Abstract
Childhood cognitively stimulating activities have been associated with higher cognitive function in late life. Whether activities in early or late childhood are more salient, and whether activities are associated with specific cognitive domains is unknown. Participants retrospectively reported cognitively stimulating activities at ages 6, 12, and 18 years. 4,198 participants were aged 55 to 77 years at cognitive testing. Six tasks measured overall cognitive function, processing speed, visual short-term memory, attention, cognitive control, episodic memory, working memory, perception, vocabulary, and verbal reasoning. Cognitively stimulating activities across childhood were associated with higher cognitive scores (highest versus lowest quartile, beta = 0.18 SD, 95% CI = 0.12, 0.23). In models adjusted for activities at each age, only age 18 activities were associated with overall cognition. The association of activities with cognitive function was strongly positive at the lowest levels of activities, with little association at middle and high levels of activities. A test of crystalized intelligence was most strongly associated with activities; tests assessing processing speed, visual short-term memory, visual working memory, and sustained attention were least associated. If the associations we found are causal, increasing cognitively stimulating activities in the late teen years among those with very few activities may benefit late life cognitive health.
Similar content being viewed by others
Cognitive function in late life is an important determinant of healthy aging. Better cognitive function improves health-related quality of life1, performance of activities of daily living2, and medication adherence3, and decreases risk of loneliness4 and risk of infection5,6,7. Identifying and supporting factors that improve late-life cognitive health may thus promote healthy aging. Several lines of evidence suggest that cognitively stimulating activities in childhood may lead to improved cognitive function in late life. Cognitively stimulating activities in early childhood, such as shared parent-child reading, have been linked to childhood literacy, numeracy, higher vocabulary comprehension and production, and improved school performance, including in experimental designs8,9,10. Cognitively simulating activities with parents and peers improve parent-child and peer relationships, as well as children’s mental health11,12,13. Mental health in turn improves childhood school performance and increases educational attainment and may thereby increase cognitive reserve into late life14,15,16,17.
Several studies have examined the association between cognitively stimulating activities in childhood, e.g., reading magazines, playing board games, and cognitive health in late life, finding associations with higher cognitive function at ages 65 years and older18,19,20. Associations of childhood cognitively stimulating activities with rate of cognitive decline have been mixed, with no association in two studies19,21, and an association with slower cognitive decline after accounting for brain pathology in another study22. Greater combined early- and mid-life cognitive activity has been associated with substantially reduced risk of Alzheimer’s disease23 and with lower levels of biomarkers of beta-amyloid deposition in the brain24. Most studies of this relationship have been conducted in a single cohort18,23,25,26.
In the present study, we sought to expand on prior studies to obtain a more granular understanding of the relationship between early-life cognitively stimulating activities and late-life cognitive health. We examined this relationship in a large cohort, the St. Louis Baby Tooth – Later Life Health Study. While many prior studies consider childhood as a single time frame without examining specific developmental periods19,25 or queried activities only in teen years20, in the present study, we queried cognitively stimulating activities at three timepoints in childhood (at ages 6, 12, and 18 years). Further, as aspects of cognitive function may be differently affected by childhood environment18,19,25, we separately examined associations with 6 tests capturing distinct components of cognitive function. Prior studies have not investigated whether associations between cognitively stimulating activities and later cognitive function may be non-linear, with threshold or saturation (ceiling) effects27,28. Here we examined non-linear associations between activities and cognitive function. Finally, most extant studies have not carefully account for childhood socioeconomic status (SES), which has been associated with cognitive function in late life and may confound an association between childhood activities and late-life cognitive function18,25,26,29. Therefore, we adjusted for childhood SES using detailed measures.
