transcriptomic database (GSE117261), VDR lung expression was downregulated in PAH-patients compared to controls (Fig. 1B, control vs all-PAH, p < 0.05, Mann–Whitney test). VDR lung expression was not significantly different among the different PAH subgroups (One-way ANOVA, Fig. 1B). The expression in women with PAH was significantly reduced compared to controls. In men, due to the smaller sample size, the difference did not achieve statistical significance (p = 0.06). We also analyzed VDR mRNA expression by qRT-PCR and VDR protein expression by Western blot in lungs from PAH-patients and in control human lungs (Fig. 1C,D). We confirmed that there was a significant VDR protein downregulation in PAH-patients (Fig. 1D).
VDR is relatively highly expressed in human lungs and it is downregulated in PAH. (A) VDR gene expression pattern in different tissues. Data from the NCBI database, project PRJEB4337. Data is log-transformed into Log2(RPKM + 1) and represented as dot plot and mean. (B) Analysis of VDR mRNA expression in transcriptome database GSE117261 in controls and PAH-patients (All PAH). The analysis was also segregated by sex and by subclass of PAH, including associated (APAH), familial PAH (FPAH), idiopathic PAH (IPAH) or other or unknown causes. (C) VDR mRNA expression by qRT-PCR and (D) VDR protein normalized by GAPDH expression by Western Blot in lungs samples from controls and PAH-patients. Multiple bands of VDR were quantified according to the manufacturer’s antibody technical sheet. Results are represented as scatter plots and bars with medians. **p < 0.01 and ***p < 0.001, non-parametric Mann Whitney test.
VDR gene expression within PASMC and PAEC
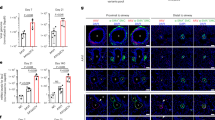
The immunohistochemistry in lung sections from controls shows that VDR colocalized with DAPI, indicating nuclear staining, in almost all cells in the vessel wall (Fig. 2A). We have also re-analyzed the VDR expression data from a published study of sc RNAseq in PAH10. Figure 2B shows the UMAP plot identifying the different cell clusters. In Fig. 2C, the data confirms that several vascular cell types express VDR, including pericytes, fibroblasts, smooth muscle and endothelial cells and it is also expressed in most types of immune cells. In PAH, VDR appears to be downregulated in PAH in vascular cells as compared to controls. VDR expression, analyzed by immunocytochemistry, was maintained in cultured PASMC from control subjects (Fig. 2D). In these cells, VDR was located mainly in the nucleus with lower expression in the cytosol. We compared VDR mRNA expression in cultured control human PASMC vs PAEC by qRT-PCR. Figure 2E shows that VDR mRNA was much higher in PASMC compared to cultured human control PAEC. PASMC from PAH-patients in culture also showed lower VDR expression compared to those from healthy donors (Fig. 2F). In contrast, VDR expression in PAEC was similar in controls and PAH-patients (Fig. 2G).
VDR is expressed in cultured human PASMC and it is upregulated by calcitriol. (A) Representative immunohistochemistry of VDR in a control human lung showing a pulmonary artery, the elastin autofluorescence is shown in green. (B) Uniform manifold approximation and projection (UMAP) plot of the pulmonary artery scRNA-Seq data of donor (Control, n = 3) and pulmonary arterial hypertension (PAH, n = 3) samples are colored by cell identity. (C) Dot plot with VDR gene expression by cell identity and condition (Control and PAH). (D) Immunocytochemistry of PASMC stained with anti-VDR. (E–G) PASMC VDR mRNA expression by qRT-PCR in (E) PAEC (n = 9) vs PASMC (n = 6) from control donors, (F) PASMC from control donors (n = 6) vs PASMC from PAH patients (n = 8), and (G) PAEC from control donors (n = 9) vs PAEC from PAH patients (n = 13). (H) VDR upregulation at 24 and 48 h by calcitriol (100 nmol/l) in PASMC from control donors (n = 6) and PASMC from PAH patients (n = 8). Results are represented as scatter plots and bars with medians. ***p < 0.001 vs PAEC, *p < 0.05 vs controls, non-parametric Mann Whitney test. In panel H, the box shows two-way ANOVA results and **p < 0.01 following Bonferroni’s multiple comparisons test.
VDR expression is rescued after calcitriol treatment
We treated human PASMC from controls subjects and PAH-patients with calcitriol for 24 and 48 h. VDR mRNA expression was strongly and time-dependently upregulated as analyzed by RT-qPCR (Fig. 2H). Notably, VDR mRNA expression was significantly lower in PASMC from PAH-patients at 24 h after calcitriol treatment. However, at 48 h, VDR mRNA expression in PAH-PASMC was similar to control-PASMC.
VDR inhibits PASMC proliferation
We then examined the antiproliferative effects of VDR stimulation with calcitriol in PASMC from PAH and controls. In the presence of vehicle, PASMC exhibited a proliferative response with a 50–100% increase in viable cells at 48 h as measured by the MTT assay. The proliferation was strongly inhibited in a concentration-dependent manner by calcitriol (1–100 nmol/l) as measured by either the BrdU or the MTT assay (Figs. 3A,B, respectively). The inhibitory effect of calcitriol on proliferation was also observed in PASMC from PAH-patients (Fig. 3C). In fact, the inhibition was significantly higher for 10 nM calcitriol in PAH (94 ± 16%, Fig. 3C) than in control cells (49 ± 11%, p < 0.05, Fig. 3B).
Calcitriol exerts antiproliferative effects in human PASMC. (A) and (B) Proliferation in human PASMC from 5 (in triplicate) controls measured by BrdU incorporation and MTT assay, respectively, and (C) proliferation in PASMC from 4 different cultures from PAH-patients, in duplicate or triplicate, by MTT assay, after exposure to calcitriol (1–100 nmol/l) for 48 h. Results are expressed as mean ± SEM. *, **, *** indicates p < 0.05, p < 0.01 and p < 0.001 vs vehicle, one-way ANOVA, Bonferroni test.