Methods
Study participants
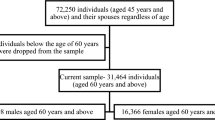
In 1958, the Baby Tooth Survey began collecting teeth from children to assess possible exposure to radiation30. Approximately 300,000 teeth were collected from these volunteers, largely in the St. Louis area, but also more widely. Recently, roughly 100,000 of these teeth were found, and in 2021, we began attempting to recontact the approximately 36,000 participants who donated these 100,000 teeth and for whom we could find potential current contact information, to invite them to participate in the St. Louis Baby Tooth – Later Life Health Study. We asked participants to complete online questionnaires regarding early-life engagement in cognitively stimulating activities and demographic factors. Participants then completed cognitive assessments. Enrollment is ongoing. As of August 10, 2023, 4,198 participants had provided sufficient responses to early-life cognitive activity questions and completed all cognitive tests and were included in our analyses. Forty-seven people declined to participate. Of the remaining non-respondents, many may have never seen our invitation, due to our having an incorrect address or other issue. The Institutional Review Board of the Harvard T. H. Chan School of Public Health approved this study. All research was performed in accordance with relevant guidelines/regulations. Written informed consent was obtained from all participants. Anonymized data not published in this article will be made available on request from corresponding author.
Early-life cognitively stimulating activities
The Rush Alzheimer’s Disease Center Lifetime Cognitive Activity scales were used to measure early-life participation in cognitively stimulating activities, hereafter termed “cognitively stimulating activities.”19,22,26,31 the scales query age-appropriate cognitively stimulating activities separately for ages 6, 12, and 18 years, e.g., playing board games, reading, writing, and doing homework. For each activity, the frequency of, or time spent in, the activity was scored on a 5-point scale from least to most frequent (e.g., once a year or less to every day; complete questionnaire in eTable 1). Points were then averaged across activities to create a mean score at each age. If more than half the age-specific items were missing, the score was considered missing. A composite early-life cognitive activity score was created by taking the mean of the three age-specific scores. This composite served as the exposure for our primary analyses. To investigate possible dose-dependent relations of early-life activities with cognitive function, we divided the age-specific and composite activity scores into quartiles.
Cognitive function
Participants completed an online cognitive assessment measuring processing speed, visual short-term memory, attention, cognitive control, working memory, perception, general intelligence, and verbal reasoning. Six tests from the TestMyBrain digital platform32,33 were used: (1) TestMyBrain Digit Symbol Matching;33,34,35 (2) TestMyBrain Gradual Onset Continuous Performance Test;33,36,37 (3) TestMyBrain Verbal Paired Associates;33,38 (4) TestMyBrain Visual Paired Associates;33 (5) TestMyBrain Multiple Object Tracking;37 (6) TestMyBrain Vocabulary35,39 (eTable 2 describes the tests and the cognitive domains covered by each test). Both Verbal and Visual Paired tests are delayed recall tests (2–3 min for verbal pairs, 4–5 min for visual pairs) that have been used in prior studies of visual and verbal long-term memory33,40. These tests had good internal reliability (0.78–0.93) and construct validity (medium to high)24,32. We created z-scores for each test by standardizing at mean = 0 and SD = 1 and formed an overall cognitive score by taking the mean of the six test scores. Higher scores indicate better cognitive function.
Covariates
Demographic and early-life factors were by self-report, and included current age, sex, racial identity (response options were: Black/African American, white, Asian, American Indian/Alaskan Native, Native Hawaiian/Pacific Islander, prefer not to say, and prefer to self-describe; participants could choose more than one option), mother and father’s educational attainment (coded as: high school or less, associate degree or some college, college graduate, or more than college), family structure at age 12 years (lived with both parents, one parent, one parent and a step-parent, or neither parent), participant’s educational attainment (coded the same as parents’). Childhood SES was queried with a set of questions. Family financial status was queried with, “How would you describe your family’s financial situation when you were a very young child,” with response options “very poor,” “poor,” “average,” “well-off,” and “very well-off.”41 Childhood food insecurity was queried with: “Were there times when your family didn’t have enough to eat (yes/no)?”42 Participants were then asked a set of questions derived from the 1960 US Census43. They were asked how many adults and children they lived with (each coded continuously) and the number of bedrooms in their home when they were age 12 years; from these responses we calculated persons per bedroom (coded continuously). They were asked whether they had a kitchen, flush toilet, bathtub or shower, a separate freezer (not a small compartment inside a refrigerator), washing machine, clothes dryer, or hot water for their family’s private use, had this amenity shared with other families, or did not have this amenity; each item was coded in three levels. Number of radios, televisions, air conditioners, and automobiles at the home were each queried as none, one, or more than one (eTable 3)43. Missingness indicators were used for missing covariate data; all covariates had less than 10% missing data.
Statistical analyses
We first examined covariates by quartile of early-life activities. Next, to estimate the association of early-life activities with cognitive function, we fit generalized additive models with composite cognitive function as the dependent variable and quartiles of composite early-life cognitive activity score (i.e., mean score across all ages) as the independent variable, using an identity link and gaussian distribution. The first model adjusted for age at cognitive assessment, sex, and racial identity. Model 2 further adjusted for potential confounders: parental education, childhood living arrangement, and family financial status44. To investigate whether more detailed adjustment for childhood indicators of SES would change associations, in Model 3 we further adjusted for childhood home environment measures (e.g., food insecurity, number of persons and bedrooms in the home, and home amenities such as telephones).
To investigate the relationship between activities at each age in childhood and late-life cognitive function we used each age-specific activity score as the independent variable in separate models, adjusted for all covariates. To estimate the association of activities at older ages in childhood independently, after accounting for activities at earlier ages, we additionally adjusted these models for cognitively stimulating activities at younger ages (e.g., we adjusted the estimates of activities at age 12 for activities at age 6 years). To examine the association of activities at each age independently of activities at other ages, we fit a model adjusted for activities at each of the three ages simultaneously.
To assess possible non-linear exposure-response relations between cognitive scores and activity scores in models adjusted for all childhood socioeconomic indicators in more detail than quartiles, we used generalized additive models using penalized cubic splines45. We fit curves for overall cognitive score and for each cognitive sub-test separately for activities at each childhood age.
Educational attainment is an important determinant of later life cognitive function. At age 18, some of our participants may have: (1) finished their education; (2) been in high school; or (3) been in college. Because of these varied circumstances, for some participants, educational attainment might both influence age 18 activities and have a direct effect on later-life cognitive function (i.e., not through age 18 activities), and therefore be a confounder. For some participants, educational attainment might be a mediator of this relationship (eFigure 1). Despite this complication, as a sensitivity analysis, we examined the association of age 18 activities with cognitive function further adjusted for educational attainment to reduce possible confounding by education.
Analyses were conducted in R software, v4.2.2. This study was approved by the Institutional Review Board of the Harvard T.H. Chan School of Public Health (IRB20-0040).
Results
Study participants were ages 55 to 77 years at cognitive test (mean age = 63). Participants in the lowest versus highest quartile of early-life activity were more likely to be male (57.0% versus 37.3%), have smoked (40.1% versus 28.9%), and have high school education or less (12.6% versus 1.7%, Table 1). Childhood circumstances differed significantly in participants with different levels of early-life activities. Participants in the lowest versus highest quartile of activities were more likely to have parents with high school education or less (mothers, 65.9% versus 38.8%; fathers, 52.5% versus 24.3%) and describe their family financial situation as poor or very poor (12.5% versus 4.4%). Activity scores at different ages were moderately to highly correlated (range = 0.52 to 0.76, eTable 4; activity score frequencies and means are shown in eFigure 2). The six cognitive tests had low to moderate correlations with each other (range = 0.14 to 0.42, eTable 5; distribution of cognitive scores are shown in eFigure 3).
In models adjusted for age at cognitive assessment, sex, and racial identity, greater childhood participation in cognitively stimulating activities was associated with higher cognitive scores in monotonically increasing fashion (highest quartile of activity, beta estimate = 0.26, 95% confidence interval (CI) = 0.21, 0.31, compared to the lowest quartile, Table 2, Model 1). Effect estimates were attenuated, but still highly statistically significant, after adjustment for parental education, childhood living arrangement, and family financial status (highest quartile, beta = 0.19, 95% CI = 0.14, 0.24, Table 2, Model 2). Further adjustment for detailed childhood socioeconomic indicators did not substantially further attenuate estimates (highest quartile, beta = 0.18, 95% CI = 0.12, 0.23, Table 2, Model 3).
In models examining cognitively stimulating activities at specific ages, more activities at each age were associated with better overall cognitive function. Associations were strongest for activities at age 18 years (Table 3, Model 1). Effect estimates for activities at ages 12 and 18 were similar in models accounting for activities at younger ages (Table 3, Model 2). In a model with all three ages entered simultaneously, effect estimates for age 18 activities remained strong and statistically significant, while estimates for activities at ages 6 and 12 had no association with late-life cognitive function (Table 3, Model 3).