Calcitriol modulates several genes of interest in PAH-proliferation
Next, we analyzed the expression of several genes potentially modulated by VDR and whose dysregulation is known to be involved in PASMC proliferation in PAH. Interestingly, in human control PASMC, calcitriol treatment for 48 h increased the expression of KCNK3 (Fig. 4A), the gene encoding TASK-1 potassium channel, as well as decreased the expression of BIRC5, gene encoding survivin protein (Fig. 4B). We also analyzed its effects on genes of the BMPR2 and TGFβ signaling pathway. Calcitriol significantly enhanced the expression of BMP4 but the effect did not achieve significance for BMP6 (p = 0.06), ligands of BMPR2, but had no effects on BMPR2 itself, or in genes related to its canonical, Smad-dependent, signaling pathway (Fig. 4C).
Calcitriol modulates the expression of genes of interest in PAH. Expression of (A) KCNK3 (gene encoding TASK1, n = 6), (B) BIRC5 (gene encoding survivin, n = 7) and (C) Genes involved in the BMP signaling pathway (n = 3–6) after exposure to vehicle o calcitriol (100 nmol/l) for 48 h. Data in panels A and B are represented as plot before-after. The grey point represents the mean. *p < 0.05, non-parametric paired test vs vehicle. Results in panel C are expressed as mean ± SEM.
Calcitriol inhibits PASMC proliferation partly via BMPR2 pathway
We analyzed whether the BMPR2 pathway was involved in the antiproliferative effect of calcitriol in control PASMC by using a gene silencing and a pharmacological approach. Forty-eight hours after targeted knockdown (siBMPR2), BMPR2 mRNA expression was reduced by ̴ 65% as compared to a scramble siRNA (Fig. 5A) leading to a significant increase in cell proliferation measured by the MTT assay (Fig. 5B). Notably, loss of BMPR2 gene partially decreased the antiproliferative effect of vitD in human cultured PASMC, analyzed both by MTT (Fig. 5C) and BrdU assays (Fig. 5D). To confirm this result, in other set of experiments, we treated human control PASMC with the BMPR2 signaling inhibitor DMH1(5 µmol/l)11. We did not find that the inhibition of BMPR2 signaling by DMH1 increased PASMC proliferation (Fig. 5E). However, and consistent with the BMPR2 silencing experiments, the antiproliferative effect of calcitriol was also partly inhibited by DMH1 as measured by either MTT or BrdU assays (Fig. 5E). Thus, calcitriol inhibited the BrdU incorporation by 55 ± 3% and 21 ± 7% in the absence and presence of DMH-1, respectively, (p < 0.001). For the MTT test the inhibition was 36 ± 5% and 18 ± 6%, respectively (p < 0.01). Because BMPR2 can also signal via a noncanonical Smad-independent pathway involving MAP kinases, we also analyzed the effects of calcitriol in the presence of MAP kinases inhibitors. The antiproliferative effect of calcitriol was not modified in the presence of the p38 MAPK inhibitor SB203580 (10 µmol/l) or the ERK MAPK selective inhibitor PD98059 (5 µmol/l) as analyzed by either BrdU and MTT assays (supplementary Fig. 1A,B).
Calcitriol inhibits PASMC proliferation via BMPR2. Human control PASMC were transfected with siRNA for BMPR2 (siBMPR2) or control siRNA (scramble). (A) BMPR2 mRNA expression assessed by qRT-PCR after 48 h post-transfection. Data is expressed as scatter plots and bars with medians, *p < 0.05, Mann–Whitney test. (B) Effects of calcitriol on proliferation in silenced BMPR2 PASMC measured by MTT assay. Results are expressed as mean ± SEM. *p < 0.05, t-test vs scramble. (C) and (D) Effects of calcitriol (1–100 nmol/l) on proliferation in PASMC transfected with siBMPR2 or scramble, measured by MTT and BrdU assays, respectively. *p < 0.05, two-way ANOVA, Bonferroni post hoc test, vs scramble. (E) Effects of calcitriol on proliferation in PASMC in the presence or absence of DMH1 (5 µmol/l), by MTT and BrdU assay; ***p < 0.001 calcitriol vs vehicle (black column), two way-ANOVA. n = 4–5 different cultures in duplicate or triplicate.
Calcitriol inhibits PASMC proliferation partly by via survivin downregulation
The antiproliferative effect-induced by calcitriol was also evaluated in human PASMC transfected with siRNA against BIRC5, the gene encoding survivin protein, or incubated with the survivin inhibitor YM155. Forty-eight hours after targeted knockdown, siRNA reduced BIRC5 mRNA expression by ~ 80% (Fig. 6A). The siBIRC5 (Fig. 6B) or 20 nM YM155 (Fig. 6C) lead to a ~ 50% decrease in cell proliferation measured by the MTT assay or by the BrdU assay. Moreover, BIRC5 knockdown abolished the calcitriol induced antiproliferative effect in human PASMC (Fig. 6D). Similarly, the survivin inhibitor YM155 also abolished the antiproliferative effect of calcitriol (Fig. 6E).
The antiproliferative effect of calcitriol is suppressed in the presence of survivin inhibition. (A) BIRC5 (gene encoding survivin) mRNA expression assessed by qRT-PCR 48 h post-transfection with siRNA against BIRC5 (siBIRC5) or scramble siRNA. Data is expressed as scatter plots and bars represent the median, *p < 0.05, Mann–Whitney test. (B, C) Proliferation of PASMC transfected with siBIRC5 or scramble (B) or YM155 (20 nmol/l) measured by MTT and BrdU assays. (D, E) Effects of calcitriol (1–100 nmol/l) on proliferation in PASMC transfected with siBIRC5 or scramble (D) or treated with YM155 (20 nmol/l) (E) measured by BrdU assay for 48 h. Data are expressed as mean ± SEM. *p < 0.05 and **p < 0.01 vs scramble or calcitriol, two-way ANOVA, Bonferroni test. n = 3–4 different cultures in triplicate.
The antiproliferative effects of calcitriol are preserved in KCNK3 silenced PASMC
To analyze the role of TASK-1 channels in the antiproliferative effect of calcitriol we transfected control human PASMC with KCNK3 siRNA (n = 5; supplementary Fig. 2). This resulted in an increase of proliferation, measured by either MTT or BrdU assays, respectively. Notably, KCNK3 silencing did not affect the antiproliferative effect of calcitriol, analyzed by MTT and BrdU.
Effects of calcitriol on PAEC proliferation
We studied the effects of calcitriol (10–100 nmol/l) on proliferation of control PAEC and PAEC derived from patients with CTEPH, measured by MTT and BrdU incorporation assays (supplementary Fig. 3). Calcitriol at 10 nmol/l increased cell growth in both control and CTEPH patient-derived PAEC. By contrast, 100 nmol/l of calcitriol had no effect on cell growth.
Calcitriol has no relaxant effect in PA
We further evaluated the direct vasodilator effect of calcitriol in PA from control subjects mounted in a wire myograph, pre-contracted with a cocktail of pulmonary vasoconstrictors: endothelin-1 (ET-1, 3 nmol/l), thromboxane A2 mimetic U46619 (30 nmol/l) and serotonin (5-HT, 3 µmol/l). Under these conditions, calcitriol (0.01–10 nmol/l) had no acute vasodilator effect (supplementary Fig. 4).
Discussion
In the present study we have studied the expression and location of VDR and the effects mediated by this receptor in the pulmonary vasculature and its potential role in PAH. For the first time, we have provided evidence that: (1) VDR is expressed in several cell types in the lung, including PASMC, (2) it is downregulated in lungs and PASMC from PAH-patients, (3) calcitriol treatment can rescue the expression of VDR, (4) it upregulates KCNK3 and BMP4 and downregulates survivin gene expression in PASMC and finally, (5) it exerts an antiproliferative effect in PASMC by modulating the survivin and the BMP signaling pathways. However, calcitriol had no antiproliferative effect in PAEC and had no direct vasodilator effect in human PA.
VDR gene expression pattern in the NCBI database is consistent with the fact that tissues with the highest VDR content are those associated with maintenance of calcium homeostasis. Thus, VDR is widely distributed throughout the small intestine from the duodenum to the ileum and the large intestine, specifically in intestinal epithelial cells to promote calcium absorption; and in distal renal tubules to regulate calcium re-absorption12. Apart from this group of organs, there is another group of tissues with lower, but considerable expression of VDR, including the lung. Previous studies have demonstrated that VDR is expressed in human bronchial epithelial cells13. In addition, VDR was expressed in human cultured PASMC while the expression in PAEC was significantly lower. Immunohistochemistry and sc RNAseq confirmed that VDR is expressed in several vascular cell types including, in decreasing order, fibroblasts and pericytes, smooth muscle and endothelial cells.
Previous studies from our group and others4,5,6,7 have demonstrated that vitD deficiency is much more frequent in PAH than in general population and, remarkably, low levels of total 25(OH)vitD are associated with poor prognosis. However, to date, none of these studies have explored the role of VDR in PAH. First, we analyzed VDR in the publicly available whole transcriptome database GSE11726114. The analysis showed that VDR expression is downregulated in lungs from PAH-patients. We also confirmed by qPCR and Western blot that VDR expression is decreased in lungs from a cohort of Spanish PAH-patients compared to lungs discarded from transplant donors. In sc RNAseq experiments, VDR seems to be reduced in vascular but not in immune cells in PAH. The data, due to the small sample size (n = 3) and limited sequencing depth, does not allow to firmly conclude whether VDR is reduced in a given vascular cell type or not. We also examined VDR expression in human cultured PASMC and PAEC from controls and PAH. VDR mRNA downregulation was confirmed in cultured PASMC. However, in PAEC the VDR expression was lower, and we found no differences between controls and PAH-patients. Notably, low levels of lung VDR is associated with several pulmonary diseases, including COPD15 and idiopathic pulmonary fibrosis16, and correlates with worse prognosis. Homozygous VDR-deficient mice present alterations in adherent junctions in the lung, suggesting that VDR may play an important role in maintaining pulmonary barrier integrity17, and pulmonary vascular alterations including upregulated Kv7 channel activity18.
VDR gene can be regulated in a tissue-specific manner by a variety of factors, which include its own most active ligand, calcitriol. Thus, there is a physiological positive feedback loop between the levels of calcitriol and VDR expression19. This autoregulation has been observed in a wide variety of cells. It can be caused by increased VDR gene transcription, concordant with the presence of a vitamin D response element (VDRE) in the promoter region of VDR gene, and by stabilization of VDR, decreasing the rate of receptor degradation. Consistent with this view, our previous results showed that PAH-patients presented severe deficit of vitD and we now also show decreased lung VDR expression in these patients. Thus, we asked whether calcitriol treatment would rescue the downregulated VDR expression in PAH-PASMC. Our results showed that VDR mRNA expression was strongly upregulated by calcitriol. Remarkably, VDR expression in PAH-PASMC reached similar levels compared to those in control PASMC after 48 h of calcitriol treatment.
The discovery of VDR in many tissues that do not participate in calcium and phosphorous homeostasis led to identify a wide variety of functions mediated by VDR of potential relevance in cardiovascular diseases, such as cell proliferation, differentiation and apoptosis, cell adhesion, oxidative stress, angiogenesis and anti-inflammatory activity9. Therefore, we studied some potential VDR-mediated effects of interest in PAH, including those on cell proliferation.
VitD metabolites have been demonstrated to alter cellular proliferation through multiple mechanisms. Calcitriol was shown to elicit antiproliferative effects in both normal and in pathologic situations, including cancer-derived cell lines and vascular smooth muscle cells (VSMC)20,21,22,23. Accordingly, we have found that calcitriol exerts antiproliferative effects in human PASMC in vitro. Notably, the inhibitory effect of calcitriol on cellular growth was also observed in PASMC from PAH-patients. In fact, calcitriol treatment attenuated pulmonary vascular remodeling in an animal model of PAH24. Our results reveal that calcitriol rescues the expression of downregulated VDR in PASMC from PAH-patients to exert antiproliferative effects. Nuclear localization of VDR seems to be necessary to exert antiproliferative actions as reported in other cell types. For instance, in breast cancer, in the absence of ligand, accumulation of VDR in the cytoplasm promotes cell growth, in contrast to the antiproliferative nuclear action of the calcitriol-VDR complex25.