We observed nonlinear relationships between early-life cognitive activity and the late-life cognitive score (Fig. 1, eFigure 4). For the composite cognitive score, at the lowest levels of activity (scores 1–2), cognitive function was most strongly associated with higher activity scores. The association between activity and cognitive function weakened across middle levels of activity (scores 2–4) and was flat or declining at the highest levels of activity (scores 4–5), although confidence intervals were wide at these activity levels. The shape of this relationship was similar for activities at each of the three ages, although activities at age 18 were more strongly associated with cognitive function than activities at younger ages.
Association of cognitive activities across early life and at ages 6, 12 and 18 years with late-life cognitive function, adjusted for parents’ education, childhood living arrangement, and detailed indicators of childhood socioeconomic status, the St. Louis Baby Tooth – Later Life Health Study, N = 4,198.
Exposure-response patterns differed for the six cognitive tests (Fig. 2). There was very little association between activities and score on TestMyBrain Multiple Object Tracking. For TestMyBrain Digit Symbol Matching, Verbal Paired Associates, and Visual Paired Associates, the association was non-linear. At low levels of activities (scores 1–2), a 1-unit increase in activity was associated with higher cognitive scores; for a 1-unit increase at the mid-range of activities levels (scores 2–4), this association was attenuated; and at high activity levels (scores 4–5), there was either no association with cognitive scores or a negative association. For both TestMyBrain Gradual Onset Continuous Performance and TestMyBrain Vocabulary, there was an approximately linear association of activities with cognitive test scores across the range of activity levels, with a steeper association of activities with Vocabulary scores.
Association of early-life cognitive activities with late-life cognitive function, separately for each cognitive test, adjusted for parents’ education, childhood living arrangement, and detailed indicators of childhood socioeconomic status, the St. Louis Baby Tooth – Later Life Health Study, N = 4,198.
In sensitivity analyses, the association of activities at age 18 with composite cognitive function was attenuated, though still robust, after adjusting for educational attainment (base model, highest quartile, beta = 0.24, 95% CI = 0.19, 0.29; further adjusted for educational attainment, highest quartile, beta = 0.13, 95% CI = 0.08, 0.18).
Discussion
Cognitively stimulating activities in childhood were positively associated with 6 tests of cognitive function and overall cognitive function in late life, at ages 55 to 77. Adjustment for parental education, family structure, and family financial status attenuated these associations, such that associations with two of 6 cognitive tests no longer reached statistical significance. Adjustment for even more detailed indicators of SES in childhood did not further attenuate associations, suggesting that confounding by SES was adequately accounted for in the simpler model.
Comparing across childhood periods, activities at age 18 were more strongly associated with cognitive function, and in models mutually adjusted for activities in the 3 childhood periods, activities at age 18 were the only childhood activities associated with late-life cognitive function. Moreover, the associations of cognitively stimulating activities at ages 6 and 12 years with cognitive function were fully attenuated in models further adjusted for activities later in childhood. This suggests that either the association with cognitively stimulating activities is a function only of activities at age 18, or that the effects of activities at the younger ages are mediated through their effects on activities at age 18.It is important to note, though, that if there were uncontrolled confounding of activity scores at age 18 and late-life cognitive function, this could introduce bias. However, such confounding would have to be unique to the age 18 scores and not affect scores at other ages, e.g., parental genetics could not confound since that would result in associations with scores at all ages46. This type of bias, when present, is generally small47. A single prior study separately examined associations of activities in early childhood (at ages 6 and 12 years combined) and at age 1826. This study found that age 18 activities explained greater variance in four of five tests of late-life cognitive function than did early childhood activities, although this study did not examine associations in mutually adjusted models.
Associations of overall cognitive function, Digit Symbol Matching, Verbal Paired Associates, and Visual Paired Associates were non-linear, with the greatest difference found between participants who experienced cognitively stimulating activities at the lowest level (“once/year or less,” “never”) versus just above the lowest level (“several times/year”, “less than one hour/day,” or “1–2 times”). Greater participation in activities above the lowest levels was not associated with further increases in cognitive function. TestMyBrain Vocabulary was a notable exception to this pattern, with a positive association of activities with test scores across the full range of activity at ages 12 and 18 years. In contrast to the other tests, TestMyBrain Vocabulary reflects knowledge acquisition. Early exposure to activities in which vocabulary is acquired, such as reading, may incline a person to continue to pursue these activities, resulting in life-long accumulation of vocabulary. In addition, our assessment of cognitively stimulating activities included many items relevant to reading (e.g., reading books, reading magazines, trips to the library), thus, may have better captured activities salient to vocabulary acquisition versus other cognitive abilities.