Next, we analyzed several possible targets of VDR involved in the antiproliferative effect. We selected the BMPR2 pathway, TASK1 and survivin, because they are known to be involved in PAH and because we found them to be dysregulated in the lungs of vitamin D deficient rats26. Loss of function mutations and downregulation of KCNK3 (gene encoding TASK-1 potassium channel) are present in hereditable and idiopathic PAH and contribute to the increased pulmonary arterial vasoconstriction and PASMC and PAEC proliferation, leading to pulmonary arterial remodeling27,28. Few reports have demonstrated that KCNK3 may be a target of VDR29,30,31 and we have also identified a VDRE in the KCNK3 gene promoter26. It must be highlighted that KCNK3 expression and activity in VSMC seem to be almost lost under culture conditions (~ 75% reduction)32. Despite this, we found that calcitriol is able to significantly upregulate KCNK3 mRNA expression in cultured PASMC from controls as well as from PAH-patients26. However, the antiproliferative effects of calcitriol were not affected by KCNK3 inhibition suggesting that the upregulation of KCNK3 does not contribute to the calcitriol-induced antiproliferative effect. Nevertheless, it is expected that the increase in KCNK3 expression may enhance and improve TASK-1 function and thereby limiting PA vasoconstriction in PAH-patients.
A misbalance between BMPRII and transforming growth factor-β (TGF-β) pathways is a well-known hallmark of PAH33. Loss of function mutation of BMPR2 gene or downregulated or dysfunctional BMPRII signaling leads to aberrant PASMC proliferation34,35. Accordingly, we found increased proliferation after BMPR2 silencing in PASMC. Interestingly, our results indicate that loss of BMPR2 gene or pharmacological inhibition of the canonical BMPRII pathway partially decreased the antiproliferative effect of calcitriol in human cultured PASMC, suggesting that the BMPRII pathway plays a role on the inhibition of cellular growth in PASMC induced by VDR. However, the inhibition of the non-canonical Smad-independent pathway of BMPR2 signaling with p38 or ERK MAPK inhibitors, did not affect the antiproliferative effect of calcitriol. The activation of BMPR2 signaling pathway may be mediated, at least in part, by the ligand BMP4, whose expression is increased after calcitriol treatment in human PASMC. Accordingly, some previous studies have identified that BMP4 may be a target of VDR in VSMC30,36 and in other cell types. In the context of PAH, BMP4 and its antiproliferative effects via a canonical Smad-dependent pathway are decreased37,38,39. Therefore, those agents enhancing BMP/Smad signaling in PASMC can restore the growth-suppressive effects of BMP4. In vitD deficient rats we also found reduced BMP4 and BMP6 expression in the lung26. In addition, calcitriol treatment attenuated the upregulation of Tgfβ2 in PAH-animals24. However, our in vitro data in control PASMC showed that calcitriol did not reduce TGFβ expression.
Survivin protein, encoded by the BIRC5 gene, belongs to the inhibitor of apoptosis family. In the context of PAH, survivin is upregulated in PA from PAH patients and in experimental models of PAH40,41. Both in vitro and in vivo, inhibition of survivin induces PASMC apoptosis, decreases proliferation and increases Kv channel activity40,41. Previous published studies have reported that calcitriol negatively regulates survivin, and this downregulation is essential for the antiproliferative effects of calcitriol in several types of cancer cells42,43. Likewise, we have found that calcitriol decreases BIRC5 gene expression in control human PASMC. In addition, silencing BIRC5 suppressed the antiproliferative effect of calcitriol measured by BrdU incorporation, despite it did not affect viability measured by MTT. We confirmed these results using the YM155 compound, which blocks the expression of this protein via inhibition its promoter and reduces right ventricle systolic pressure and pulmonary vascular remodeling in an animal model of PAH44. Altogether the data indicate that calcitriol inhibits PASMC proliferation at least partly by the suppression of survivin.
Finally, we found that calcitriol did not induce an acute vasodilation in precontracted human PA, ruling out a regulation of pulmonary vascular tone by VDR though non-genomic mechanisms. However, this does not exclude long term effects of VDR controlling vessel tone. In fact, mice with endothelial specific Vdr gene deletion and rats with and vitD deficiency show endothelial dysfunction26,45. Moreover, calcitriol did not elicit an antiproliferative effect in PAEC.
In conclusion, PAH-patients not only present severe deficit of vitD, but also a reduction in lung VDR. Calcitriol rescues VDR expression and induces an antiproliferative effect in PASMC. These data reinforce the view that vitD deficiency may contribute to the pathogenesis of PAH. From a mechanistic point of view, BMPRII signalling and survivin play a role on the antiproliferative effect of calcitriol in PASMC.
Methods
Ethics statements in human samples
All methods were performed in accordance with the National and European guidelines and regulations. NO organs/tissues were procured from prisoners. Research using human samples were approved by the Ethics Committee of the Getafe Hospital (Madrid, Spain), Ciberes Biobank (Barcelona, Spain), French institutional Ethics Committee (Protocol N8CO-08-003, ID RCB: 2008-A00485-50), Hospital Clínic of Barcelona ethics committee (HCB/2018/0837 and HCB/2018/0434). Informed consent was obtained in all cases. Human explanted lung tissues from non-PAH subjects were obtained from non-tumor lung areas of the resection specimens from patients undergoing surgery for lung carcinoma or discarded for lung transplantation. The donor lungs included in this study were thoroughly reviewed by a pathologist. PAH samples were obtained from lung transplantation. Cells, tissues and data were obtained from different sources but all statistical comparisons have been performed in the same laboratory using the same protocols and culture conditions.
Human primary pulmonary artery smooth muscle cell culture
From donor subjects, PA explants were isolated from non-tumor lung areas from patients undergoing surgery for lung cancer (from Hospital de Getafe) including carcinoid tumor (n = 2), epidermoid cancer (n = 2) and adenocarcinoma (n = 3). Primary human PASMC cultures were derived from small PA (< 1 mm internal diameter). Briefly, human PA were dissected in physiological Krebs solution, cut longitudinally into small fragments and endothelium was removed. PA explants were placed in 25 cm2 cultured flask and maintained in Smooth Muscle Cell Growth Medium supplemented with Smooth Muscle Cell Growth Supplement (310–500, Cell Applications, San Diego, USA). Around two weeks, PASMC reach 80–90% confluence and they were subcultured.
Human PASMC from patients with idiopathic PAH were used in this study. Research with these PAH-patient samples and controls undergoing surgery for localized lung cancer was part of the French Network on Pulmonary Hypertension, a program approved by the institutional Ethics Committee. PAH-PASMC were cultured as previously described28.