Our findings are consistent with several possibilities. For several aspects of cognitive function there may be a threshold of stimulation in childhood below which cognitive function is negatively impacted, and this negative impact persists into old age.
Early-life activities may raise the cognitive challenge of one’s later work or foster a lifelong habit of engaging in cognitively stimulating activities. Participation in age 18 cognitive activities may reflect the rigor of the high school education, which may be important for SES in adulthood, independently of further education48,49.
Other explanations are also consistent with these findings. To query age-appropriate activities, we assessed different types of activities at different ages. At age 6, two of the three questions tapped behavior by others, e.g., someone reading to you or telling you stories, while only one queried the participant’s own behaviors, e.g., playing games. At ages 12 and 18, in contrast, all questions probed the participant’s own behavior, and nearly all were related to reading, writing, or schoolwork. Thus, activities at ages 12 and 18 years, versus those at age 6, may be more stimulating for cognitive development. Alternatively, participation in activities at ages 12 and 18 may be more indicative of intelligence or a propensity toward lifelong learning. Children with higher cognitive function, especially as they age and gain more autonomy in selecting their activities, may choose to engage in more cognitively stimulating activities. A study of high-school graduates, for example, found that high school mental activities and high school IQ were associated, and both had direct effects on late-life cognitive function (at mean age = 74.8 years) in mutually adjusted models20. Interpreting childhood activities as causing later cognitive function rather than reflecting cognitive abilities is challenging, as children at all ages, but especially teens -- who have greater autonomy -- may select themselves into activities that reflect their interests, skills, and cognitive ability.
Our study has important limitations. Infrequent experience of cognitively stimulating activities (e.g., never reading or telling stories to a young child) may indicate parental neglect or indicate a low-resourced school or community (e.g., no nearby public library), both of which have been associated with lower cognitive function50,51,52. Childhood maltreatment or community resources may have partly driven the associations we found. We relied on retrospective reports of childhood activities, which may have been influenced by current circumstances, including cognitive function, which may have biased our results. Our measure of age 6 activities included only three items, thus, our measure of activities at age 6 may have more misclassification than our measure of activities at other ages. Non-differential misclassification would likely bias estimates of the association of age 6 activities with cognitive function toward the null hypothesis53. Our sample was predominantly comprised of white participants, and few participants identified themselves as being “very poor” in childhood, which may limit the generalizability of our findings.
Our findings concur with prior studies, in that we found childhood cognitively stimulating activities associated with late-life cognitive function. However, we found that for most cognitive domains, the positive association was primarily restricted to the low end of the activity distribution. Among participants with moderate or high levels of activities, there was little difference in cognitive performance by level of activity. These findings dovetail with prior studies indicating that low literacy and illiteracy are strong predictors of low cognitive function and dementia in late life54,55. Moreover, we found activities in late adolescence to be most robustly associated with cognitive function in late life. Should these associations prove to be causal, providing more cognitive stimulation to teens who otherwise would be at the lowest level of activities could benefit their cognitive health across the lifespan.
Data availability
Anonymized data not published in this article will be made available on request from corresponding author.
References
Wolinsky, F. D. et al. The effects of the ACTIVE cognitive training trial on clinically relevant declines in Health-Related Quality of Life. Journals Gerontology: Ser. B. 61 (5), S281–S287. https://doi.org/10.1093/geronb/61.5.S281 (2006).
Rebok, G. W. et al. Ten-Year Effects of the Advanced Cognitive Training for Independent and Vital Elderly Cognitive Training Trial on Cognition and Everyday Functioning in Older Adults. (2014). https://doi.org/10.1111/jgs.12607. Journal of the American Geriatrics Society. /01/01 2014;62(1):16–24. https://doi.org/10.1111/jgs.12607.