PASMC were seeded at 3.000 cells per well for proliferation assays in 96-well plate and 100.000 cells per well in 6-well plate. After adhesion, cells were starved of serum (0.1% of FBS) for 24 h. Then, PASMC were treated with different concentrations (1–100 nmol/l) of calcitriol (1α,25-Dihydroxyvitamin D3, D1530, Sigma-Aldrich, St. Louis, USA) or vehicle (DMSO, 0.1%). PASMC were also incubated with DMH-1 (5 µmol/l, Tocris, Bristol, UK), SB203580 (10 µmol/l, Sigma-Aldrich, St. Louis, USA) and PD98059 (5 µmol/l, Merck, Darmstadt, Germany), p38 and ERK MAPK inhibitors, respectively, and YM155 (20 nmol/l, Sigma-Aldrich, St. Louis, USA) a survivin inhibitor.
All PASMC were used within passages 2–4 and were cultured in a humidified atmosphere of 5% CO2 in air at 37 ºC. Treated cells were used to analyze gene expression, immunocytochemistry, and cell viability and proliferation assays.
Human pulmonary artery endothelial cells
Primary human PAEC were isolated from PA from controls subjects (n = 9) and PAH patients (n = 13), as previously described28 and used at passages 3–5. Research with these PAH-patient samples was part of the French Network on Pulmonary Hypertension, a program approved by the institutional Ethics Committee.
Commercial control PAEC (kindly provided by Professor Maria Jose Calzada from Hospital La Princesa, Madrid, Spain) and PAEC derived from patients with chronic thromboembolic PH (CTEPH)46 who underwent pulmonary endarterectomy at the Hospital Clinic of Barcelona, Spain, were used in this study for proliferation assays. PAEC were seeded at a density of 5.000 cells per-96-well and treated with calcitriol (1–100 nmol/l) or vehicle (DMSO, 0.1%). All human PAEC were maintained with EGM-2 Endothelial Cell Growth Medium (CC-4176, Lonza, Basel, Switzerland) and were used for this study between passages 3 and 4.
Immunofluorescence staining
Immunostaining was performed in frozen lung slices from control subjects and cultured human PASMC from and healthy donors and PAH patients (n = 3). Culture human PASMC were fixed with 4% paraformaldehyde/PBS and blocked and permeabilized with PBS containing 0.4% Triton X-100 and 3% BSA for 1 h at room temperature. Samples were subsequently incubated with the primary antibody mouse anti-VDR (1:100, SC-13133, Santa Cruz, Dallas, USA) at 4 ºC overnight and then 15 min at 37 °C. Afterward, PASMC were incubated with the secondary antibody goat anti-mouse Alexa Fluor 594 (1:200, A11032, Thermo Fisher Scientific, Massachusetts, USA) for 1 h. Nuclei were stained with DAPI. All images were taken by confocal fluorescence microscope.
siRNA transfection
Human control PASMC were transfected with specific siRNA against BMPR2 (SR300456B, OriGene, Maryland, USA), KCNK3 (gene encoding TASK-1, SASI_Hs01_00108786, Sigma-Aldrich, St. Louis, USA), BIRC5 (gene encoding survivin, SASI_Hs01_00052229, Sigma-Aldrich, St. Louis, USA), and control non-targeting siRNA (SIC001 scramble siRNA, Sigma-Aldrich, St. Louis, USA), using LipofectamineTM RNAiMAX (Thermo Fisher Scientific, Massachusetts, USA) according to the manufacturer’s instructions as previously reported47. Briefly, PASMC were seeded in a 96-well-plate or 6-well-plate at a density of 3.000 cells/well and 100.000 cells/well, respectively, with complete medium Smooth Muscle Cell Growth Medium supplemented with Smooth Muscle Cell Growth Supplement (310–500, Cell Applications, San Diego, USA). After 24 h of serum starvation, the complex of siRNA with a final concentration of 10 nmol/l and lipofectamine were mixed with Opti-MEM Reduced Serum Media (Thermo Fisher Scientific, Massachusetts, USA) supplemented with 2% FBS and 1% antibiotic/antimycotic solution and transfected into PASMC. Twenty-four hours after transfection, the medium was replaced with Smooth Muscle Cell Growth Medium supplemented with 5% FBS, non-essential amino acid solution (1x), pyruvate solution (1x), penicillin (100 U/ml), streptomycin (0.1 mg/ml) and amphotericin B (0.25 µg/ml) plus calcitriol (1–100 nmol/l) or vehicle (DMSO, 0.1%). After 48 h, PASMC were collected for RNA isolation and to perform cell viability and proliferation assays.
Cell viability and cell proliferation assays
Cell viability was assessed by the colorimetric MTT assay (1 mg/ml; Sigma-Aldrich, St. Louis, USA) in active proliferating cells. After one hour of incubation at 37 ºC, absorbance was recorded at a wavelength of 540 nm by a colorimetric plate reader (EZ Read 400 Microplate Reader, Biochrom, Cambridge, UK). Data were expressed as percentage of absorbance values at baseline time or vehicle. In addition, cell proliferation was also analyzed by 5-bromo-2’-deoxyuridine (BrdU) incorporation assay, following the manufacturer’s protocol (Roche Applied Science, Penzberg, Germany). The absorbance was measured at dual wavelength of 450–620 nm. Results were normalized as a percentage of the vehicle’s values. Each experimental condition was performed at least in triplicate.
Vascular reactivity
Human donor PA rings (1.7–2 mm long, ~ 0.8 mm internal diameter) were mounted in a wire myograph with Krebs solution maintained at 37 °C and bubbled with 95% O2 and 5% CO2. PA were stretched to give an equivalent transmural pressure of 30 mmHg. After equilibration, the effect of calcitriol was assessed by cumulative doses (0.01–10 nmol/l) in pre-contracted PA rings with a cocktail of pulmonary vasoconstrictors: endothelin-1 (ET-1, 3 nmol/l, Sigma-Aldrich, St. Louis, USA), thromboxane A2 mimetic U46619 (30 nmol/l, Sigma-Aldrich, St. Louis, USA) and serotonin (5-HT, 3 µmol/l, Sigma-Aldrich, St. Louis, USA).