Hayes, T. L., Larimer, N., Adami, A. & Kaye, J. A. Medication Adherence in Healthy Elders: Small Cognitive Changes Make a Big Difference. Journal of Aging and Health. /06/01 2009;21(4):567–580. doi: (2009). https://doi.org/10.1177/0898264309332836
Yin, J., Lassale, C., Steptoe, A. & Cadar, D. Exploring the bidirectional associations between loneliness and cognitive functioning over 10 years: the English longitudinal study of ageing. Int. J. Epidemiol. 48 (6), 1937–1948 (2019).
Shah, F. A. et al. Bidirectional relationship between cognitive function and pneumonia. Am. J. Respir. Crit Care Med. 188 (5), 586–592. https://doi.org/10.1164/rccm.201212-2154OC (2013).
Wang, Y., Li, M., Kazis, L. E. & Xia, W. Clinical outcomes of COVID-19 infection among patients with Alzheimer’s disease or mild cognitive impairment. Alzheimer’s Dementia: J. Alzheimer’s Association May. 18 (5), 911–923. https://doi.org/10.1002/alz.12665 (2022).
Salive, M. E., Satterfield, S., Ostfeld, A. M., Wallace, R. B. & Havlik, R. J. Disability and cognitive impairment are risk factors for pneumonia-related mortality in older adults. Public Health Rep. 108 (3), 314 (1993).
O’Farrelly, C., Doyle, O., Victory, G. & Palamaro-Munsell, E. Shared reading in infancy and later development: Evidence from an early intervention. Journal of Applied Developmental Psychology. (2018). /01/01/ 2018;54:69–83. https://doi.org/10.1016/j.appdev.2017.12.001.
Fikrat-Wevers, S., van Steensel, R. & Arends, L. Effects of Family Literacy Programs on the Emergent Literacy Skills of Children From Low-SES Families: A Meta-Analysis. Review of Educational Research. /08/01 2021;91(4):577–613. doi: (2021). https://doi.org/10.3102/0034654321998075
Koshy, B. et al. Home environment: short-term trends and predictors in early childhood from an Indian community birth cohort. https://doi.org/10.1111/cch.12846. Child Care Health Dev. 2021/05/01 2021;47(3):336–348. https://doi.org/10.1111/cch.12846.
Oberle, E., Ji, X. R., Guhn, M., Schonert-Reichl, K. A. & Gadermann, A. M. Benefits of extracurricular participation in early adolescence: associations with peer belonging and Mental Health. J. Youth Adolesc. 2019/11/01 (11), 2255–2270. https://doi.org/10.1007/s10964-019-01110-2 (2019).
Hoyne, C. & Egan, S. M. Shared Book reading in early childhood: a review of influential factors and developmental benefits. Leanbh Og. 12 (1), 77–92 (2019).
Weisleder, A. et al. Links between shared reading and play, parent psychosocial functioning, and child behavior: evidence from a randomized controlled trial. J. Pediatr. 213, 187–195 (2019). e1.
Agnafors, S., Barmark, M. & Sydsjö, G. Mental health and academic performance: a study on selection and causation effects from childhood to early adulthood. Social Psychiatry and Psychiatric Epidemiology. /05/01 2021;56(5):857–866. doi: (2021). https://doi.org/10.1007/s00127-020-01934-5
Gräf, C. et al. Mental health problems and school performance in first graders: results of the prospective cohort study ikidS. Eur. Child. Adolesc. Psych. 10 (10), 1341–1352. https://doi.org/10.1007/s00787-019-01296-7 (2019). /01 2019.
Iraniparast, M. et al. Cognitive Reserve and mild cognitive impairment: predictors and rates of reversion to Intact Cognition vs Progression to Dementia. Neurology. 98 (11), e1114–e1123. https://doi.org/10.1212/wnl.0000000000200051 (2022).
Dekhtyar, S., Wang, H-X., Fratiglioni, L. & Herlitz, A. Childhood school performance, education and occupational complexity: a life-course study of dementia in the Kungsholmen Project. Int. J. Epidemiol. 45 (4), 1207–1215. https://doi.org/10.1093/ije/dyw008 (2016).
Wilson, R. S., Boyle, P. A., Yang, J., James, B. D. & Bennett, D. A. Early life instruction in foreign language and music and incidence of mild cognitive impairment. Neuropsychology. 29 (2), 292 (2015).
Everson-Rose, S. A., Mendes de Leon, C. F., Bienias, J. L., Wilson, R. S. & Evans, D. A. Early Life Conditions and Cognitive Functioning in Later Life. Am J. Epidemiol. 158(11), 1083–1089. https://doi.org/10.1093/aje/kwg263 (2003).
Fritsch, T. et al. Cognitive functioning in healthy aging: the role of reserve and lifestyle factors early in life. Gerontologist. 47 (3), 307–322 (2007).
Wilson, R. S. et al. Early life socioeconomic status and late life risk of Alzheimer’s disease. Neuroepidemiology 25 (1), 8–14 (2005).
Wilson, R. S., Boyle, P.A., Yu, L., Barnes, L. L., Schneider, J. A. & Bennett. D. A. Life-span cognitive activity, neuropathologic burden, and cognitive aging. Neurology 81(4), 314–321 (2013).
Wilson, R. S., Scherr, P. A., Schneider, J. A., Tang, Y. & Bennett, D. A. Relation of cognitive activity to risk of developing Alzheimer disease. Neurology. 69 (20), 1911–1920 (2007).
Landau, S. M. et al. Association of lifetime cognitive engagement and low β-amyloid deposition. Arch. Neurol. 69 (5), 623–629 (2012).
Oveisgharan, S., Wilson, R. S., Yu, L., Schneider, J. A. & Bennett, D. A. Association of early-life cognitive enrichment with Alzheimer disease pathological changes and cognitive decline. JAMA Neurol. 77 (10), 1217–1224 (2020).
Wilson, R. S., Barnes, L. L., Krueger, K. R., Hoganson, G., Bienias, J. L. & Bennett, D. A. Early and late life cognitive activity and cognitive systems in old age. J. Int. Neuropsychol. Soc. 11(4), 400–407 (2005).
Diekelmann, S., Biggel, S., Rasch, B. & Born, J. Offline consolidation of memory varies with time in slow wave sleep and can be accelerated by cuing memory reactivations. Neurobiology of Learning and Memory. (2012). /09/01/ 2012;98(2):103–111. https://doi.org/10.1016/j.nlm.2012.07.002.
Vemuri, P. et al. Association of lifetime intellectual enrichment with cognitive decline in the older population. JAMA Neurol. 71 (8), 1017–1024 (2014).
Cermakova, P., Formanek, T., Kagstrom, A. & Winkler, P. Socioeconomic position in childhood and cognitive aging in Europe. Neurology. 91 (17), e1602–e1610 (2018).
Reiss, L. Z. Strontium-90 absorption by deciduous teeth. Sci. Nov. 134(3491), 1669–1673 (1961).
Wilson, R. S., Barnes, L. L. & Bennett, D. A. Assessment of lifetime participation in cognitively stimulating activities. Cognitive Reserve Psychology, 173–186 (2013).
Passell, E. et al. Digital cognitive assessment: Results from the TestMyBrain NIMH Research Domain Criteria (RDoC) field test battery report. (2019).
Singh, S. et al. The TestMyBrain digital neuropsychology toolkit: development and psychometric characteristics. J. Clin. Exp. Neuropsychol. 43(8), 786–795 (2021).
Chaytor, N. S. et al. Construct validity, ecological validity and acceptance of self-administered online neuropsychological assessment in adults. Clin. Neuropsychol. 35(1), 148–164 (2021).
Hartshorne, J. K. & Germine, L. T. When does cognitive functioning peak? The asynchronous rise and fall of different cognitive abilities across the life span. Psychol. Sci. 26(4), 433–443 (2015).
Fortenbaugh, F. C. et al. Sustained attention across the life span in a sample of 10,000: dissociating ability and strategy. Psychol. Sci. 26 (9), 1497–1510 (2015).
Treviño, M. et al. How do we measure attention? Using factor analysis to establish construct validity of neuropsychological tests. Cogn. Research: Principles Implications. 6, 1–26 (2021).
Germine, L. et al. Is the web as good as the lab? Comparable performance from web and lab in cognitive/perceptual experiments. Psychon. Bull. Rev. 19, 847–857 (2012).
Richler, J. J., Wilmer, J. B. & Gauthier, I. General object recognition is specific: evidence from novel and familiar objects. Cognition. 166, 42–55 (2017).