Gene expression by qRT-PCR
Frozen lungs used for lung VDR expression were obtained from the Ciberes Biobank; controls from organ donors (n = 10, 4 women/6 men, 61[8] years (mean [SD]), 10% smokers, 30% ex-smokers) were discarded for lung transplantation because were > 65y (n = 4) or were smokers or recent ex-smokers (n = 2), had atelectasia (n = 1), or had long time stay at ICU (n = 3) and PAH lungs were explanted from idiopathic PAH patients (n = 10, 4 women/6 men, 45[9] years, 70% ex-smokers). Total RNA from human lung and PASMC were extracted with the NucleoSpin RNA kit (Macherey–Nagel, Düren, Germany), Absolutely RNA Microprep kit (Stratagene Cloning Systems, Agilent Technologies, CA, USA), depending on the sample, according to the supplier’s instructions in all cases and including DNAse digestion. RNA quantity and quality were assessed with NanoDropTM 1000 Spectrophotometers (Thermo Fisher Scientific). One microgram of total RNA extracted was reverse transcribed into cDNA using iScriptTM cDNA Synthesis Kit (Biorad) or High Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific), following manufacturer’s instructions. Gene expression was determined by quantitative real-time PCR (qRT-PCR) with a TaqMan Gene Expression Master Mix (Thermo Fisher Scientific), using specific primers from Applied Biosystems databases (Thermo Fisher Scientific, Massachusetts, USA) listed in supplementary Table 1. Amplifications, detections and analyses were performed in a 7.900HT Fast Real-time OCR System (Centro de Genomica, Universidad Complutense, Madrid, Spain). The delta-delta Ct method was used to quantify relative changes in mRNA expression. Gene expression was normalized against 18S, β-ACTIN or GNB2L1.
Western blot
Six frozen human lungs from controls and PAH patients as described above were homogenized with a lyses buffer containing Trizma Pre-set crystals pH 7.5, DL-dithiothreitol (DTT) 1 M, NP40 1% and supplemented with protease and phosphatase inhibitor cocktail (Roche Diagnosis GmbH, Mannheim, Germany) in a TissueLyser device (Qiagen, Hilden, Germany). Twenty-five µg from lung homogenates were run on a sodium dodecyl sulphate–polyacrylamide electrophoresis and proteins were transferred to polyvinylidene difluoride membranes. They were incubated with the primary antibody against VDR (1:200, SC-13133, Santa Cruz, Dallas, USA)48 and then with the appropriate secondary horseradish peroxidase conjugated antibody. Results were normalized by the relative signal of Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH; 1:10.000, Sigma-Aldrich, St. Louis, USA). Antibody binding was detected by an ECL system (SuperSignal West Fento Chemiluminescent Substrate, Thermo Fisher Scientific Massachusetts, USA). Blots were imaged using an Odissey Fc System (Li-COR, Biosciences, Nebraska, USA) and were quantified by densitometry using the Quantity One software (Bio-Rad laboratories, CA, USA). Full length Western blots are shown in supplementary Fig. 5.
VDR analysis in transcriptomic databases
Tissue distribution of VDR expression was analyzed using RNA-Seq data from 27 human healthy tissues provided by the National Center for Biotechnology Information (NCBI) portal (bioproject PRJEB4337, ID: 231263). To compare normal vs PAH VDR lung expression, RNA-Seq data was downloaded from the publicly available Gene Expression Omnibus (GEO) dataset GSE117261 (https://www.ncbi.nlm.nih.gov/gds/). This dataset is the largest published transcriptome in PAH, containing the gene expression of 58 patients with PAH who underwent lung transplantation14. It included explanted lungs from 32 patients with idiopathic PAH (IPAH), 17 patients with associated PAH (APAH), 5 patients with familial PAH (FPAH) and 4 with other or unknown forms of PAH. Control lung samples were obtained from 25 transplant failed donors, i.e. those who did not find an appropriate recipient, but still meeting physiologic standards. The patient demographics and the detailed methods using Affymetrix human HuGene1.0-ST microarrays are described in the original paper and its supplement14. VDR expression profile at single cell level was extracted from the publicly available dataset GSE210248. This dataset derives from a sc RNAseq from human pulmonary arteries of controls and PAH lungs from Crnkovic et al10. All analyses were performed using R (R version 4.3.3) and Seurat (Seurat_5.0.3). We performed differential expression analysis using DESeq2 on the sample level, treating samples as independent observations by pseudobulk approach. Cell annotation was done according to Crnkovic et al. 2022.
Statistics
Statistical analyses were performed using GraphPad Software v7 (GraphPad Software Inc., USA). All data were tested for normal distribution (D’Agostino-Pearson normality test) and parametric or non-parametric statistics were used as appropriate. Multiple samples comparisons were analyzed by one-way or two-way ANOVA following by Bonferroni post hoc test. Data are presented either as scatter plots and medians or as means ± sem. p-values less than 0.05 were considered statistically significant.
Data availability
Data available on request from F.P-V, fperez@med.ucm.es.
References
Simonneau, G. et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 53, 1801913. https://doi.org/10.1183/13993003.01913-2018 (2019).
Wilkins, M. R. Pulmonary hypertension: The science behind the disease spectrum. Eur. Respir. Rev. 21, 19–26. https://doi.org/10.1183/09059180.00008411 (2012).
Pullamsetti, S. S., Savai, R., Seeger, W. & Goncharova, E. A. Translational advances in the field of pulmonary hypertension. From cancer biology to new pulmonary arterial hypertension therapeutics. Targeting cell growth and proliferation signaling hubs. Am. J. Respir. Crit. Care Med. 195, 425–437. https://doi.org/10.1164/rccm.201606-1226PP (2017).
Callejo, M. et al. Total, bioavailable, and free vitamin d levels and their prognostic value in pulmonary arterial hypertension. J. Clin. Med. 9, 448. https://doi.org/10.3390/jcm9020448 (2020).
Tanaka, H. et al. Therapeutic impact of dietary vitamin D supplementation for preventing right ventricular remodeling and improving survival in pulmonary hypertension. PLoS One 12, e0180615. https://doi.org/10.1371/journal.pone.0180615 (2017).
Callejo, M., Blanco, I., Barbera, J. A. & Perez-Vizcaino, F. Vitamin D deficiency, a potential cause for insufficient response to sildenafil in pulmonary arterial hypertension. Eur. Respir. J. 58, 2101204. https://doi.org/10.1183/13993003.01204-2021 (2021).
Mirdamadi, A. & Moshkdar, P. Benefits from the correction of vitamin D deficiency in patients with pulmonary hypertension. Caspian J. Intern. Med. 7, 253–259 (2016).
Moore, D. D. et al. The NR1H and NR1I receptors: Constitutive androstane receptor, pregnene X receptor, farnesoid X receptor alpha, farnesoid X receptor beta, liver X receptor alpha, liver X receptor beta, and vitamin D receptor. Pharmacol. Rev. 58, 742–759. https://doi.org/10.1124/pr.58.4.6 (2006).
Bikle, D. & Christakos, S. New aspects of vitamin D metabolism and action: Addressing the skin as source and target. Nat. Rev. Endocrinol. https://doi.org/10.1038/s41574-019-0312-5 (2020).
Crnkovic, S. et al. Single-cell transcriptomics reveals skewed cellular communication and phenotypic shift in pulmonary artery remodeling. JCI Insight 7, 153471. https://doi.org/10.1172/jci.insight.153471 (2022).
Gaskill, C. F. et al. Disruption of lineage specification in adult pulmonary mesenchymal progenitor cells promotes microvascular dysfunction. J. Clin. Invest. 127, 2262–2276. https://doi.org/10.1172/JCI88629 (2017).
Wang, Y., Zhu, J. & DeLuca, H. F. Where is the vitamin D receptor?. Arch. Biochem. Biophys. 523, 123–133. https://doi.org/10.1016/j.abb.2012.04.001 (2012).
Menezes, R. J. et al. Vitamin D receptor expression in normal, premalignant, and malignant human lung tissue. Cancer Epidemiol. Biomark. Prev. 17, 1104–1110. https://doi.org/10.1158/1055-9965.Epi-07-2713 (2008).
Stearman, R. S. et al. Systems analysis of the human pulmonary arterial hypertension lung transcriptome. Am. J. Respir. Cell Mol. Biol. 60(6), 637–649. https://doi.org/10.1165/rcmb.2018-0368OC (2019).
Fei, J. et al. Low vitamin D status is associated with epithelial-mesenchymal transition in patients with chronic obstructive pulmonary disease. J. Immunol. 203, 1428–1435. https://doi.org/10.4049/jimmunol.1900229 (2019).
Tzilas, V. et al. Vitamin D prevents experimental lung fibrosis and predicts survival in patients with idiopathic pulmonary fibrosis. Pulm. Pharmacol. Ther. 55, 17–24. https://doi.org/10.1016/j.pupt.2019.01.003 (2019).
Chen, H., Lu, R., Zhang, Y. G. & Sun, J. Vitamin D receptor deletion leads to the destruction of tight and adherens junctions in lungs. Tissue Barriers 6, 1–13. https://doi.org/10.1080/21688370.2018.1540904 (2018).
Olivencia, M. A. et al. Vitamin D receptor deficiency upregulates pulmonary artery Kv7 channel activity. Int. J. Mol. Sci. 24, 12350. https://doi.org/10.3390/ijms241512350 (2023).
Pike, J. W. & Meyer, M. B. The vitamin D receptor: New paradigms for the regulation of gene expression by 1,25-dihydroxyvitamin D(3). Endocrinol. Metab. Clin. North Am. 39, 255–269. https://doi.org/10.1016/j.ecl.2010.02.007 (2010).
Jeon, S. M. & Shin, E. A. Exploring vitamin D metabolism and function in cancer. Exp. Mol. Med. 50, 20. https://doi.org/10.1038/s12276-018-0038-9 (2018).
Somjen, D. et al. 25-hydroxyvitamin D3–1alpha-hydroxylase is expressed in human vascular smooth muscle cells and is upregulated by parathyroid hormone and estrogenic compounds. Circulation 111, 1666–1671. https://doi.org/10.1161/01.Cir.0000160353.27927.70 (2005).
Wu-Wong, J. R., Nakane, M., Ma, J., Ruan, X. & Kroeger, P. E. Effects of Vitamin D analogs on gene expression profiling in human coronary artery smooth muscle cells. Atherosclerosis 186, 20–28. https://doi.org/10.1016/j.atherosclerosis.2005.06.046 (2006).
Zhang, Z. et al. Preventive effects of vitamin D treatment on bleomycin-induced pulmonary fibrosis. Sci. Rep. 5, 17638. https://doi.org/10.1038/srep17638 (2015).
Yu, H. et al. 1,25(OH)2D3 attenuates pulmonary arterial hypertension via microRNA-204 mediated Tgfbr2/Smad signaling. Exp. Cell. Res. 362, 311–323. https://doi.org/10.1016/j.yexcr.2017.11.032 (2018).
Trivedi, T. et al. The vitamin D receptor is involved in the regulation of human breast cancer cell growth via a ligand-independent function in cytoplasm. Oncotarget 8, 26687–26701. https://doi.org/10.18632/oncotarget.15803 (2017).
Callejo, M. et al. Vitamin D deficiency downregulates TASK-1 channels and induces pulmonary vascular dysfunction. Am. J. Physiol. Lung Cell. Mol. Physiol. 319, L627–L640. https://doi.org/10.1152/ajplung.00475.2019 (2020).
Antigny, F. et al. Potassium channel subfamily K member 3 (KCNK3) contributes to the development of pulmonary arterial hypertension. Circulation 133, 1371–1385. https://doi.org/10.1161/circulationaha.115.020951 (2016).
Lambert, M. et al. Characterization of Kcnk3-mutated rat, a novel model of pulmonary hypertension. Circ. Res. 125, 678–695. https://doi.org/10.1161/CIRCRESAHA.119.314793 (2019).
Milani, C. et al. Transcriptional effects of 1,25 dihydroxyvitamin D-3 physiological and supra-physiological concentrations in breast cancer organotypic culture. BMC Cancer 13, 119. https://doi.org/10.1186/1471-2407-13-119 (2013).
Shalhoub, V. et al. Chondro/osteoblastic and cardiovascular gene modulation in human artery smooth muscle cells that calcify in the presence of phosphate and calcitriol or paricalcitol. J. Cell Biochem. 111, 911–921. https://doi.org/10.1002/jcb.22779 (2010).
Campos, L. T. et al. Differences in transcriptional effects of 1α,25 dihydroxyvitamin D3 on fibroblasts associated to breast carcinomas and from paired normal breast tissues. J. Steroid Biochem. Mol. Biol. 133, 12–24. https://doi.org/10.1016/j.jsbmb.2012.08.002 (2013).