Wilmer, J. B. et al. Capturing specific abilities as a window into human individuality: the example of face recognition. Cognit. Neuropsychol. 29 (5–6), 360–392 (2012).
Straughen, J. K., Caldwell, C. H., Osypuk, T. L., Helmkamp, L. & Misra, D. P. Direct and Proxy Recall of Childhood Socio-Economic Position and Health. Paediatr. Perinat. Epidemiol. 27 (3), 294–302. https://doi.org/10.1111/ppe.12045 (2013). /05/01 2013.
McWhorter, K. L. et al. Traumatic childhood experiences and multiple dimensions of poor sleep among adult women. Sleep. 42 (8), zsz108. https://doi.org/10.1093/sleep/zsz108 (2019).
United States Census Through the Decades Index of Questions. United States Department of Commerce. Accessed December 5. (2023). https://www.census.gov/history/www/through_the_decades/index_of_questions/
Bennett, D. A. et al. Religious orders study and rush memory and Aging Project. J. Alzheimers Dis. 64 (s1), S161–s189. https://doi.org/10.3233/jad-179939 (2018).
Wood, S. N. Generalized Additive Models: An Introduction with R (CRC, 2017).
Weisskopf, M. G., Tchetgen Tchetgen, E. J., Raz, R. & Commentary On the Use of Imperfect negative control exposures in epidemiologic studies. Epidemiol. May. 27 (3), 365–367. https://doi.org/10.1097/ede.0000000000000454 (2016).
Groenwold, R. Three types of bias: distortion of research results and how that can be prevented. Ned. Tijdschr. Geneeskd. 157 (40), A6497–A6497 (2013).
Burris, C. C., Welner, E. D. W. K. & Murphy, J. Accountability, Rigor, and Detracking: Achievement effects of embracing a challenging curriculum as a Universal Good for all students. Teachers Coll. Record. 2008/03/01(3), 571–607. https://doi.org/10.1177/016146810811000301 (2008).
Morgan, T. L., Zakhem, D. & Cooper, W. L. From High School Access to Postsecondary Success: an exploratory study of the impact of high-rigor coursework. Educ. Sci. 8(4). https://doi.org/10.3390/educsci8040191 (2018).
Vargas, T., Damme, K. S. F. & Mittal, V. A. Neighborhood deprivation, prefrontal morphology and neurocognition in late childhood to early adolescence. NeuroImage 2020/10/15/ 2020;220:117086. https://doi.org/10.1016/j.neuroimage.2020.117086
Strathearn, L. et al. Long-term cognitive, psychological, and health outcomes associated with child abuse and neglect. Pediatrics 146(4), e20200438 (2020).
Young-Southward, G., Eaton, C., O’Connor, R. & Minnis, H. Investigating the causal relationship between maltreatment and cognition in children: a systematic review. Child Abuse Negl. https://doi.org/10.1016/j.chiabu.2020.104603 (2020). /09/01/ 2020;107:104603.
Sorahan, T. & Gilthorpe, M. S. Non-differential misclassification of exposure always leads to an underestimate of risk: an incorrect conclusion. Occup. Environ. Med. Dec. 51 (12), 839–840. https://doi.org/10.1136/oem.51.12.839 (1994).
Rentería, M. A. et al. Illiteracy, dementia risk, and cognitive trajectories among older adults with low education. Neurology 2019/12/10 2019;93(24):e2247–e2256. https://doi.org/10.1212/WNL.0000000000008587
Lee, J-Y. et al. Illiteracy and the incidence of Alzheimer’s disease in the Yonchon County survey, Korea. Int. Psychogeriatr. 20 (5), 976–985. https://doi.org/10.1017/S1041610208007333 (2008).
Author information
Authors and Affiliations
Contributions
ALR led the writing and contributed to the analytic design. MGW conceptualized and supervised the study and edited the manuscript. XYQ conducted data analyses, prepared figures and tables, contributed to the analytic design, and edited the manuscript. KAM managed the data collection and edited the manuscript. LTG and RSR contributed to analytic design and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Roberts, A.L., Qiu, X., McAlaine, K.A. et al. Early-life cognitively stimulating activities and late-life cognitive function in the St. Louis Baby Tooth Later Life Health Study. Sci Rep 15, 2105 (2025). https://doi.org/10.1038/s41598-024-79083-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-79083-x