Manoury, B., Etheridge, S. L., Reid, J. & Gurney, A. M. Organ culture mimics the effects of hypoxia on membrane potential, K(+) channels and vessel tone in pulmonary artery. Br. J. Pharmacol. 158, 848–861. https://doi.org/10.1111/j.1476-5381.2009.00353.x (2009).
Sharmin, N., Nganwuchu, C. C. & Nasim, M. T. Targeting the TGF-beta signaling pathway for resolution of pulmonary arterial hypertension. Trends Pharmacol. Sci. 42, 510–513. https://doi.org/10.1016/j.tips.2021.04.002 (2021).
Morrell, N. W. et al. Genetics and genomics of pulmonary arterial hypertension. Eur. Respir. J. https://doi.org/10.1183/13993003.01899-2018 (2019).
Morrell, N. W. Pulmonary hypertension due to BMPR2 mutation. Proc. Am. Thoracic Soc. 3, 680–686. https://doi.org/10.1513/pats.200605-118SF (2006).
Panizo, S. et al. RANKL increases vascular smooth muscle cell calcification through a RANK-BMP4-dependent pathway. Circ. Res. 104, 1041–1048. https://doi.org/10.1161/circresaha.108.189001 (2009).
Cai, P., Kovacs, L., Dong, S., Wu, G. & Su, Y. BMP4 inhibits PDGF-induced proliferation and collagen synthesis via PKA-mediated inhibition of calpain-2 in pulmonary artery smooth muscle cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 312, L638–L648. https://doi.org/10.1152/ajplung.00260.2016 (2017).
Jeffery, T. K., Upton, P. D., Trembath, R. C. & Morrell, N. W. BMP4 inhibits proliferation and promotes myocyte differentiation of lung fibroblasts via Smad1 and JNK pathways. Am. J. Physiol. Lung Cell. Mol. Physiol. 288, L370–L378. https://doi.org/10.1152/ajplung.00242.2004 (2005).
Yang, J. et al. Id proteins are critical downstream effectors of BMP signaling in human pulmonary arterial smooth muscle cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 305, L312–L321. https://doi.org/10.1152/ajplung.00054.2013 (2013).
McMurtry, M. S. et al. Gene therapy targeting survivin selectively induces pulmonary vascular apoptosis and reverses pulmonary arterial hypertension. J. Clin. Invest. 115, 1479–1491. https://doi.org/10.1172/jci23203 (2005).
Zhang, S. et al. Targeted inhibition of survivin with YM155 promotes apoptosis of hypoxic human pulmonary arterial smooth muscle cells via the upregulation of voltage-dependent K(+) channels. Mol. Med. Rep. 13, 3415–3422. https://doi.org/10.3892/mmr.2016.4977 (2016).
Diaz, R. et al. Differential regulation of TP73 isoforms by 1alpha,25-dihydroxyvitamin D3 and survivin in human colon and breast carcinomas. Genes Chromosome Cancer 49, 1135–1142. https://doi.org/10.1002/gcc.20821 (2010).
Koike, H. et al. Survivin is associated with cell proliferation and has a role in 1a,25-dihydroxyvitamin D3 induced cell growth inhibition in prostate cancer. J. Urol. 185, 1497–1503. https://doi.org/10.1016/j.juro.2010.12.005 (2011).
Blanco, I. et al. Survivin inhibition with YM155 ameliorates experimental pulmonary arterial hypertension. Front. Pharmacol. 14, 1145994. https://doi.org/10.3389/fphar.2023.1145994 (2023).
Ni, W. et al. Elimination of vitamin D receptor in vascular endothelial cells alters vascular function. Hypertension 64, 1290–1298. https://doi.org/10.1161/hypertensionaha.114.03971 (2014).
Tura-Ceide, O. et al. Derivation and characterisation of endothelial cells from patients with chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 44, P2327 (2014).
Morales-Cano, D. et al. Activation of PPARbeta/delta prevents hyperglycaemia-induced impairment of Kv7 channels and cAMP-mediated relaxation in rat coronary arteries. Clin. Sci. 130, 1823–1836. https://doi.org/10.1042/CS20160141 (2016).
Srikuea, R., Hirunsai, M. & Charoenphandhu, N. Regulation of vitamin D system in skeletal muscle and resident myogenic stem cell during development, maturation, and ageing. Sci. Rep. 10, 8239. https://doi.org/10.1038/s41598-020-65067-0 (2020).
Funding
This study was supported by grants SAF2016-77222-R and PID2019-107363RB-I00 to FPV and PID2020-117939RB-I00 ta AC funded by MCIN/AEI/https://doi.org/10.13039/501100011033 and by FEDER Una manera de hacer Europa, Instituto de Salud Carlos III (PI15/01100 to LM, PI18/00960 to O.T-C., Miguel Servet grant CP17/00114 to O.T-C.) with funds from the European Union and Fundación Contra la Hipertensión Pulmonar (Empathy grant). M.C. and M.A.O. are funded by Universidad Complutense grants.
Author information
Authors and Affiliations
Contributions
M.C. and D.M.-C. coordinated the study. M.C., D.M.-C., M.A.O., G.M.-P., V.M., B.B., A.S.-N. did the experiments. A.C., L.M., A.V., F.P. contributed to the study design and relevant discussions. F.P., N.M, O.T.-C. contributed cells from controls and PAH patients. F.P.-V. conceived the study. M.C. and F.P-V. wrote a draft and the final version of the manuscript, respectively, with substantial contributions from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Research using human samples were approved by the Ethics Committee of the Getafe Hospital (Madrid, Spain), Ciberes Biobank (Barcelona, Spain) and French institutional Ethics Committee (Protocol N8CO-08-003, ID RCB: 2008-A00485-50). Informed consent was obtained in all cases. Human explanted lung tissues from non-PAH subjects were obtained from non-tumor lung areas of the resection specimens from patients undergoing surgery for lung carcinoma or discarded for lung transplantation. The donor lungs included in this study were thoroughly reviewed by a pathologist. PAH samples were obtained from lung transplantation.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Callejo, M., Morales-Cano, D., Olivencia, M.A. et al. Vitamin D receptor and its antiproliferative effect in human pulmonary arterial hypertension. Sci Rep 14, 27445 (2024). https://doi.org/10.1038/s41598-024-78380-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-78380-9